Abstract
A 760-kb YAC was constructed by homologous recombination in yeast, containing the genes located in the distal portion of the DMD gene. The YAC was introduced in mouse LA-9 cells by PEG-mediated cell fusion. One transformant accommodated an intact DMD-YAC, i.e. a full copy of the DMD internal Dp 116, Dp 71 and Dp 40 genes (apo-dystrophin-2, -1 and -3, respectively). We have studied the expression of the various gene products derived from the introduced DMD-YAC. RT-PCR revealed expression of human Dp 71 but not of Dp 116 or Dp 40. Remarkably, differences were observed in processing of the 3′ region of the endogenous mouse and the human transcripts, due to different splicing of exons 71 (absent in human and present in mouse transcript) and 78 (present in human and absent in mouse transcript). The splicing pattern of the human transcript is the same as that of the major Dp 71 (apo-dystrophin-1) product in human blood. The observed splicing differences may be caused by either species-specific exon use and/or by cis-acting factors, e.g. the upstream transcript composition, because we have no evidence for endogenous Dp 71 expression.
Similar content being viewed by others
Introduction
Duchenne and Becker muscular dystrophy (DMD/BMD) are X-linked disorders caused by mutations in the dystrophin gene, the largest gene found so far in nature. At present, six independent promoters of the DMD gene have been identified, each directing gene expression from a different first exon. The brain promoter [1–4] and the muscle promoter [3, 5–7] are located in the 5′ region of the gene as is a third Purkinje cell promoter, located between muscle exons one and two [8]. These promoters give rise to C-, M-, and P-dystrophins, respectively. Recently, Nishio et al. [9] have identified a novel promoter, located far upstream from the brain promoter, its transcript designated L-dystrophin. Finally, two promoters are present at a distal position in the gene. One of them lies between exons 55 and 56 and is only active in peripheral glia cells [10]. Its transcript encodes a 116-kD polypeptide, called Dp 116, apo-dystrophin-2 or S-dystrophin, containing exons 56–79. The major carboxy-terminal promoter, however, lies more distally, between exons 62 and 63 [11–13]. This so-called ‘liver promoter’ controls the expression of a 4.8-kb transcript, which is ubiquitously transcribed [11, 12, 14]. The transcript is colinear with exons 63–79 and encodes a 70.8-kD polypeptide, called Dp 71 or apo-dystrophin-1 or G-dystrophin. More recently, an even shorter 2.2-kb mRNA, initiated at the same promoter as Dp 71, has been described [15]. This mRNA comprises exons 63–70 and encodes a protein of 40 kD, termed apo-dystrophin-3. In addition to the use of alternative promoters, mRNA diversity is also further generated by alternative splicing of the 5′- and 3′-terminal regions of the DMD locus, giving rise to a combinatorial variety of isoforms [for a review, see ref. 16, 17].
The complexity of the transcriptional control of the DMD gene suggests that the isoforms perform a number of distinct cellular functions, in conjunction with the variety of dystrophin-associated membrane glycoproteins that are currently being discovered [14, 18–21]. It has been suggested that dystrophin mutations affecting Dp 71 and/or Dp 116 expression may be involved in mental retardation affecting roughly one third of DMD patients [22]. However, extensive studies have as yet failed to find systematic association with the position of mutations and mental retardation.
To gain more insight into the regulation of the expression of the DMD gene and the biological significance of the dystrophin isoforms, we aim to study the gene expression in a controlled environment. We intend to reintroduce into mammalian cells a construct containing the 2.4 Mb of the human DMD gene, including all genomic regulatory elements. In this paper we report the first step on this way, i.e. the introduction into mammalian cells of a 760-kb DMD-YAC, called yneo(18–25)C, which covers the distal portion of the DMD gene and harbours the complete genes for Dp 71 (apo-dystrophin-1), Dp 116 (apo-dystrophin-2) and Dp 40 (apo-dystrophin-3), including their promoters, and thus represents a good test system. After introduction of the YAC into mouse LA-9 cells and verification of its integrity, we examined transcription and splicing of the human mRNAs derived from it and compared expression and splicing with the endogenous mouse transcripts.
