Abstract
Although association between multiple sclerosis (MS) and HLA-DR2, DQw6 has been well documented, family studies have not established linkage to HLA. Here we have (1) carried out an HLA-DQA1, -DQB1 association study in unrelated patients and controls, and (2) analyzed linkage between MS and HLA in multiplex families using both nonparametric and parametric methods. The subjects and families were derived from the genetically homogeneous Finnish population, and 14 of the 21 families came from a high-risk area with exceptional familial clustering of cases. In the association study, the frequencies of the alleles DQA1*0102 and DQB1*0602 (encoding DR2-associated DQw6 antigen) were significantly increased in MS patients compared to controls. In the families, we observed that the segregation of MS with DQA1*0102 and DQB1*0602 was not HLA haplotype specific, i.e., these alleles were frequently transmitted to MS relatives on different parental haplotypes. Consequently, we found strong evidence for linkage between MS and HLA only when the haplotype-independent segregation of the MS-associated alleles was controlled. This observation may partially explain the lack of linkage evidence in previous family studies. The highest LOD scores were obtained to the DQA1 locus (LODmax = 6.43, θ = 0.00). The linkage analyses suggest that both the patients’ HLA haplotypes may contribute to MS susceptibility. In one of a patient’s haplotypes, the susceptibility locus was closely associated with DQA1*0102 and DQB1*0602, whereas in the other haplotype no association with any of the individual candidate loci was found. These results demonstrate, for the first time, a close linkage between MS and HLA, and raise the possibility of distinct HLA-linked susceptibility genes in MS.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterized by loss of the myelin sheath and gliosis. Although both humoral and cell-mediated immune abnormalities have been observed in MS patients, their relation to the demyelination process is not understood. Both genetic and environmental factors have been implicated in the etiology of MS [1]. The evidence for genetic predisposition is mainly based on the higher concordance rate observed in monozygotic compared to dizygotic twins and other siblings [2], and on associations between MS and HLA. The most prominent HLA association has been demonstrated with DR2, DQw6 in populations of northern European ancestry [reviewed in ref. 3]. These populations show the highest incidence of the disease, whereas considerably lower incidences are found in other racial and ethnic groups [4]. The mode of inheritance has not been established, and the susceptibility is probably conferred by several interacting loci. In addition to HLA, a role for the T cell receptor β germline genes has been proposed [5, 6], although conflicting results have also been published [7, 8]. Apart from abnormalities in the immune response, defects in the myelinforming system have been suspected in MS. Recent association [9, 10] and linkage [10] data by us and others suggest close linkage between MS and the myelin basic protein (MBP) gene, located on chromosome 18.
The highly polymorphic HLA region is located on chromosome 6 (6p21.3) covering about 4 Mb of genomic DNA. HLA genes are subdivided into class I (HLA-A, -B and -C) and class II (HLA-DR, -DQ, -DP) genes. The gene products are heterodimeric cell surface glycoproteins required for the recognition of both self and foreign antigens by T lymphocytes. A hallmark of the HLA gene complex is linkage disequilibrium between alleles at different loci, which greatly complicates any attempt to pinpoint disease susceptibility to a specific locus.
A striking inconsistency exists between HLA association and linkage studies in MS. Despite the associations observed in several population studies, family linkage studies have not unequivocally established linkage between MS and HLA. Some affected sib-pair studies have reported increased sharing of HLA haplotypes among affected sib-pairs [11, 12], whereas others have found no deviation from random inheritance of HLA haplotypes [13–15]. LOD score analyses have provided evidence for linkage between MS and HLA [16–18], but the maximum LOD scores were obtained at recombination distances θ = 0.10–0.20, which are incompatible with the association maintained at the population level.
On the basis of shared HLA-DQ antigen residues in DR2, DQw6 and non-DR2, non-DQw6 patients, it has been suggested that the DQA1 and DQB1 genes coding for α and β chains of the DQ antigens, respectively, could be the primary HLA genes in MS susceptibility [19]. In the present study, therefore, we have analyzed linkage between MS and HLA-A, C, B, DR, DQA1, DQB1 haplotypes and also, for the first time, between MS and HLA candidate loci (DQA1 and DQB1) in 21 Finnish multicase families. We have also carried out an HLA-DQA1, -DQB1 association study. Since MS may be a genetically heterogeneous disease, it is advantageous to focus the analyses on a homogeneous population such as the Finns [20]. Furthermore, most of the families came from a high-risk area in western Finland demonstrating exceptional familial clustering of MS [21, 22].
