Abstract
Dysregulation of the hematopoietic niche during hyperlipidemia facilitates pathologic leukocyte production, driving atherogenesis. Although definitive hematopoiesis occurs primarily in the bone marrow, during atherosclerosis this also occurs in the spleen. Cells of the bone marrow niche, particularly endothelial cells, have been studied in atherosclerosis, although little is known about how splenic endothelial cells respond to the atherogenic environment. Here we show unique dysregulated pathways in splenic compared to bone marrow endothelial cells during atherosclerosis, including perturbations of lipid metabolism and endocytic trafficking pathways. As part of this response, we identify the mixed lineage kinase domain-like (MLKL) protein as a repressor of splenic, but not bone marrow, myelopoiesis. Silencing MLKL in splenic endothelial cells results in inefficient endosomal trafficking and lipid accumulation, ultimately promoting the production of myeloid cells that participate in plaque development. These studies identify endocytic trafficking by MLKL as a key mechanism of splenic endothelial cell maintenance, splenic hematopoiesis and, subsequently, atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text or included in the supplementary materials. RNA sequencing data generated in this study were deposited in the Gene Expression Omnibus database using accession number GSE216341. Other publicly available RNA sequencing data can be found using the following accession numbers: GSE144498 and GSE134663.
References
Bjorkegren, J. L. M. & Lusis, A. J. Atherosclerosis: recent developments. Cell 185, 1630–1645 (2022).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Rasheed, A. Niche regulation of hematopoiesis: the environment is ‘micro,’ but the influence is large. Arterioscler. Thromb. Vasc. Biol. 42, 691–699 (2022).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012).
Rauch, P. J. et al. Innate response activator B cells protect against microbial sepsis. Science 335, 597–601 (2012).
Emami, H. et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 8, 121–130 (2015).
Mendez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 20, 285–298 (2020).
Mercier, F. E., Ragu, C. & Scadden, D. T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 12, 49–60 (2012).
Leimkuhler, N. B. & Schneider, R. K. Inflammatory bone marrow microenvironment. Hematology Am. Soc. Hematol. Educ. Program 2019, 294–302 (2019).
Rohde, D. et al. Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nat. Cardiovasc. Res. 1, 28–44 (2022).
Inra, C. N. et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527, 466–471 (2015).
Rasheed, A. et al. Loss of MLKL (mixed lineage kinase domain-like protein) decreases necrotic core but increases macrophage lipid accumulation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 40, 1155–1167 (2020).
Ketelhuth, D. F. J. et al. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc. Res. 115, 1385–1392 (2019).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Yoon, S., Kovalenko, A., Bogdanov, K. & Wallach, D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47, 51–65 (2017).
Gong, Y. N. et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300 (2017).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Zhao, J. et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl Acad. Sci. USA 109, 5322–5327 (2012).
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Asai, K., Kuzuya, M., Naito, M., Funaki, C. & Kuzuya, F. Effects of splenectomy on serum lipids and experimental atherosclerosis. Angiology 39, 497–504 (1988).
Lee, M. K. S. et al. Defective AMPK regulation of cholesterol metabolism accelerates atherosclerosis by promoting HSPC mobilization and myelopoiesis. Mol. Metab. 61, 101514 (2022).
Cesta, M. F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 34, 455–465 (2006).
Lewis, S. M., Williams, A. & Eisenbarth, S. C. Structure and function of the immune system in the spleen. Sci. Immunol. 4, eaau6085 (2019).
Samson, A. L. et al. A toolbox for imaging RIPK1, RIPK3, and MLKL in mouse and human cells. Cell Death Differ. 28, 2126–2144 (2021).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Mahley, R. W. & Rall, S. C. Jr. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537 (2000).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
Alvarez-Diaz, S. et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45, 513–526 (2016).
Morrison, S. J., Wright, D. E. & Weissman, I. L. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc. Natl Acad. Sci. USA 94, 1908–1913 (1997).
Alva, J. A. et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767 (2006).