Materials and Methods
Cell Culture
Mouse L cell line A-9 (GM00346B) was cultured in DMEM containing 10% fetal bovine serum. The mouse/yeast fusion cell lines were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin and 600 µg/ml G418.
Fusion
Fusion of yeast spheroplasts with LA-9 cells was carried out as described elsewhere [23]. 48 h after fusion, selection was initiated by addition of 700 µg/ml G418 to the medium. After 1 week of selection, the G418 concentration was lowered to 600 µg/ml. Colonies appeared after 2 weeks with changes of medium every 2–3 days.
DNA Staining
Mouse/yeast cell lines were cultured on a coverslip for 6 h after fusion. Cells were fixed for 3 × 5 min with methanol:acetic acid (1:1) and air-dried. The cell nuclei were stained for 15 min in a 0.2 µ1/ml bisbenz-imide (Hoechst 33258, Boehringer Mannheim) solution in staining buffer (22 mM citric acid and 55.5 mM Na2HPO4, pH 5.5–5.8) and washed with the same buffer.
Exon PCR
DNA from the mouse/yeast fusion cell lines was isolated by boiling the cells isolated from one well (24-well culture plates) for 10 min in 250 µl phosphate-buffered saline (PBS) with 5% Chelex-100 (Biorad). PCR was performed with 5–10 µl DNA solution in 50 µl containing 5 µl 10 × PCR buffer (Supertaq buffer, HT Biotechnology), 3 µl 5 mM dNTPs, 50 pmol forward primer, 50 pmol reverse primer and 0.2 U Supertaq polymerase (HT Biotechnology). Forty cycles of PCR (at 93 ° C for 1 min, 58 ° C for 1 min and 72 ° C for 4 min on a Perkin-Elmer-Cetus DNA thermal cycler) were performed on the sample followed by an incubation at 72 ° C for 5 min.
The primers used for the neomycin gene and exons 54, 59, 66 and 79 are as follows (nucleotide numbering according to Koenig et al. [24]):
-
Neo-F: 5′-TGAATGAACTGCAGGACGAG-3′
-
Neo-R: 5′-CAAGCTCTTCAGCAATATCACG-3′
-
Ex54-F: 5′-GACCTCCGCCAGTGGCAGAC-3′ (8098–8112)
-
Ex54-R: 5′-GAATGCTTCTCCAAGAGGC-3′ (8228–8210)
-
Ex59-F: 5′-GAGGCCACGGATGAGCTGG-3′ (9029–9047)
-
Ex59-R: 5′-GGTGATCTTGGAGAGAGT-3′ (9131–9113)
-
Ex66-F: 5′-CAGGGAGGATCCGTGTCCTG-3′ (9780–9799)
-
Ex66-R: 5′-GTCTTCCAAATGTGCTTTAC-3′ (9847–9828)
-
Ex79-F: 5′-ggtaccGTCAAAAGGAACTGGGTGGT-TTGG-3′(12591–12614)
-
Ex79-R: 5′-ctgcagATTTGTTACCTTAGAGCTTTG-GGT-3′(13356–13333)
The primers used for exons 52 and 60 were described by Beggs et al. [25].
Southern Blotting
HindIII digestions of the mouse/yeast hybrid clones were separated on an agarose gel, transferred to nylon membrane and hybridized with left and right YAC vector arm-specific probes [26] as well as with specific cDNA subclones.
Fluorescent in situ Hybridization (FISH)
FISH experiments, including two-colour FISH, were performed essentially according to Dauwerse et al. [27]. The mouse/yeast hybrids were checked for human DNA content by hybridization with Alu-PCR products, derived from a PCR of 50–100 ng of the yeast clone yneo(18–25)C using the human-specific Alu primer pDJ34 [28]. Alu-PCR products and total yeast DNA were labelled by nick translation in the presence of Dig-11-dUTP and biotin-14-dATP, respectively, and detected by FITC and TRITC, respectively.
RNA Preparation
Peripheral blood lymphocytes were isolated using Histopaque 1077 (Sigma) followed by rinsing in cold PBS. Mouse LA-9 cells and the mouse/yeast fusion cells were isolated by scraping in cold PBS. The resulting cell pellets were subjected to extraction with RNA-zol B as described by the manufacturer (Cinna, Biotecx Laboratories).