Subjects and Methods
Subjects and Families
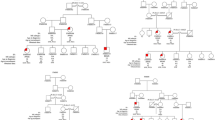
Seventy-two nonrelated Finnish patients with definite [23] MS (both relapsing-remitting and progressive) and 85 Finnish control subjects were studied in the association analysis. Twenty-one patients had living family members with MS; these were probands of the 21 families (with 2–6 MS cases per family), which were studied in the linkage analysis [for more details of the patients and families see ref. 10]. Fourteen of the families were from a high-risk area in western Finland, where up to 30% of MS cases are familial [21]. Five of the families have been partially analyzed in a previous HLA-A, C, B, DR haplotype study [12]; all the members of these families were retyped for this study. There were also 2 patients with monosymptomatic optic neuritis (ON), a condition which usually leads to MS [24], and these subjects were classified as affected in the linkage analysis. Since very mild and subclinical forms of MS may occur, attempts were made to identify such cases. Both the patients and the asymptomatic siblings of 9 families were examined using cranial magnetic resonance imaging (MRI) [25]. Three of the asymptomatic siblings showed lesions strongly suggestive of MS, and they were classified either as ‘affected’ or ‘unknown’ in the linkage analyses.
HLA Typing
Serological HLA-A, B, C and DR typing was performed in 15 of the 21 families on peripheral blood lymphocytes, using the antibody-mediated cytotoxicity test [26]. The reagents used define 14 A, 22 B, 7 C and 9 DR locus specificities. All families, nonrelated patients and controls were analyzed for DQA1 and DQB1. Six DQA1 alleles were determined using the AmpliType- Forensic DQα DNA Typing Kit (Perkin-Elmer Cetus) [27]. The alleles DQA1*04,*05, and *06 [28] could not be discriminated, and alleles typed as DQAl*04-05-06 were assigned as two different alleles in those families where they were present both on a DQB1*0201 and on a DQB1*0402 haplotype. The DQB1 alleles were determined using an oligotyping method essentially identical to the one described in the 11th HLA Workshop protocols [29]. The main deviation from the protocol was the use of PCR primers GLPDQβl and GAMPDQXβ [30]. The prehybridization (30 min at 56°C) and hybridization (1.5 h at 56 °C) were carried out in 3.0 M tetramethylammonium chloride, 50 mM Tris HCl pH 8.0, 2 mM EDTA pH 8.0, 5 × Denhardt’s solution, 0.1% SDS, and 100 µl/ml denatured herring DNA. The oligonucleotide probes were:
-
DQB230N, 5′-AACGGGACCGAGCGCGTG-3′
-
DQB2602, 5′-CGTTATGTGACCAGATAC-3′
-
DQB2603, 5′-CGTCTTGTGACCAGATAC-3′
-
DQB2604, 5′-CGTCTTGTAACCAGACAC-3′
-
DQB3701, 5′-AGGAGTACGTGCGCTTCG-3′
-
DQB4501, 5′-GACGTGGAGGTGTACCGG-3′
-
DQB5701, 5′-GCGGCCTGTTGCCGAGTA-3′
-
DQB5702, 5′-GCGGCCTAGCGCCGAGTA-3′
-
DQB5704, 5′-GCGGCCTGATGCCGAGTA-3′
-
DQB5705, 5′-GGCTGCCTGCCGCCGAGT-3′
-
DQB5707, 5′-GGCCGCCTGCCGCCGAGT-3′
-
DQB5708, 5′-GCGGCTTGACGCCCGAGTA-3′
with specificity for DQB1 alleles 0303+ 0402 (DQB2302N), 0601+0301 (DQB2602), 0602+ 0302+0303 (DQB2603), 0603+0604 (DQB2604), 0501+0502+0503 (DQB3701), 0301 (DQB4501), 0501+0604+0605 (DQB5701), 05032+0602+0603 (DQB5704), 0201 (DQB5705), 0302 (DQB5707), and 0401+0402 (DQB5708). The final wash was at 59°C in the presence of 3 M tetramethylammonium chloride.
