Featured
-
-
Letter |
 Tbx3 improves the germ-line competency of induced pluripotent stem cells
Tbx3 improves the germ-line competency of induced pluripotent stem cells
The transcription factor Tbx3 is shown to significantly improve the quality of induced pluripotent stem (iPS) cells. Tbx3 binding sites in embryonic stem cells are present in genes involved in pluripotency and reprogramming factors. Furthermore, there are intrinsic qualitative differences in iPS cells generated by different methods in terms of their pluripotency, thus highlighting the need to rigorously characterize iPS cells beyond in vitro studies.
- Jianyong Han
- , Ping Yuan
- & Bing Lim
-
Letter |
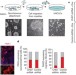 CHD7 cooperates with PBAF to control multipotent neural crest formation
CHD7 cooperates with PBAF to control multipotent neural crest formation
Heterozygous mutations in the gene encoding CHD7, an ATP-dependent chromatin-remodelling protein, result in CHARGE syndrome — a disorder characterized by malformations of the craniofacial structures, peripheral nervous system, ears, eyes and heart. In humans and Xenopus, CHD7 is now shown to be essential for the formation of multipotent migratory neural crest and for activating the transcriptional circuitry of the neural crest; shedding light on the pathoembryology of CHARGE syndrome.
- Ruchi Bajpai
- , Denise A. Chen
- & Joanna Wysocka
-
News |
 Britain grants patent for iPS cells
Britain grants patent for iPS cells
The first issued outside Japan for reprogrammable stem cells credits different inventors.
- Sabin Russell
-
-
Research Highlights |
Regenerative biology: New nerve cells connect
-
Article |
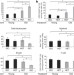 Systemic signals regulate ageing and rejuvenation of blood stem cell niches
Systemic signals regulate ageing and rejuvenation of blood stem cell niches
Age-associated changes in stem cell supportive niche cells are shown to deregulate normal haematopoiesis by causing haematopoietic stem cell dysfunction. Age-dependent defects in niche cells are systemically regulated and can be reversed by exposure to a young circulation or by neutralization of the conserved longevity regulator, insulin-like growth factor-1, in the marrow microenvironment.
- Shane R. Mayack
- , Jennifer L. Shadrach
- & Amy J. Wagers
-
-
Article |
 Direct conversion of fibroblasts to functional neurons by defined factors
Direct conversion of fibroblasts to functional neurons by defined factors
Mouse and human fibroblasts can be reprogrammed to a pluripotent state with a combination of four transcription factors. Here, mature differentiated cells are directed, via a combination of a few transcription factors (distinct from those described for generating iPS cells), to form functional neurons in vitro, without having to revert the fibroblasts to an embryonic state.
- Thomas Vierbuchen
- , Austin Ostermeier
- & Marius Wernig
-
Letter |
 Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency
Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency
The extent of epigenetic reprogramming in mammalian primordial germ cells (PGCs) and in early embryos, and its molecular mechanisms, are poorly understood. DNA methylation profiling in PGCs now reveals a genome–wide erasure of methylation, with female PGCs being less methylated than male ones. A deficiency of the cytidine deaminase AID interferes with the genome–wide erasure of DNA methylation, indicating that AID has a critical function in epigenetic reprogramming.
- Christian Popp
- , Wendy Dean
- & Wolf Reik
-
News |
 Health benefits of red-wine chemical unclear
Health benefits of red-wine chemical unclear
Sceptics continue to ask whether resveratrol really can delay the effects of ageing.
- Lizzie Buchen
-
Letter |
 DNMT1 maintains progenitor function in self-renewing somatic tissue
DNMT1 maintains progenitor function in self-renewing somatic tissue
Progenitor cells sustain the capacity of self-renewing tissues for proliferation while suppressing cell cycle exit and terminal differentiation. DNA methylation is one potential epigenetic mechanism for the cellular memory needed to preserve the somatic progenitor state through cell divisions. The DNA methyltransferase 1 and other regulators of DNA methylation are now shown to be essential for epidermal progenitor cell function.
- George L. Sen
- , Jason A. Reuter
- & Paul A. Khavari
-
Research Highlights |
 Molecular biology: Flowering time unravelled
Molecular biology: Flowering time unravelled
-
-
Letter |
 Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate
Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate
Immune homeostasis relies on tight control over the size of a population of regulatory T cells (Treg) that can suppress over-exuberant immune responses. Cells commit to the Treg lineage by upregulating the transcription factor Foxp3. Conserved non-coding DNA sequence elements at the Foxp3 locus are now shown to control the composition, size and maintenance of the Treg cell population.
- Ye Zheng
- , Steven Josefowicz
- & Alexander Y. Rudensky
-
Article |
 Opposing microRNA families regulate self-renewal in mouse embryonic stem cells
Opposing microRNA families regulate self-renewal in mouse embryonic stem cells
The differentiation of an embryonic stem cell (ESC) requires both suppression of the self-renewal process and activation of the specific differentiation pathway. The let-7 family of microRNAs (miRNAs) are now shown to suppress the self-renewal program in cells that are normally unable to silence this program, whereas introduction of ESC cell cycle regulating miRNAs blocks the action of let-7. Thus, the interplay between these two groups of miRNAs dictates cell fate.
- Collin Melton
- , Robert L. Judson
- & Robert Blelloch
Browse broader subjects
Browse narrower subjects
- Ageing
- Angiogenesis
- Bone development
- Bone remodelling
- Cartilage development
- Cell growth
- Cell proliferation
- Ciliogenesis
- Differentiation
- Disease model
- Embryogenesis
- Embryology
- Epigenetic memory
- Experimental organisms
- Germline development
- Haematopoiesis
- Intrauterine growth
- Lymphangiogenesis
- Morphogenesis
- Neurogenesis
- Organogenesis
- Pattern formation
- Pluripotency
- Reprogramming
- Self-renewal
- Senescence
- Stem-cell niche
- Stem cells
- Transdifferentiation

 Rfx6 directs islet formation and insulin production in mice and humans
Rfx6 directs islet formation and insulin production in mice and humans Chromatin remodelling during development
Chromatin remodelling during development The art of animal colouring
The art of animal colouring