Reverse Transcription and Nested PCR (RT-PCR)
Samples of total RNA (1–3 µg) were incubated in a volume of 32 µl TE, containing 100 ng random primer (Promega) at 65 °C for 10 min. The sample was then snap-chilled on ice and made up to 60 µl with a premix containing 12 µl 5 × reverse transcriptase buffer (BRL), 6 µl 100 mMDTT (BRL), 6 µl 10 mM dNTPs (Pharmacia), 1 µl RNasin (Promega) and 600 U MMLV reverse transcriptase (BRL). The reaction was incubated at 42 °C for 1 h. For the first PCR, a 20-µl mixture containing 2.5 µl 10 × PCR buffer [166 mM (NH4)2SO4, 670 mM Tris-HCl pH 8.8, 67 mM MgCl2, 100 mM ß-mercaptoethanol], 1,500 mM dNTPs, 20 pmol forward primer, 20 pmol reverse primer, 12.5% vol/vol DMSO, 25 µg BSA, 1 U Amplitaq Taq polymerase (Perkin-Elmer-Cetus) and 0.06 U Deep Vent (Biolabs) was added to 5 µl of RT reaction and covered with mineral oil. Thirthy cycles of PCR (at 93 °C for 1 min, at 58 °C for 1 min, and at 72 °C for 4 min on a Perkin-Elmer-Cetus thermal cycler) were followed by an incubation at 72 °C for 5 min. For the nested PCR, samples of the previously obtained product (3 µl) were added to a 50-µl mixture containing 5 µl 10 × PCR Supertaq buffer, 5 µl 2 mM dNTPs, 20 pmol forward primer, 20 pmol reverse primer and 0.2 Units Supertaq polymerase.
Nested PCR was carried out similarly to the first PCR. The primers used are shown in table 1.
Protein Truncation Test
Modified primers, containing a T7 promoter and a eukaryotic translation initiation sequence were used for nested PCR (see table 1). In vitro transcription/translation and analysis of the products was performed using a TnT kit (Promega) as described before [29].
Direct Sequencing
PCR products were purified from agarose gels using a QIAEX gel extraction kit (Qiagen). The products were directly sequenced using a DNA Sequencing Kit (Sequenase Version 2.0, USB). The primers used are listed in table 1.
Results
Construction of yneo (18–25)C and Introduction in LA-9 Cells
To obtain a YAC with the C-terminal promoters of the DMD gene and the apo-dystrophin-1 to -3 genes, we constructed YAC y(18–25)C in two subsequent recombination steps. Each recombination involved mating between two partly overlapping YACs, plating on sporulation agar and dissection of individual spores. Spore cultures were grown and their YAC content was analysed in several ways: (1) size of the YAC; (2) recombination between vector arms; (3) exon content, and (4) long-range physical map (see Den Dunnen et al., [30] for DMD-YAC nomenclature and experimental details). y(l 8–25)C is a correctly recombined YAC, which measures 760 kb and contains the distal 660 kb (23%) of the DMD gene, from exon 52–79, and 100 kb downstream of the 3′ end (fig. 1).
Stepwise construction scheme. Black squares indicate the position of the centromeric vector arm of the YAC. Individual recombination steps are indicated by arrows. The construct of 2.4 Mb, containing genomic sequences from the entire muscle-specific DMD gene, with the exception of a 100-kb region surrounding exon 60, was obtained as described elsewhere [30]. The non-chimeric YACs 10H8 and 16BH2 were obtained from the CEPH and ICRF YAC libraries, respectively. The DMD gene is drawn schematically (top). Location of genomic probes is indicated by vertical arrows, while each vertical bar represents an exon. Every tenth exon is numbered.