Nosaka, T. et al. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 18, 4754–4765 (1999).
Poller, W. C., Nahrendorf, M. & Swirski, F. K. Hematopoiesis and cardiovascular disease. Circ. Res. 126, 1061–1085 (2020).
Morita, Y. et al. Functional characterization of hematopoietic stem cells in the spleen. Exp. Hematol. 39, 351–359 e353 (2011).
Hosseini, Z. et al. Resolvin D1 enhances necroptotic cell clearance through promoting macrophage fatty acid oxidation and oxidative phosphorylation. Arterioscler. Thromb. Vasc. Biol. 41, 1062–1075 (2021).
Linton, M. F., Atkinson, J. B. & Fazio, S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science 267, 1034–1037 (1995).
Amersfoort, J., Eelen, G. & Carmeliet, P. Immunomodulation by endothelial cells—partnering up with the immune system? Nat. Rev. Immunol. 22, 576–588 (2022).
Rickard, J. A. et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157, 1175–1188 (2014).
Hildebrand, J. M. et al. A missense mutation in the MLKL brace region promotes lethal neonatal inflammation and hematopoietic dysfunction. Nat. Commun. 11, 3150 (2020).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
FastQC Version 0.11.3 (QUBES, 2015).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Putri, G. H., Anders, S., Pyl, P. T., Pimanda, J. E. & Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 38, 2943–2945 (2022).
Acknowledgements
We thank the Animal Care and Veterinary Service staff for their support of this work as well as R. Seymour for performing the splenectomies. We also thank X. Zhao for assistance with the histology and B. Ye for supervising the flow cytometry sorting for the adoptive transfer studies. All images were made using BioRender. We also acknowledge the assistance of the Ottawa Bioinformatics Core Facility (uOttawa/OHRI, RRID: SCR_022466), the Cell Biology and Image Acquisition Core (uOttawa, RRID: SCR_021845) and the Louise Pelletier Histology Core (uOttawa, RRID: SCR_021737).
This work was supported by the Scientific and Technological Research Council of Turkey (H.K.), the University of Ottawa Cardiac Endowment Fund (A.R.), a Canadian Institutes of Health Research Fellowship (A.R.), the CI3 Big Data Award (K.J.R.) and the Canadian Institutes of Health Research (M.O. and K.J.R.).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.R. and K.J.R. Methodology: A.R. and K.J.R. Investigation: A.R., S.R., T.D., M.-A.N., M.G., J.N.R., H.J.W., Y.M. and A.B. Resources: R.L., H.K., M.C., C.v.S., M.O. and K.J.R. Writing—original draft: A.R. and K.J.R. Writing—review and editing: A.R., H.K., M.C., C.v.S., M.O. and K.J.R. Visualization: A.R. and K.J.R. Supervision: A.R. and K.J.R. Project administration: A.R. and K.J.R. Funding: K.J.R.
Corresponding authors
Ethics declarations
Competing interests
R.L. was an employee of Ionis Pharmaceuticals. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks David Wallach and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bone marrow and splenic endothelial cells respond differently to an atherosclerotic environment.
a-c, Gene set enrichment analyses of pathways related to Endothelial Cell Biology (a), Cell Cycle (b) and Cell Death (c) related pathways. Red circles = bone marrow and blue circles = spleen. Filled circles represent normalized enrichment scores of pathways with P-adj<0.05 and empty circles as P-adj≥0.05. Full dataset found in Supplementary Table 1.
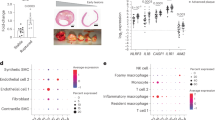
Extended Data Fig. 2 Atherosclerosis does not affect splenic stromal cells.
Representative images and quantification of PDGFRβ+ area in the splenic red pulp after 4, 8 or 16 weeks of HCD feeding in Apoe-/- mice. PDGFRβ shown in green and nuclei in blue. Week 4: n = 2 mice, Weeks 8 & 16: n = 3 mice. Scale bar = 500μm. Quantification is represented as a percent of the red pulp area. WP: white pulp. Data are shown as mean ± s.e.m. Statistical significance was determined by one-way ANOVA followed by Holm-Sidak’s post-hoc test for multiple comparisons.