Statistical Analyses
The HLA-DQA1 and -DQB1 allele frequencies in nonrelated MS patients, in the unaffected parents of the MS families, and in controls were compared using the χ2 method. The allele frequencies were calculated by gene counting. Those unaffected parents who were typed for DQA1 and DQB1, or for whom both alleles could be deduced, were chosen for this analysis. Relative risk (RR) was calculated by the method of Woolf using the formula (A × D)/(B × C). When one element of the equation was zero, RR was calculated with the formula of Haldane:
Linkage analysis between MS and HLA was carried out using both nonparametric and parametric methods. The transmission disequilibrium test was used to analyze the transmission of the MS-associated allele DQA1*0102 in the families by combining all affected and nonaffected offspring in the data [31]. In the sib-pair analysis, the sharing of HLA haplotypes was determined in affected siblings and the distortion from random segregation was evaluated using the χ2 method. Two-point linkage analyses using the LOD score method were carried out using the computer program MLINK (version 5.10) of the LINKAGE package [32]. Equal recombination fractions were used for males and females. Autosomal dominant, intermediate and recessive models with reduced age-dependent penetrance [10] (f = 0.05, 0.38 and 0.76, which cover the reasonable penetrance estimates in MS) were applied in the linkage analyses. An intermediate model was utilized because dose effect or interaction of HLA susceptibility determinants have been demonstrated in HLA-associated diseases [33–36]. Because three genetic models and three penetrance estimates were tested, the LOD score significance level was corrected, conservatively, for 9 tests [37]: Z0 + log9 = 3 + 0.95 = 3.95. Statistical tests for heterogeneity were carried out with the admixture test as implemented in the HOMOG program [32]. The estimate of the MS-susceptibility-gene frequency (p) was based on the prevalence data [21], Q = 1/1000, using the formula f[p2 + 2p(1−p] = Q in the autosomal dominant model, fp2 +1/10 × f2p(1−p) = Q in the intermediate model, which allows a 10-fold increased penetrance when both chromosomes carry the susceptibility gene, and fp2 = Q in the recessive model. For the linkage analysis, the HLA haplotypes were assigned as seven different alleles without any decrease in informativeness. Linkage disequilibrium (LD) between MS and DQA1 was adapted to the linkage analyses and was calculated using the association analysis data (table 1): the allele frequencies in the MS population were multiplied by the susceptibility-gene frequency (p), and the allele frequencies in the control population were multiplied by the wild-type gene frequency (1−p). When LD is allowed for the linkage analysis, the susceptibility gene is transmitted with an increased probability on HLA haplotypes carrying DQA1*0102. In the analyses not allowing for LD, the gene frequencies for HLA haplotypes, DQA1, DQB1 and DR were extrapolated from the DQA1 allele frequencies in the control population (table 1).
Results
Association with DQA1 and DQB1
The frequencies of both DQA1*0102 and DQB1*0602 were significantly increased in MS patients, when compared to controls (table 1). To gain sensitivity in the detection of other MS-associated alleles, the allele frequencies were also analyzed after exclusion of DQA1*0102- and DQBl*0602-positive subjects, but no significant differences were found between patients and controls in these comparisons (data not shown). Interestingly, when the allele frequencies in MS patients were compared to those observed in the unaffected parents of MS families, there was no significant deviation, and the frequencies of both DQA1*0102 and DQB1*0602 were almost identical (table 1).
In order to detect possible genetic heterogeneity, the group of MS patients was subdivided with respect to five determinants: (1) familial versus nonfamilial MS; (2) males versus females; (3) ON versus other presenting symptoms; (4) relapsing-remitting versus progressive (both primarily and secondarily progressive) disease course, and (5) birth place in the high-risk ares versus other regions of Finland. No statistically significant differences in the DQA1 and DQB1 allele frequencies were found in these subdivisions (p > 0.1 in all comparisons, data not shown).
The DQA1*0102 phenotype was found in 46 (63.8%) of the patients versus 27 (31.8%) of the controls [RR = 3.8, 95% confidence interval (CI) = 1.9–7.2], and the DQB1*0602 phenotype in 42 (58.3%) of the patients versus 22 (25.9%) of the controls (RR = 4.0, 95% CI = 2.0–7.6). Genotype-wise the highest RRs were associated with DQA1*0102, DQB1*0602 homozygotes (7 patients versus 0 controls: RR = 19.6, 95% CI = 2.3–160) and DQA1*0102/04-05-06, DQB1*0602/0201 heterozygotes (10 patients versus 2 controls: RR = 6.7, 95% CI = 1.5–21.0). The frequency of the inferred haplotype DQA1*05, DQB1*0201, included in the latter genotype, was similar in patients (11%) and in controls (12%). Spurkland et al. [19] have reported that 96% of Norwegian MS patients and 60% of controls carry one of the DQA1 alleles (0102, 0103, 0401, 0501) encoding glutamine at residue 34 and one of the specific DQB1 alleles (0602, 0603, 0604, 0302, 0303), conferring a RR of 13. In our study, the above-mentioned allele combinations were found in 67% of the patients and in 55% of the controls, the RR being only 1.6. We analyzed the combinations of a number of polymorphic amino acid residues on the hypervariable domain of DQα and DQβ chains, encoded in cis or in trans [34]. However, we did not find any combinations (other than those observed in DQA1*0102, DQB1*0602 homozygotes and DQA1*0102/04-05-06, DQB1*0602/ 0201 heterozygotes), which would have conferred a higher RR than DQA1*0102 and DQB1*0602 alone (analysis not shown).