To allow selection for the YAC in mammalian cells, a neomycin resistance gene was introduced in the right vector-arm of y(18–25)C by retrofitting with vector pRVl [31, 32]. Yeast spheroplasts containing the resultant YAC, yneo(18–25)C, were subsequently fused to mouse LA-9 cells using polyethylene glycol (PEG) [23]. Six hours after fusion, we stained the cell nuclei to monitor cell fusion efficiency. Although about 5% of the mouse LA-9 cells showed fusion with yeast sphero-plasts, only a few G418-resistant colonies were obtained (frequency of 1 in 5 × 10−7).
G418-resistant clones were further analysed by PCR. All clones contained the neomycin gene (not shown). Segments of the human DMD gene were present in four out of six clones (table 2). Clone 3A was positive for exons 52, 54, 59, 60, 66 and 79, indicating that it contained the entire 660-kb human DMD gene segment. Detailed analysis by Southern blotting, using human dystrophin cDNAs as probe, confirmed the presence of yneo(18–25)C in clone 3A, without any detectable rearrangements (fig. 2, and data not shown).
Southern blot analysis of two mouse/yeast hybrid clones. HindIII digests of mouse LA-9 cells, the mouse/yeast hybrid clones 3A and 4D and yneo(1825)C were hybridized with dystrophin cDNA subclones [39, 40] 63-1/e (A) and 44-1 (B). The HindIII bands containing the human exons 52 or 79 are indicated. In mouse LA-9 cells as well as in the mouse/yeast hybrid clones, additional bands derived from the endogenous mouse DMD gene are visible. * = Vector band in yneo(18–25)C.
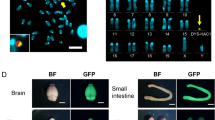
FISH analysis of metaphase chromosomes of clone 3A (fig. 3A) with Alu-PCR products from yneo(18–25)C revealed only one hybridization signal (fig. 3B), indicating that the YAC integrated in a single locus of the mouse genome. In addition, two-colour FISH with total yeast DNA showed integration of yeast DNA at the same locus (fig. 3C). We noticed that at this site, the mouse chromosome shows a secondary constriction (fig. 3A, arrowhead), an indication that the integrated yeast and/or human DNA has a different conformation compared to mouse DNA.
Visualization of human and yeast DNA in the mouse/yeast hybrid clone 3A by two-colour FISH. A DAPI (blue) staining of the mouse chromosomes. The arrowhead indicates the secondary constriction at the site where the human and yeast sequences have integrated. B Hybridization with FITC-labelled Alu-PCR products derived from yneo(18–25)C (green). C Hybridization with TRITC-labelled total yeast DNA (red).
Human RNA Is Transcribed from the 760-kb YAC
The expression of the human DMD gene in clone 3A was investigated by RT-PCR of two overlapping segments of the region between exons 63 and 79 (fig. 4A: exon 63–68; fig. 4B: exon 67–79). To check for amplification of mouse transcripts, the parent LA-9 cells were used as a control. Human peripheral blood lymphocytes served as a control for the human RT-PCR reaction. A nested PCR was necessary to visualize products on agarose gels, both in blood as well as in LA-9 and clone 3A. We were unable to amplify the segment between exons 63 and 68 in the LA-9 sample. This is expected, due to several mismatches between the human primer sequences and the mouse cDNA sequence (table 2). To discriminate further between human and mouse products, the samples were analysed by restriction enzyme digestion (fig. 4C: exon 63–68; fig. 4D: exon 67–79). Fig. 4C shows that the pattern of clone 3A was identical to that seen in human blood cells and consistent with the human cDNA sequence [24], indicating that the product was derived from the DMD-YAC. Analysis of the products comprising exons 67–79 demonstrates that the products amplified in clone 3A seemed to be a mixture of human and mouse transcripts in an estimated ratio of 1:2. A similar result was obtained using primer sets completely identical to the human and the mouse cDNA sequence (not shown).
RT-PCR amplification and restriction enzyme digestion of two segments of the DMD transcript derived from human blood, mouse LA-9 cells and mouse/YAC hybrid clone 3A. A Amplification product of exons 63–68 (primers: 1st PCR: D63F1 and D68R2; nested PCR: D63F2 and D68R1). B Amplification product of exons 67–79 (primers: 1st PCR: D67F1 and D79R4; nested PCR: D67F6 and D79R3). C Digestion of the products from panel A with PstI. D Digestion of the products from panel B with NcoI.