Extended Data Fig. 3 Splenic expansion and MLKL knockdown is achieved by two independent sequences of MLKL-targeting antisense oligonucleotides.
a-b, Spleen weight (left) and spleen ratio to body weight (right) (a; n = 13 mice per group) and pulp area quantification (b; n = 6 mice per group) during Mlkl KD in Apoe-/- mice after 16 weeks of HCD feeding and administration of control (scrambled) or two individual Mlkl-targeting ASOs. Control and Mlkl KD Seq. 1 data presented in Fig. 2e & 2g. c, Increased magnification of representative images from Fig. 2g. Scalebar = 200μm. WP: white pulp. d-f, Knockdown of MLKL in the spleen by Mlkl-targeting ASO sequences after 16 weeks of treatment was confirmed at the gene expression (d; n = 6 mice per group) and proteins levels by Western blotting (e; control n = 6 mice, Mlkl KD Seq. 1 & 2 n = 7 mice per group) and at 6 weeks of treatment by confocal microscopy (f; MLKL shown in purple, CD45 shown in green and nuclei in blue; scale bar = 500μm). Western blotting samples were run on separate gels, with one sample run on all gels to ensure the results between gels were comparable. Data are shown as mean ± s.e.m. Statistical significance was determined by one-way ANOVA followed by Holm-Sidak’s post-hoc test for multiple comparisons.
Extended Data Fig. 4 Mlkl knockdown decreases necrotic core but not overall lesion area.
a, Representative images of H&E stained aortic sinuses from Apoe-/- mice after 16 weeks of HCD feeding and Mlkl KD. Scale bar = 600μm. b-d, Quantification of necrotic core content and total area of atherosclerotic plaques from male and female mice. Male: control n = 7 mice, Mlkl KD n = 6 mice; Female: control n = 3 mice, Mlkl KD n = 4 mice; Combined: n = 10 mice per group. Data are shown as mean ± s.e.m. Statistical significance was determined by two-tailed unpaired t-test.
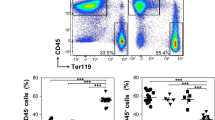
Extended Data Fig. 5 Flow cytometry gating scheme.
a-c, Representative flow cytometry plots for gating of hematopoietic and non-hematopoietic cell types in the bone marrow, spleen and liver. Bone marrow and splenocytes were first gated on viable cells and liver progenitors were gated on viable CD45+ cells. All gatings were determined by FMO controls. d, CD45.1 positive populations were determined by FMO controls where indicated. Lin2: Lineage2, LKS-: Lin2- CD117+ Sca1-, LSK: Lin2- Sca1+ CD117+, MPP2/3/4: multipotent progenitor 2/3/4, CMP: common myeloid progenitor, MEP: megakaryocyte-erythrocyte progenitor, GMP: granulocyte-monocyte progenitor, CLP: common lymphoid progenitor, FMO: fluorescence minus one.
Extended Data Fig. 6 Mlkl knockdown potentiates the expansion of splenic stem and progenitor cells.
a,b, Flow cytometry quantification of splenic hematopoietic stem, progenitor (a) and mature (b) populations in Apoe-/- mice after 16 weeks of treatment. n = 10 mice per group. c,d, Total number of splenocytes per spleen (c; control n = 7 mice, Mlkl KD n = 8 mice) and bone marrow cells per leg (d; control n = 18 mice, Mlkl KD n = 11 mice). e, Flow cytometry quantification of hematopoietic stem and progenitors in the livers of Apoe-/- mice after 8 weeks of treatment. n = 5 mice per group. Data are shown as mean ± s.e.m. Statistical significance was determined by two-tailed unpaired t-test (a, c & e) or two-tailed Mann-Whitney test (b & d).