HLA Haplotypes in the MS Families
We analyzed whether there would be any predominant antigen combinations in the HLA-A, B, DR, DQA1, DQB1 haplotypes found in the familial patients, most of whom come from the high-risk area in western Finland. The MS-associated alleles DQA1*0102 and DQB1*0602 appeared to be found in heterogeneous haplotypic backgrounds. DQA1*0102 was transmitted to the patients on 15, and DQB1*0602 on 12 different HLA-A, B, DR, DQA1, DQB1 haplotypes in the 15 families typed for these HLA loci. All DR2 haplotypes carried DQA1*0102 and DQB1*0602, the common Caucasian haplotype B7, DR2, DQA1*0102, DQB1*0602 being the most frequent. This haplotype constituted 41% of all individual DR2, DQA1*0102, DQB1*0602 haplotypes, which is close to the frequency observed in European Caucasians [38], and in the general population of Finland [J. Partanen, unpubl. data]. DQA1*0102 was also found on three different DRw6, DQA1*0102, DQB1*0604 haplotypes. In three families, DQA1*0102 was found in patients either on a DRw6, DQAl*0102, DQB1*0604, or on a DR2, DQA1*0102, DQB1*0602 haplotype. As to the non-DQA1*0102, non-DQB1*0602 haplotypes transmitted to the patients, they were also heterogeneous with no predominant A, B or DR locus antigens or DQA1, DQB1 alleles (data not shown).
Nonparametric Linkage Analysis
To analyze the segregation of the MS-associated allele DQA1*0102 in the families, we applied the transmission test for LD [31]. The transmission of DQA1*0102 from heterozygous parents to both affected and nonaffected offspring was analyzed. In these meioses, the transmission of DQA1*0102 to MS patients was significantly increased, while its transmission to unaffected offspring showed no deviation (table 2). Then we carried out an affected sib-pair analysis, first, in a conventional way, in families fully informative for HLA haplotypes, and second, by excluding families with a parent homozygous for the associated allele DQA1*0102, analogous to a study on diabetes-associated insulin gene polymorphisms by Julier et al. [39]. In 15 families, sibships were fully informative for HLA haplotypes. These included 10 affected sib-pairs, 3 trios, 1 quartet and 1 quintet. The remaining 6 families were not included in the sib-pair analysis because they only included affected relative-pairs other than sib-pairs (5 families), or because they were not informative (in 2 sibships only 3 haplotypes could be unequivocally assigned). Some of the parents were deceased and therefore not available for typing, but the typing of patients’ children helped to assign the haplotypes in these families. Since there were many sibships with more than two affected sibs, the sib-pairs formed in these families were weighted [40]. The sharing of HLA haplotypes was slightly increased in the MS sib-pairs (table 3). When the transmission of DQA1*0102 was analyzed in each pair, we observed that of the 23 pairs (weighted), 7.59 pairs (33%) inherited this allele on different HLA haplotypes. Consequently, when the families with DQA1*0102 homozygous parents were excluded, the evidence for linkage was much more significant (table 3). When the observed haplotype sharing was compared to expected values under recessive and dominant models with different disease gene frequencies, the minimum χ2 values were 0.05 and 1.86 for recessive and dominant models, respectively. Thus, none of the models could be rejected, although the observed values fit slightly better with a recessive model.
LOD Score Analysis
Three models of inheritance (dominant, intermediate and recessive) with three penetrance estimates (0.05, 0.38 and 0.76) were tested. Two intra-HLA recombinations were observed in unaffected relatives, one between A and B, the other between B and DR. In these cases, the DR-DQ segment was considered as the segregating HLA haplotype. Pairwise LOD scores for linkage using either whole HLA haplotypes or DQA1 alleles as segregating markers are presented in table 4. Adapting the LD observed between MS and DQA1, we obtained significant LOD scores in the linkage analyses between MS and DQA1 (table 4). The LOD scores obtained between MS and the DQA1 locus (LODmax = 6.43, θ = 0.00, f = 0.05, intermediate model) were considerably higher than those obtained between MS and HLA haplotypes (LODmax = 3.48, θ = 0.10, f = 0.05, recessive model). The LOD scores between MS and DQA1 were also slightly higher than those between MS and DQB1 (LODmax = 5.39, θ = 0.00, f = 0.05, intermediate model). Recessive and intermediate models yielded consistently higher LOD scores than dominant models. When the 3 asymptomatic siblings with abnormal MRI were classified as ‘unknown’, the LOD scores slightly increased (MS vs. HLA: LODmax = 3.73, θ = 0.10, f = 0.05, recessive model; MS vs. DQA1: LODmax = 6.68, θ = 0.00, f = 0.05, intermediate model) but the differences between models were retained as presented in table 4. No evidence for genetic heterogeneity was obtained with the family admixture test (HOMOG program).