Only Human Dp 71 Is Expressed in Clone 3A
To investigate which apo-dystrophin transcripts were generated from the DMD-YAC, the first round of RT-PCR was carried out with primer sets specific for Dp 71/apo-dystrophin-1 (fig. 5A), Dp 40/apo-dystrophin-3 (fig. 5B) and Dp 116/apo-dystrophin-2 (fig. 5C). The results demonstrate that Dp 71 is present in clone 3A (fig. 5A) and in human blood, but not in mouse LA-9 cells. Restriction analysis showed that the Dp 71 product in clone 3A is of human origin (not shown). In contrast to human blood, we could not detect Dp 40 transcripts in clone 3A or in LA-9 cells. Furthermore, we could not detect human Dp 116 transcripts, as can be seen in fig. 5C. Even after amplification of other cDNA segments specific for Dp 116 (fig. 5D), the products found in clone 3A and LA-9 were identical and of mouse origin (not shown). Further investigation showed that this mouse DMD gene transcript at least includes exon 56 and may originate either from the mouse Dp 116 promoter or from promoters located yet more upstream.
Identification of the human transcripts in clone 3a. The following primer sets were used. A Dp 71,1st round: APO1/3-Fl and D74R1; nested PCR: D67F6 and D73R1. B Dp 40, 1st round: APO1/3-Fl and DI70R2; nested PCR: D67F6 and DI70R1. C Dp 116, 1st round: AP02-F1 and D74R1; nested PCR: D67F6 and D73R1. D Exon 59–74, 1st round: D59F2 and D74R1; nested PCR: D67F6 and D73R1. E Exon 67–74, 1st round: D67F1 and D74R1; nested PCR: D67F6 and D73R1. These results serve as a positive control for the RT-PCR.
We performed the so-called protein truncation test [29] to check for potential alterations affecting the coding region of Dp 71, which might have occurred during the manipulations of the DMD-YAC-For this purpose, nested RT-PCRs were carried out using forward human DMD-specific primers, containing a T7 promoter and a eukaryotic translation initiation sequence. The RT-PCR products (exons 63–68 and 67–79) were subsequently used to generate protein products by a combined in vitro transcription/translation reaction. SDS-PAGE analysis showed that the translation products derived from clone 3A migrated at a similar position as the products obtained from blood (not shown). This clearly demonstrates that the reading frame of the RNA derived from yneo(18–25)C is still intact.
Differences in Splicing of the C-Terminal Region
Since alternative splicing of exons 71 and 78 has been observed in a variety of tissues [5, 12, 13, 33, 34], we sequenced these regions in the RT-PCR products of clone 3A and the LA-9 cells. In addition, we determined the sequences of the RT-PCR product from blood and of a cDNA clone derived from human muscle [35]. Comparison of the nucleotide sequence of the human muscle cDNA with that of the human product in clone 3A revealed complete identity except for exon 71, which is absent in the product of clone 3A (not shown). The RT-PCR product obtained from human peripheral blood lymphocytes did not contain this exon either. However, exon 71 was present in the RT-PCR product of LA-9 cells and in muscle cDNA. We observed that exon 78 was absent from the RT-PCR product of mouse LA-9 cells, but present in the human product in clone 3A, human blood, and human muscle cDNA (not shown). These results imply that splicing of both exons 71 and 78 differs in the opposite sense for mouse and human transcripts, even though they arise in one and the same cell.
Discussion
The dystrophin gene encodes a variety of transcripts that are directed by at least six different promoters. Ideally, one would like to analyse the transcription of the gene in the context of all genomic sequences. In this paper, we have shown the first step toward this goal, by the construction of a 760-kb DMD-YAC, which contains the genes encoding Dp 116, Dp 71 and Dp 40, and its subsequent reintroduction into mammalian cells. Since we aim for the introduction of extremely large YACs, namely a 2.4-Mb DMD-YAC, we have chosen a transformation method that does not require DNA purification, i.e. PEG-mediated cell fusion [23]. Fusion of yneo(18–25)C containing spheroplasts with mouse LA-9 seemed to be efficient (5%) as judged by DNA staining (after 6 h). However only a few colonies remained after G418 selection, indicating that the vast majority of the hybrids lose the exogenous DNA.
