Abstract
Cryptosporidium spp. are significant zoonotic intestinal parasites that induce diarrhea and even death across most vertebrates, including humans. Previous studies showed that sheep are important hosts for Cryptosporidium and that its distribution in sheep is influenced by geography, feeding patterns, age, and season. Environmental factors also influence the transmission of Cryptosporidium. Molecular studies of Cryptosporidium in sheep have been conducted in only a few regions of China, and studies into the effect of sheep-housing environments on Cryptosporidium transmission are even rarer. To detect the prevalence of Cryptosporidium in large-scale sheep-housing farms, a total of 1241 fecal samples were collected from sheep, 727 environmental samples were taken from sheep housing, and 30 water samples were collected in six regions of China. To ascertain the existence of the parasite and identify the species of Cryptosporidium spp., we conducted nested PCR amplification of DNA extracted from all samples using the small-subunit (SSU) rRNA gene as a target. For a more in-depth analysis of Cryptosporidium spp. subtypes, C. xiaoi-and C. ubiquitum-positive samples underwent separate nested PCR amplification targeting the 60 kDa glycoprotein (gp60) gene. The amplification of the Cryptosporidium spp. SSU rRNA gene locus from the whole genomic DNA of all samples yielded a positive rate of 1.2% (20/1241) in fecal samples, 0.1% (1/727) in environmental samples, and no positive samples were found in water samples. The prevalence of Cryptosporidium spp. infection in large-scale housed sheep was 1.7%, which was higher than that in free-ranging sheep (0.0%). The highest prevalence of infection was found in weaning lambs (6.8%). Among the different seasons, the peaks were found in the fall and winter. The most prevalent species were C. xiaoi and C. ubiquitum, with the former accounting for the majority of infections. The distribution of C. xiaoi subtypes was diverse, with XXIIIc (n = 1), XXIIId (n = 2), XXIIIe (n = 2), and XXIIIl (n = 4) identified. In contrast, only one subtype, XIIa (n = 9), was found in C. ubiquitum. In this study, C. xiaoi and C. ubiquitum were found to be the predominant species, and Cryptosporidium was found to be present in the environment. These findings provide an important foundation for the comprehensive prevention and management of Cryptosporidium in intensively reared sheep. Furthermore, by elucidating the prevalence of Cryptosporidium in sheep and its potential role in environmental transmission, this study deepens our understanding of the intricate interactions between animal health, environmental contamination, and public health dynamics.
Similar content being viewed by others
Introduction
Cryptosporidium spp. are an intestinal parasite of medical and veterinary significance with the capacity to infect a wide range of vertebrates, including humans1,2. Cryptosporidium spp. infection are frequently asymptomatic, yet it has the potential to induce a range of gastrointestinal disorders in the host and can become life-threatening in severe instances3. An expert committee formed by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) identified Cryptosporidium spp. as one of the 24 most detrimental foodborne parasites4. Cryptosporidium spp. oocysts exhibit remarkable resilience, enabling them to retain their viability in water and other environmental media over extended durations. These oocysts are transmitted through direct contact with infected individuals (human-to-human transmission) or animals (zoonotic transmission), as well as by the consumption of contaminated food (foodborne transmission) and water (waterborne transmission)5,6.
Sheep are included among the primary hosts of Cryptosporidium, and the parasite is capable of inducing diarrhea and, in severe cases, mortality in lambs. This, in turn, leads to substantial economic losses for farmers7. Cryptosporidium infection rates in sheep vary from 0.9 to 76.9%. Approximately 46 distinct Cryptosporidium species have been identified, with 14 species displaying the capacity to infect sheep. Among these, C. ubiquitum, C. xiaoi, C. andersoni, and C. parvum are the most significant8,9,10. The types and occurrences of Cryptosporidium infections in sheep exhibit geographical diversity. Of particular concern for public health is the presence of C. parvum and C. ubiquitum, both prevalent in sheep, because of their association with Cryptosporidiosis11.