To facilitate comparison with previously published linkage analyses we also performed linkage analyses without allowing for LD. In these analyses (table 4), all LOD scores were nonsignificant when HLA haplotypes were used as markers, LODmax = 1.54 (θ = 0.10), and the LOD scores were mostly negative at θ = 0.00. This LOD score profile is similar to many previous reports [see especially ref. 18]. In the analyses between MS and DQA1 the highest LOD scores peaked at θ = 0.00, and were very close to the conservative significance level of 3.95 (LODmax = 3.95, θ = 0.00, when subjects with abnormal MRI were classified as ‘unknown’).
Discussion
The finding of an increased frequency of HLA-DQA1*0102 and DQB1*0602 (encoding the DR2-associated DQw6 antigen) in MS patients appears to be remarkably consistent between different studies in populations of northern European descent [3, 19, 41]. Other findings have been controversial. Paradoxically, family studies have not established linkage between MS and HLA despite this well-documented association. The increased frequency of specific DQA1 and DQB1 allele combinations observed in Norwegian patients has not been confirmed in a Swedish [3], a British [41], or our present study. Mainly on the basis of association studies, three major theories have emerged to explain the connection between MS and HLA: (1) a rare, as yet unidentified, susceptibility gene exists in the HLA region in linkage disequilibrium with DR2, DQw6; (2) susceptibility is conferred by certain allele combinations in specific haplotypes, such as B7, DR2, DQw6, not only by one locus, and (3) the DR or DQ loci are themselves relevant in MS susceptibility. The two latter hypotheses are not mutually exclusive. Linkage strategies utilized to date based on HLA-A, B or HLA-A, B, DR haplotype segregation are most powerful on the assumption of a rare susceptibility gene and have satisfactorily tested only the first hypothesis, with controversial results [11–18]. Several factors may have confounded previous analyses, including biased assumptions of the susceptibility-gene frequency [15, 42] and locus heterogeneity. An additional crucial open question is the contribution of individual HLA candidate loci in MS, which has not been addressed in previous family studies. In the results of the present study, there are several overlapping aspects to be discussed.
First, in the linkage analyses, the strongest evidence for linkage was obtained when the haplotype-independent segregation of the MS-associated alleles was controlled. This was accomplished in the nonparametric analyses by excluding families in which a parent was homozygous for DQA1*0102 (and in most cases also for DQB1*0602 and DR2), and in the LOD score analysis by using DQA1 and DQB1 loci as segregating markers. It is of note that the LOD score analysis did not provide statistically significant evidence for linkage on the basis of haplotype segregation when not allowing for LD (LODmax = 1.54, θ = 0.10). In these analyses, the negative contribution to the LOD scores came mainly from families in which the MS relatives inherited different HLA haplotypes carrying the MS-associated alleles. The haplotype-independent transmission of DR2, DQB1*0602 and, most prominently, of DQA1*0102 in this data set, may partially explain the absence of close linkage on the basis of previous haplotype segregation data [13–18]. This finding emphasizes the importance of estimating LD between the disease and specific alleles by performing association analysis along with linkage analysis. This is probably especially important in polygenic diseases, in which the susceptibility gene frequencies may be relatively high. An analogous situation has been described by Julier et al. [39] who studied the segregation of insulin IGF2 haplotypes in insulin-dependent diabetes mellitus (IDDM) families. They found that when a parent carried fully informative haplotypes but was homozygous for a candidate site, both haplotypes were equally transmitted to the affected offspring providing no evidence for linkage. Evidence for linkage was obtained only after several family selection criteria were applied, including parental heterozygosity for candidate sites.
Second, the MS-associated alleles were present on a heterogeneous group of HLA haplotypes, which showed variation not only at the A and B loci but in some cases also at the DR and DQB1 loci. There were MS relatives, who inherited DQA1*0102 on different parental chromosomes, but did not carry the same DQB1 alleles and DR antigens. Whether this indicates that DQA1*0102 is an HLA-linked susceptibility factor by itself, rather than being in linkage disequilibrium with an allele at another locus, cannot be solved in this context due to the small number of observations. DQA1*0102 was most frequently found on the haplotype DR2, DQA1*0102, DQB1*0602, and there were only three exceptions to this in our families (DRw6, DQA1*0102, DQB1*0604 haplotype). The question of the primary role of the individual alleles on the DR2, DQA1*0102, DQB1*0602 haplotype has been very difficult to clarify in association studies. Therefore, family studies, as here, and the identification of susceptibility haplotypes with only one common denominator may provide an alternative approach to this question. The overall heterogeneity of HLA haplotypes in these families demonstrated that the HLA-linked susceptibility was not restricted to only a few haplotypes such as B7, DR2, DQA1*0102, DQB1*0602. This divergence of HLA haplotypes among patients conforms with the results of Hauser et al. [43], and suggests that the DR2, DQAl*0102, DQB1*0602- associated susceptibility determinants in MS may be quite common instead of being present only on a limited set of A, B, DR, DQA1, DQB1 haplotypes.