G418-resistant mouse/YAC hybrids were analysed by DMD exon PCR, which proved to be a rapid method to investigate the DMD-YAC content of the clones. Clone 3A contained all exons screened and had no detectable rearrangements as shown by Southern analysis. Thus complete integration of the 760-kb DMD-YAC in the mouse genome does occur, albeit at low frequency. FISH analysis showed that both the yeast and YAC DNA were integrated at one site in the mouse genome. In approximately 5% of the clone 3 A cells we observed an additional FISH signal, suggesting that the mouse cell line LA-9 is unstable (chromosomal alterations were frequently observed) and/or that integrated yeast DNA is prone to further rearrangements. The observed secondary constriction at the integration site might support the latter explanation.
We could show that clone 3A expresses human Dp 71 transcripts. This implies that transcription from the human promoter does occur within the mouse background. It has to be noted, however, that the PCR products for both the human and mouse mRNAs were only visible after nested PCR. Consequently, the expression levels of the transcripts are very low. Determination of the steady-state levels of the human and the mouse transcripts showed that the mouse transcripts in clone 3A and in LA-9 cells are expressed 2-fold higher. Remarkably, although human Dp 71 is transcribed from the DMD-YAC, we have no evidence for endogenous Dp 71 expression in these cells. At the moment, we do not know whether this difference is caused by the exogenous DNA having a different structure, as indicated by the secondary constriction, to a position effect at the site of integration, or to differences in the regulation of human and mouse promoters.
We recently developed a novel system, the protein truncation test, to identify mutations leading to premature stop codons [29]. Besides establishing the integrity of the DMD gene by Southern blotting, this test shows that the manipulation of YACs in yeast, necessary for the construction of yneo(18–25)C, and the integration of the DMD-YAC in the murine genome had not affected the reading frame of the DMD gene. This was further supported by sequence analysis of parts of the human transcript, showing no difference with the published cDNA sequence.
Although the DMD gene contains 79 exons, only a limited number, encoding portions of the C-terminal region of the protein, are subject to alternative splicing [5, 12, 13, 33, 34]. In this study, we observed that splicing of exons 71 and 78 in the human and mouse DMD mRNAs is not identical in the LA-9 cell line. The human and the mouse transcripts showed an opposite pattern for splicing of both exons, i.e. exon 71 was absent and exon 78 was present in the human mRNA, while exon 71 was present and exon 78 was absent in the mouse mRNA. The absence of exon 78 in the mouse mRNA modifies the reading frame, resulting in a replacement of the last 14 amino acids by 32 different amino acids, which confer hydrophobicity to the region [13, 33]. The human product still contains the dystrophin carboxy-terminal amino acid sequence and could thus be identical to one of the Dp 71 subfamilies described by Kramarcy et al. [14]. There are two possible explanations for the differences in splicing of the human and mouse DMD transcripts. First, not all factors and/or signal regulating splicing may function across species. Second, as the transcripts originate from different promoters, splicing might be dependent on cis-acting structural elements in the transcript itself (e.g. the specific exon 1 attached. A combinatorial effect is also possible.
This present study illustrates the great potential of YAC-based reconstruction of large genomic regions containing megabase genes or multigene families and their reintroduction into mammalian cells. Recently, it has been demonstrated that YACs can be successfully introduced into embryonic stem cells [36, 37]. Furthermore, it was shown that yeast DNA, which is simultaneously introduced during cell fusion, has no effect on the generation and development of transgenic mice. Thus, introduction of human DMD-YACs in ES cells now seems feasible and is currently the only possibility to study transcriptional regulation of full-size and manipulated DMD genes. Clearly, future gene therapy studies will greatly benefit from the additional insights gained from such studies.