The sheep industry is a cornerstone of China’s animal husbandry sector, having experienced rapid growth in recent years. The most common rearing approach has transitioned from free-range grazing to large-scale-housed feeding, which mitigates the impact of external environmental conditions and seasonal fluctuations. While this shift has enhanced production efficiency, the elevated rearing density also creates an environment conducive to the occurrence of Cryptosporidiosis outbreaks12. There have been many studies on Cryptosporidium infections in sheep, but little is known about Cryptosporidium contamination in the living environment of housed sheep. In this study, we investigated Cryptosporidium infections and environmental contamination in large-scale-housed sheep farms by collecting fresh fecal samples from sheep as well as samples from the sheep's living environment. The results of the study help characterize the regional distribution of Cryptosporidium species and subtypes, which may be useful for the prevention and control of Cryptosporidium infections.
Results
Prevalence of Cryptosporidium species
In total, 1241 sheep fecal samples were gathered, of which 20 were positive for Cryptosporidium, yielding an infection rate of 1.6% (95% CI 0.9–2.3%). Within the six designated collection areas, Cryptosporidium infection prevalence among confined sheep in four regions was as follows: Henan, 1.5% (11/743); Ningxia, 1.9% (5/259); Jiangxi, 3.3% (2/60); and Tianjin, 6.1% (2/33). Notably, a significant difference in infection rates was observed between Tianjin and Henan (P < 0.05). A combined total of 727 environmental samples were gathered from Henan and Ningxia, with merely one sample (0.1%, 95% CI 0.0–0.4%) yielding a positive result. This sample originated from a large-scale sheep farm in Henan. Thirty water samples were collected and subjected to testing but yielded no positive readings for Cryptosporidium (Table 1).
Cryptosporidium infection in sheep at different physiological stages
Following the examination of 1127 fecal samples for the presence of Cryptosporidium in large-scale-housed sheep that were divided into nine well-defined physiological stages, the most remarkable occurrence of Cryptosporidium infection was in weaning lambs (6.8%, 95% CI 2.7–10.9%), which exhibited a considerably higher infection rate compared with fattening lambs (1.6%, 95% CI 0.0–3.5%) (P < 0.05). No instances of Cryptosporidium infection were identified among adult sheep, except for breeding rams, as shown in Table 2.
Cryptosporidium infection of sheep and contamination of the environment in different seasons
In the context of studying seasonal dynamics, we focused on Ruzhou-scale farms situated in Henan Province, China. The findings revealed that the infection rate reached its peak during winter (2.6%, 95% CI 0.0–2.2%) and hit its nadir in spring (0.9%, 95% CI 0–2.22%). Among the environmental samples, a single positive sample was identified during the summer, while the presence of Cryptosporidium was absent throughout the remaining seasons (Table 3).
Genotyping and subtyping of Cryptosporidium spp. infecting sheep
The SSU rRNA gene fragments from the 21 Cryptosporidium-positive samples obtained in this experiment were effectively sequenced. The outcomes unveiled the existence of four distinct species: C. xiaoi (n = 10), C. ubiquitum (n = 9), C. parvum (n = 1), and C. andersoni (n = 1). For C. xiaoi, two sequences were procured, wherein six isolates exhibited complete congruence with a sequence identified in Algerian sheep (LC414392), while four isolates bore a resemblance to a sequence detected in Tibetan sheep (OL376597). In the case of C. ubiquitum, all nine isolates exhibited complete similarity to a sequence identified in British sheep (KM199742). A C. parvum isolate showed a complete resemblance to a sequence (OQ456120) identified in Chinese cows. Likewise, an isolate of C. andersoni exhibited complete congruity with a sequence (MK841325) detected in Chinese camels.
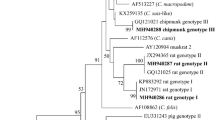
The gp60 gene was effectively amplified from nine isolates of C. xiaoi and an additional nine isolates of C. ubiquitum. Among these, C. xiaoi presented four distinct subtypes (XXIIIc, n = 1; XXIIId, n = 2; XXIIIe, n = 2; XXIIIl, n = 4), while C. ubiquitum exhibited a solitary subtype, XIIa. A phylogenetic tree was constructed to evaluate the genetic relationships between the gp60 subtypes of C. xiaoi and C. ubiquitum (Fig. 1).