Third, although the mode of inheritance of the HLA-linked susceptibility determinants cannot be clearly defined, both the sib-pair analysis, and the LOD score analysis, to some extent, suggest that both HLA chromosomes of an individual contribute to MS susceptibility. In the sib-pair analysis, an excess of MS sib-pairs shared both haplotypes when the DQA1*0102 homozygous parents were excluded. In the LOD score analysis, the recessive and intermediate models consistently provided the strongest evidence for linkage. DQA1*0102 and DQB1*0602 were almost invariably present on one HLA haplotype in the familial patients, while in the case of the patient’s other haplotype not only the DQA1 and DQB1 alleles but the whole haplotype from A to DQB1 was typically the same among the patients within a family. No predominant DQA1 or DQB1 alleles were present on the other haplotype, a situation also observed in the nonfamilial patients and which could not be explained by any preferential DQα, DQβ chain amino acid combinations as proposed by Spurkland et al. [19] for MS and, analogously, by Khalil et al. [34] for IDDM. These observations do not support any simple recessive one-locus model for the HLA susceptibility factors, and moreover, population data indicate that the DR2, DQA1*0102, DQB1*0602 haplotype has a dominant effect [3, 15]. The question of the contribution of the other haplotype is intriguing, and based on our data, another susceptibility locus, distinct from the DQA1*0102-associated locus, may exist. The heterogeneous DQA1, DQB1 genotypes and the absence of any MS-associated DQ alleles other than DQA1*0102 and DQB1*0602 indicate that the other putative susceptibility locus does not show linkage disequilibrium with DQA1 or DQB1. Additional complexity is provided by the DQA1*0102, DQB1*0602 homozygotes since this genotype, although relatively infrequent (10%) among the patients, conferred the highest RR for MS, which may indicate a dose effect.
Available data do not provide many clues for the localization of the other putative HLA-linked susceptibility locus, given the lack of any consistent associations other than those with DR2, DQA1*0102 and DQB1*0602. The increased frequency of DQA1*0102/04-05-06, DQB1*0602/0201 heterozygous patients in our series suggests a possible role for the DQA1*05, DQB1*0201 haplotype (DR3 associated) in MS susceptibility. This haplotype has an increased frequency among patients in several HLA-associated diseases, and slightly increased frequencies of this haplotype have also been occasionally observed in MS patients [3]. In our series, its frequency was increased only in combination with DQA1*0102, DQB1*0602. In a few HLA-class-II-associated diseases it has been suggested that both HLA chromosomes contribute to the disease, as in the case of IDDM, celiac disease and systemic lupus erythematosus (SLE) [32–35]. In IDDM and in celiac disease, specific α and β chain combinations of the DQαβ heterodimer encoded in cis or in trans have been proposed, and an interplay between distinct loci within HLA has been suggested in SLE (DQ and the complement genes) and in celiac disease (DQ and DP).
Finally, it is of interest that in the same set of Finnish families, MS shows linkage both to HLA on chromosome 6 and to the MBP gene on chromosome 18 [10]. Further investigation on the interaction of the genes within HLA, and their joint inheritance with the MBP-linked predisposing gene and perhaps with still other genes, such as those coding for T cell receptors which functionally interact with HLA antigens, may help to identify the components of genetic susceptibility in MS. Such an approach will eventually provide insight into the pathological mechanisms in MS, yield tools to identify high-risk individuals for therapeutic or preventive strategies, and aid the development of a genetically susceptible animal model.
References
McDonald WI: The mystery of the origin of multiple sclerosis. J Neurol Neurosurg Psychiatry 1986;49:434–445
Ebers GC, Bulman DE, Sadovnick AD, Paty DW, Warren S, Hader W, Murray TJ, Seland TP, Duquette P, Grey T, Nelson R, Nicolle M, Brunet D: A population-based study of multiple sclerosis in twins. N Engl J Med 1986;315:1638–1642
Olerup O, Hillert J: HLA class II-associated genetic susceptibility in multiple sclerosis: A critical evaluation. Tissue Antigens 1991;38:1–15
Kurtzke JF: Epidemiology of multiple sclerosis; in Hallpike JF, Adams CWM, Tourtellotte WW (eds): Multiple Sclerosis. Baltimore, Williams and Wilkins, 1983, pp 47–96.