Extended Data Fig. 7 MLKL represses hyperlipidemic but not acute inflammatory models of splenic hematopoiesis.
a-d, Spleen weight (left) and spleen:body weight ratios (right) (a), plasma cholesterol (b) and quantification of splenic hematopoietic populations (c,d) in C57Bl/6 mice fed an HCD while receiving ASOs for 15 weeks. control n = 5 mice, Mlkl KD n = 6 mice. e-h, Mlkl knockdown in WT chow fed C57Bl/6 mice after 12 weeks of ASO administration. Splenic weight (left), spleen-to-body weight ratio (right) (e), plasma cholesterol (f), hematopoietic progenitors (g) and mature hematopoietic splenic cell types (h) after Mlkl knockdown during chow feeding. n = 6 mice per group. i-l, G-CSF administration to WT mice during Mlkl knockdown. Spleen weight (left) and ratio to body weight (right) (i; control n = 9 mice, Mlkl KD n = 10 mice), plasma cholesterol (j; n = 6 mice per group) and splenic hematopoietic progenitor (k; LSK, CMP, MEP, GMP: n = 10 mice per group; CLP: control n = 6 mice, Mlkl KD n = 9 mice) and mature populations (l; n = 10 mice per group) were evaluated after myeloablation and G-CSF treatment for 2 weeks. Data are shown as mean ± s.e.m. Statistical significance was determined by two-tailed unpaired t-test.
Extended Data Fig. 8 Loss of Mlkl does not alter bone marrow endothelial or splenic stromal populations.
a,b, Representative flow cytometry plots and quantification of bone marrow endothelial cells (a; control n = 8 mice, Mlkl KD n = 6 mice) and splenic stromal cells (b; n = 5 mice per group) after 16 and 8 weeks of treatment in Apoe-/- mice, respectively. Data are shown as mean ± s.e.m. Statistical significance was determined by two-tailed unpaired t-test.
Extended Data Fig. 9 Splenic endothelial cells have greater lipid availability and regulate HSPC activation.
a, Representative flow cytometry plots and quantification of bone marrow and splenic lipid content (BODIPY+) from Apoe-/- mice after 4 weeks of HCD feeding and Mlkl knockdown. n = 4 mice per group. b, DiI-labelled acetylated low-density lipoprotein (DiI-acLDL) incorporation in bone marrow and splenic endothelial cells was assessed 4 hours after in vivo administration to 5-week HCD-fed Apoe-/- mice. n = 5 mice per group. c,d, Representative images and quantification of primary mouse splenic (c; siControl n = 6 replicates, siMLKL n = 5 replicates) and bone marrow (d; siControl n = 10 replicates, siMLKL n = 9 replicates) endothelial cells transfected with non-targeting (siControl) or Mlkl-targeting (siMLKL) siRNA. Data representative from 3 independent experiments. MLKL shown in purple and nuclei shown in blue. Scalebar = 10μm. Cells are outlined by the white dashed lines. e, Representative flow cytometry plots and quantification of pStat5 expression in CMFDA+ HSPCs after co-culture with transfected splenic endothelial cells. siControl n = 3 replicates, siMLKL n = 4 replicates. Representative of 3 individual experiments. f, Myeloid colony formation from HSPCs after co-culture in transwells with transfected splenic endothelial cells. n = 3 replicates per group. Data representative from 3 independent experiments. Data are shown as mean ± s.e.m. Statistical significance was determined by two-way ANOVA followed by Holm-Sidak’s post-hoc test for multiple comparisons (a), two-tailed paired t-test (b), two-tailed Mann Whitney test (c) or two-tailed unpaired t-test (d-f).
Supplementary information
Supplementary Tables 1–4
Combined supplementary tables.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed Western blot.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig.8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rasheed, A., Robichaud, S., Dennison, T. et al. Hyperlipidemia-induced hematopoiesis is repressed by MLKL in endothelial cells of the splenic niche. Nat Cardiovasc Res 3, 594–611 (2024). https://doi.org/10.1038/s44161-024-00470-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00470-8