Molecular phylogenetic tree illustrating the genetic relationship between the gp60 subtypes of C. xiaoi and C. ubiquitum. The evolutionary history was inferred using the maximum likelihood method, and evolutionary analyses were conducted in MEGA 7.0. Bootstrap values > 50% from 1000 replicates are shown on the nodes. The sequences detected in this study are shown with triangles; known sequences observed in this study are marked with open triangles, and new sequences are indicated by filled triangles.
Discussion
This study aimed to ascertain the prevalence of Cryptosporidium infections in large-scale-housed sheep and assess the presence of environmental contamination. Additionally, it was designed to evaluate the infection-related risk factors and identify the predominant species/genotypes of Cryptosporidium spp. in housed sheep. The average Cryptosporidium infection rate in this study was 1.6% (20/1241), which is generally lower than many other findings in China. For instance, Mi et al. reported a rate of 28.5% across 10 provinces in China, while Wu et al. observed a rate of 4.5% in Tibetan sheep from Gansu7,13,14. The overall infection rate in our study surpassed the 0.94% infection rate discovered in Xinjiang’s sheep in China by Qi et al. Furthermore, the results resembled Lang et al.’s findings for Inner Mongolian sheep (1.2%) and Penglin Wang et al.’s findings for sheep across eight provinces in China (1.9%)9,15,16. The results obtained in this study are also relatively low within the global context10. The variations in the aforementioned outcomes could potentially have stemmed from disparities in sampling locations, seasons, testing methodologies, feeding environments, and/or experimental frameworks.
Cryptosporidium is transmitted through the fecal–oral route, involving the ingestion of contaminated water or food, as well as animal-to-animal transmission. Among these, waterborne transmission is widely recognized as the primary mode of dissemination17,18,19,20. This study involved the collection of 714 environmental samples and 30 samples of sheep drinking water. Despite the identification of just a single positive sample for Cryptosporidium on the fecal leakage board among the environmental samples, this observation effectively underscores the existence of Cryptosporidium contamination of the housing of extensively barn-reared sheep in farming establishments. The reason that Cryptosporidium was not detected in environmental samples from other parts of the sheep barn may have been due to burial of the soil, resulting in Cryptosporidium oocysts not being collected at the time of sampling. Cryptosporidium was not detected in the water samples, a result that diverges from the findings of prior research21. This discrepancy might be linked to the utilization of sheep drinking bowls at this farm, which can diminish the introduction of impurities, like mud and dirt, by sheep while drinking, ultimately guaranteeing cleaner water. This cleaner drinking water, in turn, curbs pathogen transmission and diminishes the vulnerability of sheep to diseases.
In this study, the Cryptosporidium infection prevalence among large-scale-housed sheep (1.7%) surpassed that in free-ranging sheep (0.0%), aligning with findings from earlier research16. These outcomes could be attributed to several factors. Firstly, free-range sheep are spread across a broader expanse and thus experience limited inter-flock contact, consequently curtailing the likelihood of the disease spreading. Additionally, extensive rearing often integrates elevated intensification and commercialization, potentially subjecting sheep to persistent stress. This chronic stress might contribute to immune suppression, heightening the sheep’s vulnerability to pathogenic agents capable of inducing illness.
The majority of studies have consistently demonstrated that Cryptosporidium infection is more commonly observed in immature sheep than adult sheep10,14,22. This pattern was partially mirrored in the current study, as Cryptosporidium infections were absent in adult sheep, excluding the breeding rams. The high rate of infection in breeding rams may be due to the more fixed living enclosure and the contamination of the environment with Cryptosporidium, which can lead to persistent and repeated infections. It may also be related to the fact that breeding rams are involved in the task of mating, which leads to long-term stress and reduced immunity, increasing their risk of infection. The peak prevalence of Cryptosporidium infection (6.8%) surfaced among weaning lambs, which can be potentially attributed to three factors: (1) Post-weaning, lambs lose a source of immune factors from maternal milk, but their immune systems remain in the developmental stages, rendering them more vulnerable to pathogens23. (2) Following weaning, lambs commonly part from their mothers to initiate independent living, exposing them to environmental alterations that might provoke physical adjustments or maladaptation in the juveniles, thereby elevating disease susceptibility24. (3) After weaning, lambs undergo a significant change in their diet from mother’s milk to solid food. If the transition is inappropriate or the food is not suitable, it may lead to malnutrition, which in turn affects the functioning of the immune system.