Beall SS, Concannon P, Charmley P, McFarland HF, Gatti RA, Hood LE, McFarlin DE, Biddison WE: The germline repertoire of T cell receptor β-chain genes in patients with chronic progressive multiple sclerosis. J Neuroimmunol 1989;21:59–66
Seboun E, Robinson MA, Doolittle TH, Chilla TA, Kindt TJ, Hauser SL: A susceptibility locus for multiple sclerosis is linked to the T cell receptor β chain complex. Cell 1989;57:1095–1100
Lynch SG, Rose JW, Petajan JH, Staufer D, Kamerath C, Leppert M: Discordance of T-cell receptor β-chain genes in familial multiple sclerosis. Ann Neurol 1991;30:402–410
Hillert J, Chunmao L, Olerup O: No association with germline T cell receptor β-chain gene alleles or haplotypes in Swedish patients with multiple sclerosis. J Neuroimmunol 1991;31:141–147
Boylan KB, Takahashi N, Paty DW, Sadovnick AD, Diamond M, Hood LE, Prusiner SB: DNA length polymorphism 5′ to the myelin basic protein gene is associated with multiple sclerosis. Ann Neurol 1990;27:291–297
Tienari PJ, Wikström J, Sajantila A, Palo J, Peltonen L: Genetic susceptibility to multiple sclerosis linked to myelin basic protein gene. Lancet 1992;340:987–991
Visscher B, Detels R, Dudley JP, Haile RW, Malmgren RM, Terasaki PI, Park MS: Genetic susceptibility to multiple sclerosis. Neurology 1979;29:1354–1360
Kinnunen E, Koskimies S, Lagerstedt A, Wikström J: Histocompatibility antigens in familial multiple sclerosis in a high-risk area of the disease. J Neurol Sci 1984;65:147–155
Ebers GC, Paty DW, Stiller CR, Nelson RF, Seland TP, Larsen B: HLA typing in multiple sclerosis sibling pairs. Lancet 1982;ii:88–90
Govaerts A, Gony J, Martin-Mondiere C, Poirier JC, Schmid M, Schuller E, Degos JD, Dausset J: HLA and multiple sclerosis: Population and families study. Tissue Antigens 1985;25:187–199
Stewart GJ, McLeod JG, Basten A, Bashir HV: HLA family studies in multiple sclerosis: A common gene, dominantly expressed. Hum Immunol 1981;3:13–29
Haile RW, Hodge SE, Visscher BR, Spence MA, Detels R, McAuliffe TL, Park MS: Genetic susceptibility to multiple sclerosis: A linkage analysis with age-of-onset corrections. Clin Genet 1980;18:160–167
Ho HZ, Tiwari JL, Haile RW, Terasaki PI, Morton NE: HLA-linked and unlinked determinants of multiple sclerosis. Immunogenetics 1982;15:509–517
Tiwari JL, Hodge SE, Terasaki PI, Spence MA: HLA and inheritance of multiple sclerosis: Linkage analysis of 72 pedigrees. Am J Hum Genet 1980;32:103–111
Spurkland A, Ronningen KS, Vandvik B, Thorsby E, Vartdal F: HLA-DQA1 and HLA-DQB1 genes may jointly determine susceptibility to develop multiple sclerosis. Hum Immunol 1991;30:69–75
Nevanlinna H: The Finnish population structure. A genetic and genealogical study. Hereditas 1972;71:195–236
Wikström J, Kinnunen E, Porras J: The age-specific prevalence ratio of familial multiple sclerosis. Neuroepidemiology 1984;3:74–82
Wikström J: Studies on the clustering of multiple sclerosis in Finland. II. Microepidemiology in one high-risk county with special reference to familial cases. Acta Neurol Scand 1975;51:173–183
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davies FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW: New diagnostic criteria for multiple sclerosis: Guide-lines for research protocols. Ann Neurol 1983;13:227–231
Sandberg-Wollheim M, Bynke H, Cronqvist S, Holtas S, Platz P, Ryder LP: A long term prospective study of optic neuritis: Evaluation of risk factors. Ann Neurol 1990;27:386–393
Tienari PJ, Salonen O, Wikström J, Valanne L, Palo J: Familial multiple sclerosis: MRI findings in clinically affected and unaffected siblings. J Neurol Neurosurg Psychiatry 1992;55:883–886
Amos D, Badir H, Boyle W, McQueen M, Tiilikainen A: A simple microcytotoxicity test. Transplantation 1969;7:220–223
Saiki R, Walsh PS, Levenson CH, Erlich HA: Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci USA 1989;86:6230–6234
Bodmer J, Marsh SGE, Albert ED, Bodmer WF, Dupont B, Erlich HA, Mach B, Mayr WR, Sasazuki T, Schreuder GMT, Strominger JL, Parham P, Svejgaard A, Terasaki P: The nomenclature for factors of the HLA system, 1991. Tissue Antigens 1992;39:161–173
Kimura A, Sasazuki T: Eleventh international histocompatibility workshop reference protocol for the HLA DNA-typing technique; in Tsuji K, Aizawa M, Sasazuki T (eds): HLA 1991. Oxford, Oxford University Press, 1992, pp 197–418.