References
Boyce FM, Beggs AH, Feener CA, Kunkel LM: Dystrophin is transcribed in brain from a distant upstream promoter. Proc Natl Acad Sci USA 1991;88:1276–1280
Den Dunnen JT, Casula L, Makover A, Bakker E, Yaffe D, Nudel U, Van Ommen GJB: Mapping of dystrophin brain promoter: A deletion of this region is compatible with normal intellect. Neuromusc Disord 1991;1:327–331
Nudel U, Robzyk K, Yaffe D: Expression of the putative Duchenne muscular dystrophy gene in differentiated myogenic cell cultures and in the brain. Nature 1988;331:635638.
Chelly J, Hamard G, Koulakoff A, Kaplan JC, Kahn A, Berwald-Netter Y: Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature 1990;344:64–65
Feener CA, Koenig M, Kinkel LM: Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature 1989;338:509–511
Klamut HJ, Gangopadhyay SB, Worton RG, Ray PN: Molecular and functional analysis of the Duchenne muscular dystrophy gene. Mol Cell Biol 1990;10:193–205
Nudel U, Zuk D, Einat P, Zeelon E, Levy Z, Neuman S, Yaffe D: Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature 1989;337:76–78
Gorecki DC, Monaco AP, Derry JMJ, Walker AP, Barnard EA, Barnard PJ: Expression of four alternative dystrophin transcripts in brain regions regulated by different promoters. Hum Mol Genet 1992;1:505–510
Nishio H, Takeshima Y, Narita N, Yanagawa H, Suzuki Y, Ishikawa Y, Minami R, Nakamura H, Matsuo M: Identification of a novel first exon in the human dystrophin gene and a new promoter located more than 500 kb upstream of the nearest known promoter. J Clin Invest 1994;94:1037–1042
Byers TJ, Lidov HGW, Kunkel LM: An alternative dystrophin transcript specific to peripheral nerve. Nat Genet 1993;4:77–81
Blake DR, Love DR, Tinsley J, Morris GE, Turley H, Gatter K, Dickson G, Edwards YH, Davies KE: Characterization of a 4.8 kb transcript from the Duchenne muscular dystrophy locus expressed in Schwannoma cells. Hum Mol Genet 1992; 1: 103–109.
Lederfein D, Levy Z, Augier N, Mornet D, Morris G, Fuchs O, Yaffe D, Nudel U: A 71 kd protein is a major product of the Duchenne muscular dystrophy gene in brain and other non-muscle tissues. Proc Natl Acad Sci USA 1992;89:5346–5350.
Rapaport D, Lederfein D, Den Dunnen JT, Grootscholten PM, Van Ommen GJB, Fuchs O, Nudel U, Yaffe D: Characterization and cell type distribution of a novel, major transcript of the Duchenne muscular dystrophy gene. Differentiation 1992;49:187–194
Kramarcy NR, Vidal A, Froehner SC, Sealock R: Association of utrophin and multiple dystrophin short forms with the mammalian Mr 58,000 dystrophin-associated protein (syntrophin). J Biol Chem 1994;269:2870–2876
Tinsley JM, Blake DJ, Davies KE: Apo-dystrophin-3: A 2.2 kb transcript from the DMD locus encoding the dystrophin glycoprotein binding site. Hum Mol Genet 1993;2:521–524.
Ahn AH, Kunkel LM: The structural and functional diversity of dystrophin. Nat Genet 1993;3:283–291
Love DR, Byth BC, Tinsley JM, Blake DJ, Davies KE: Dystrophin and dystrophin-related proteins: A review of protein and RNA studies. Neuromusc Disord 1993;3:5–21
Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC: Two forms of mouse syntrophin, a 5 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 1993;11:531–540
Ervasti JM, Campbell KP: Membrane organization of the dystrophin-glycoprotein complex. Cell 1991;66:1121–1131
Ervasti JM, Campbell KP: A role for the dystrophin-glycoprotein complex as transmembrane linker between laminin and actin. J Cell Biol 1993;122:809–823
Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S: Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem 1993;268:14972–14980
Lenk U, Hanke R, Thiele H, Speer A: Point mutations at the carboxy terminus of the human dystrophin gene: Implications for an association with mental retardation in DMD patients. Hum Mol Genet 1993;2:1877–1881
Huxley C, Hagino Y, Schlessinger D, Olson MV: The human HPRT gene on a yeast artificial chromosome is functional when transferred to mouse cells by cell fusion. Genomics 1991;9:742–750
Koenig M, Monaco AP, Kunkel LM: The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 1988;53:219–228
Beggs AH, Koenig M, Boyce FM, Kunkel LM: Detection of 98-percent DMD-BMD gene deletions by polymerase chain reaction. Hum Genet 1990;86:45–48
Burke DR, Carle GR, Olson MV: Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science 1987;236:806–812
Dauwerse JG, Jumelet EA, Wessels JW, Saris JJ, Hagemeijer A, Beverstock GC, Van Ommen GJB, Breuning MH: Extensive cross-homology between the long and short arm of chromosome 16 may explain leukemic inversion and translocations. Blood 1992;79:1299–1304
De Jong PJ, Yokobota K, Chen C, Lohman F, Pederson L, McNinch J, van Dilla M: Human chromosome-specific partial digest libraries in lambda and cosmid vectors (abstract). Cytogenet Cell Genet 1989; 51:985.