In this study, we selected a large-scale-housed sheep farm in Ruzhou, Henan Province to investigate the seasonal dynamics of Cryptosporidium infection in sheep. When examining fecal samples, we observed a heightened infection prevalence during the autumn and winter seasons, in contrast to the lower prevalence during the spring and summer periods. This trend aligns with previous research outcomes7. This phenomenon could potentially have arisen from the heightened durability of Cryptosporidium oocysts in the cooler temperatures characteristic of autumn and winter25,26. Additionally, the inclination of sheep to huddle together for warmth in cooler temperatures might contribute to heightened contact and transmission rates. The reduced infection rates observed during spring (0.9%) and summer (0.9%) might be attributed to the elevated temperatures characteristic of the Ruzhou region in Henan Province, China, during these periods. Such temperatures may prove inhospitable to the ex vivo persistence of Cryptosporidium oocysts27. The seasonal dynamics of Cryptosporidium-contaminated environments were then investigated in this study. Only one positive sample for Cryptosporidium was detected in environmental samples collected from a manure leakage plate during the summer months. This may have been caused by the collection of samples that happened to come into contact with fresh sheep fecal samples containing Cryptosporidium oocysts.
C. ubiquitum is prevalent among ruminants, rodents, carnivores, and primates, encompassing approximately nine distinct genotypes (XIIa–XIIi)28,29,30,31. Despite C. ubiquitum exhibiting a wide host spectrum, certain subtypes display signs of host specificity. Notably, ruminants often contract subtype XIIa, which concurrently serves as the dominant subtype of C. ubiquitum in human infections28. In this investigation, we detected nine isolates of C. ubiquitum and determined that all nine were categorized under subtype XIIa at the gp60 gene locus. This observation suggests that sheep could serve as significant intermediaries in the transmission of C. ubiquitum to humans, potentially facilitated by their direct interactions with infected animals or exposure to contaminated environments and drinking water sources.
C. xiaoi is usually found in sheep and goats and is the dominant Cryptosporidium species in sheep in many parts of China7,32 as well as the United States and Australia33,34. There are 12 subtypes (XXIIIa–XXIIIl) currently identified35. In this research, nine isolates of C. xiaoi were effectively subjected to subtyping, revealing the presence of four distinct subtypes: XXIIIc (n = 1), XXIIId (n = 2), XXIIIe (n = 2), and XXIIIl (n = 4). Yingying Fan et al. did not detect sheep infections involving XXIIIc; however, this study’s outcomes disclosed the presence of C. xiaoi XXIIIc infection in sheep35. These findings align with Lang et al.’s observations in Inner Mongolia16, effectively broadening the known spectrum of hosts susceptible to C. xiaoi XXIIIc.
C. parvum and C. andersoni constitute the predominant species of Cryptosporidium known to infect humans36. However, it is worth noting that C. andersoni predominantly targets primates and equids, whereas C. parvum displays a considerably wider spectrum of hosts, encompassing primates, ruminants, equids, and rodents37. Therefore, zoonotic Cryptosporidiosis is mainly caused by C. parvum. Simultaneously, the increasing occurrence of C. andersoni in humans is contributing to an incremental escalation in its potential as a public health hazard38,39,40,41. While this study unearthed merely one positive sample for each of C. parvum and C. andersoni, it is essential to remain vigilant to the potential public health and safety complications that might arise from outbreaks involving these two Cryptosporidium species.