Todd J, Bell JI, McDevitt HO: HLA-DQβ gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987:329:599–604.
Spielman RS, McGinnis RE, Ewens WJ: Transmission test for linkage disequilibrium: The insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 1993;52:506–516
Ott J: Analysis of Human Genetic Linkage. Baltimore, Johns Hopkins University Press, 1991.
Morton NE, Green A, Dunsworth T, Svejgaard A, Barbosa J, Rich SS, Iselius L, et al: Heterozygous expression of insulin-dependent diabetes mellitus (IDDM) determinants in the HLA system. Am J Hum Genet 1983;35:201–213
Khalil I, Deschamps I, Lepage V, Al-Daccak R, Degos L, Hors J: Dose effect of cis and trans encoded HLA-DQαβ heterodimers in IDDM susceptibility. Diabetes 1992;41:378–384
Arnett FC: Genetic aspects of human lupus. Clin Immunol Immunopathol 1992;63:4–6
Bugavan TL, Angelini G, Larrick J, Auricchio S, Ferrara GB, Erlich HA: A combination of particular HLA-DPβ allele and an HLA-DQ heterodimer confers susceptibility to coeliac disease. Nature 1989;339:470–473
Kidd KK, Ott J: Power and sample size in linkage studies. Cytogenet Cell Genet 1984;37:510–511
Baur MP, Danilovs JA: Reference tables of two and three locus haplotype frequencies for HLA-A, B, C, DR, BF and GLO; in Terasaki PI (ed): Histocompatibility Testing 1980. Los Angeles, UCLA Press, 1980, pp 994–1210.
Julier C, Hyer RN, Davies J, Merlin F, Soularue P, Briant L, Cathelineau G, Deschamps I, Rotter JI, Froguel P, Boitard C, Bell JI, Lathrop GM: Insulin-IGF2 region on chromosome 11p encodes a gene implicated in HLA-DR4 dependent diabetes susceptibility. Nature 1991;354:155–159
Hodge S: The information contained in multiple sibling pairs. Genet Epidemiol 1984;1:109–122
Francis DA, Thompson AJ, Brookes P, Davey N, Lechler RI, McDonald WI, Batchelor JR: Multiple sclerosis and HLA: Is the susceptibility gene really HLA-DR or -DQ? Hum Immunol 1991;32:119–124
Clerget-Darpoux F, Govaerts A, Feingold N: HLA and susceptibility to multiple sclerosis. Tissue Antigens 1984;24:160–169
Hauser SL, Fleischnick E, Weiner HL, Marcus D, Awdeh Z, Yunis EJ, Alper CA: Extended major histocompatibility complex haplotypes in patients with multiple sclerosis. Neurology 1989;39:275–277
Acknowledgements
We are indebted to the patients and their families for their assistance and cooperation, to Ms. Kaarina Lindberg for her technical assistance, and to Professor Kimmo Aho, MD, for his valuable help in the preparation of the manuscript. This study was supported by grants from the Paulo Foundation, the Sigrid Juselius Foundation, the Orion Inc. Research Foundation, the Finnish Neurology Foundation, the Finnish Medical Society Duodecim, the Oskar Öflund Foundation, and the University of Helsinki 350 Years Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tienari, P.J., Wikström, J., Koskimies, S. et al. Reappraisal of HLA in Multiple Sclerosis: Close Linkage in Multiplex Families. Eur J Hum Genet 1, 257–268 (1993). https://doi.org/10.1159/000472423
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1159/000472423
Key Words
This article is cited by
-
Linkage disequilibrium between the MBP tetranucleotide repeat and multiple sclerosis is restricted to a geographically defined subpopulation in Finland
Genes & Immunity (2003)
-
Linkage analysis suggests a region of importance for multiple sclerosis in 3p14–13
Genes & Immunity (2001)
-
Multiple loci for multiple sclerosis
Nature Genetics (1996)
-
A putative vulnerability locus to multiple sclerosis maps to 5p14–p12 in a region syntenic to the murine locus Eae2
Nature Genetics (1996)