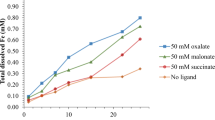
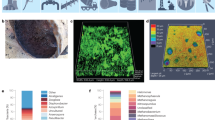
Abstract
IN NATURE of May 14, Dr. J. Newton Friend alludes to Gallionella ferruginea as obtaining its life's energy by oxidising ferrous carbonate and organic ferrous salts, causing the precipitation of rust, or ferric hydroxide. May I point out that Gallionella ferruginea can live and grow well without any iron at all, and so cannot be a vital factor in the metabolism of the bacterium, using the term “bacterium” in its widest sense? The oxidation which takes place can be simply explained by the fact that ferrous carbonate in solution is very unstable, becoming very rapidly oxidised.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MACFADYEN, W. The Corrosion of Iron and Steel. Nature 78, 55 (1908). https://doi.org/10.1038/078055d0
Issue Date:
DOI: https://doi.org/10.1038/078055d0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.