Conclusions
This study determined that there was a cumulative Cryptosporidium infection rate of 1.7% (20/1153) in large-scale-housed sheep across certain regions of China. Moreover, four distinct Cryptosporidium species were discovered infecting the sheep: C. xiaoi, C. ubiquitum, C. parvum, and C. andersoni. Notably, C. xiaoi and C. ubiquitum emerged as the prevailing species. A singular instance of Cryptosporidium positivity was identified in an environmental sample, signifying the existence of Cryptosporidium-associated environmental contamination. Cryptosporidium was not detected in water samples. These findings establish a crucial foundation for the comprehensive prevention and management of Cryptosporidium in intensively reared sheep. Furthermore, by elucidating the prevalence of Cryptosporidium in sheep and its potential role in environmental transmission, this study enhances our comprehension of the intricate interplay among animal health, environmental pollution, and public health dynamics.
Materials and methods
Fecal sample collection
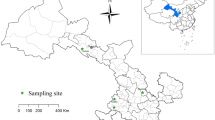
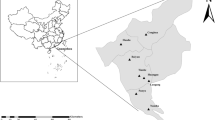
Between March 2021 and March 2023, a total of 1,998 samples were gathered from six distinct regions across China: Henan, Ningxia, Heilongjiang, Jiangxi, Inner Mongolia, and Tianjin. This comprehensive compilation included 1,241 sheep fecal samples (1153 from large-scale-housed sheep farms and 88 from free-range farms), 727 samples from the sheep living environments (714 from large-scale-housed sheep farms and 13 from free-range farms), and 30 water samples collected in a large-scale-housed sheep farm situated in Henan Province. These details are presented in Table 1.
Fecal samples were collected from sheep at nine physiological and developmental stages: lactating lambs, weaned lambs, fattening lambs, young sheep, empty ewes, pre-pregnant ewes, post-pregnant ewes, lactating ewes, breeding rams. Samples of 5–30 g of feces were collected rectally one at a time, placed in a clean plastic bag, numbered, registered, returned to the laboratory, and stored in a refrigerator at 4 °C for examination.
Collection of environmental samples
Environmental samples of 5–30 g were randomly collected at the different physiological stages sheep house entrance, fecal leakage board, sheep house exit, and corridor. The samples were placed in clean plastic bags, numbered, registered, and returned to the laboratory to be refrigerated (4 °C) before testing.
Water sample collection
A Ruzhou-scale farm in Henan Province was selected, and 2–4 water samples were collected from enclosures of sheep at different physiological stages, with a total of 30 samples collected. Each sample collected was 50 mL, which was placed in a clean centrifuge tube, numbered, registered, and brought back to the laboratory to be stored in the refrigerator at 4 °C before examination.
Seasonal dynamic survey of scale farms
To assess for sheep digestive tract infections and environmental contamination of Cryptosporidium spp. across various seasons within large-scale farms, we gathered samples from farms in Ruzhou City every quarter, resulting in a total of 573 fecal samples and 411 environmental samples. The samples were placed in clean plastic bags, numbered, registered, and returned to the laboratory to be refrigerated (4 °C) before testing.
DNA extraction and PCR amplification
DNA extraction from fecal and environmental samples involved placing approximately 100 mg of each sample into a 1.5 ml centrifuge tube. The E.Z.N.A. Stool DNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA) was utilized for whole-genome DNA extraction. Subsequently, 200 μL of DNA extract was collected, labeled, and stored at − 20 °C before subsequent testing.
DNA was extracted from the water samples as follows: a centrifuge tube containing 50 mL of water sample underwent centrifugation at 3500 rpm for 10 min. The supernatant was then discarded, leaving the precipitate. The DNeasy PowerSoil Pro kit (QIAGEN Inc., Hilden, Germany) was employed to extract the entire genomic DNA from the precipitate. Subsequently, 50 μL of DNA extract was collected, labeled, and stored at − 20 °C.
To ascertain the existence of the parasite and identify the species of Cryptosporidium spp., we conducted nested PCR amplification of DNA extracted from all samples using the small-subunit (SSU) rRNA gene as a target42. For a more in-depth analysis of Cryptosporidium spp. species and subtypes, C. xiaoi- and C. ubiquitum-positive samples underwent separate nested PCR amplification targeting the 60 kDa glycoprotein (gp60) gene28,35. The amplified products underwent 1% agarose gel electrophoresis using DNA Green dye for visualization, and the analysis was conducted using a Tanon 3500 gel image analysis system.