Roest PAM, Roberts RG, Sugino S, Van Ommen GJB, Den Dunnen JT: Protein truncation test (PTT) for rapid detection of translation-terminating mutations. Hum Mol Genet 1993;2:1719–1721
Den Dunnen JT, Grootscholten PM, Dauwerse JD, Monaco AP, Walker AP, Butler R, Anand R, Coffey AJ, Bentley DR, Steensma HY, Van Ommen GJB: Reconstruction of the 2.4 Mb human DMD-gene by homologous YAC recombination. Hum Mol Genet 1992;1:19–28
Srivastava AK, Schlessinger D: Vectors for inserting selectable markers in vector arms and human DNA inserts of yeast artificial chromosomes (YACs). Gene 1991;103:53–59.
Heikoop JC, Steensma HY, Van Ommen GJB, Den Dunnen JT: A simple and rapid method for separating co-cloned YACs. Trends Genet 1994; 10:40.
Bies RD, Phelps SF, Cortez MD, Roberts R, Caskey CT, Chamberlain JS: Human and murine dystrophin mRNA transcripts are differentially expressed during skeletal muscle, heart, and brain development. Nucleic Acids Res 1992;20:1725–1731
Roberts RG, Coffey AJ, Bobrow M, Bentley DR: Exon structure of the human dystrophin gene. Genomics 1993;16:536–538
Dickson G, Love DR, Davies KE, Wells KE, Piper TA, Walsh FS: Human dystrophin gene transfer: Production and expression of a functional recombinant-based gene. Hum Genet 1991;88:53–58
Peterson KR, Clegg CH, Huxley C, Josephson BM, Haugen HS, Furukawa T, Stamatoyannopoulos G: Transgenic mice containing a 248 kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci USA 1993;90:7593–7597.
Jakobovits A, Moore AL, Green LL, Vergara GJ, Maynard-Curie CE, Austin HA, Klapholz S: Germ-line transmission and expression of a human-derived yeast artificial chromosome. Nature 1993,362:255–258.
Hoffman EP, Monaco AP, Feener CA, Kunkel LM: Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science 1987;238:347–350
Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener CA, Kunkel LM: Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987;50:509–517
Grootscholten PM, Den Dunnen JT, Monaco AP, Anand R, Van Ommen GJB: YAC mapping strategies applied to the DMD-gene. Technique 1991;3:41–50
Acknowledgements
We would like to thank Dr. Clare Huxley and Dr. Y. de Steensma for helpful discussions, Denis le Paslier (CEPH) for additional unrearranged YACs and Drs. M. Koenig and D. Yaffe for sharing unpublished sequences. This research was supported by the Muscular Dystrophy Group of Great Britain and Northern Ireland, the Dutch Prevention Fund and the Prinses Beatrix Fonds.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heikoop, J.C., Hogervorst, F.B.L., Meershoek, E.J. et al. Expression of the Human Dp 71 (Apo-Dystrophin-1) Gene from a 760-kb DMD-YAC Transferred to Mouse Cells. Eur J Hum Genet 3, 168–179 (1995). https://doi.org/10.1159/000472292
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1159/000472292