Sequencing and phylogenetic analyses
Secondary positive amplified fragments were forwarded to either Beijing SinoGenoMax Co. Ltd. or Sangon Bioengineering Co. (Shanghai) for purification and bi-directional sequencing. The BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was employed to analyze all sequences through homology searches. Representative nucleotide sequences obtained during this study have been deposited in the GenBank database and assigned accession numbers. The accession number for the gp60 gene is OR345287–OR345291.
Statistical analysis
Statistical analysis was carried out using SPSS version 22.0 (SPSS Inc.) and utilizing a chi-square test with a 95% confidence interval. This analysis was conducted to compare Cryptosporidium spp. infection rates across distinct collection sites, physiological stages, and seasons. A significance level of P value < 0.05 was employed to determine the statistical significance of the differences between groups.
Ethics approval and consent to participate
The study was conducted in accordance with the Chinese Law on the Management of Laboratory Animals (1988) and was reviewed and approved by the Research Ethics Committee of Henan Agricultural University. Permission was obtained from the farm owner before faecal samples were collected. In this study, all faecal samples were carefully collected from the rectum of each dairy cattle without causing harm.
Data availability
The datasets supporting the conclusions of this article are included within the article. Representative sequences are submitted to the GenBank database under the following accession numbers: OR345287–OR345291.
Abbreviations
- C.:
-
Cryptosporidium
- Spp.:
-
Species
- PCR:
-
Polymerase Chain Reaction
- SSU:
-
Small-subunit
- gp60 :
-
60 KDa glycoprotein
References
Kotloff, K. L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. N. Am. 64, 799–814. https://doi.org/10.1016/j.pcl.2017.03.006 (2017).
Khan, A., Shaik, J. S. & Grigg, M. E. Genomics and molecular epidemiology of Cryptosporidium species. Acta Trop. 184, 1–14. https://doi.org/10.1016/j.actatropica.2017.10.023 (2018).
Bouzid, M., Hunter, P. R., Chalmers, R. M. & Tyler, K. M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 26, 115–134. https://doi.org/10.1128/CMR.00076-12 (2013).
Bouwknegt, M., Devleesschauwer, B., Graham, H., Robertson, L. J. & van der Giessen, J. W. Euro-FBP workshop participants. Prioritisation of food-borne parasites in Europe, 2016. Euro Surveill. 23(9), 17–00161. https://doi.org/10.2807/1560-7917.ES.2018.23.9.17-00161 (2018).
Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 124, 80–89. https://doi.org/10.1016/j.exppara.2009.03.018 (2010).
Zahedi, A. & Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 132, 500–512. https://doi.org/10.1016/j.rvsc.2020.08.002 (2020).
Mi, R. et al. Sheep as a potential source of zoonotic Cryptosporidiosis in China. Appl. Environ. Microbiol. 84, e00868-e918. https://doi.org/10.1128/AEM.00868-18 (2018).
Ryan, U. M., Feng, Y., Fayer, R. & Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 51, 1099–1119. https://doi.org/10.1016/j.ijpara.2021.08.007 (2021).
Qi, M. et al. Distribution and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi amongst grazing adult sheep in Xinjiang, China. Parasitol. Int. 71, 80–86. https://doi.org/10.1016/j.parint.2019.04.006 (2019).
Chen, Y., Qin, H., Huang, J., Li, J. & Zhang, L. The global prevalence of Cryptosporidium in sheep: A systematic review and meta-analysis. Parasitology. 149, 1652–1665. https://doi.org/10.1017/S0031182022001196 (2022).
Xiao, L. & Feng, Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 8–9, 14–32. https://doi.org/10.1016/j.fawpar.2017.09.002 (2017).
Santin, M. Cryptosporidium and Giardia in ruminants. Vet. Clin. N. Am. Food Anim. Pract. 36, 223–238. https://doi.org/10.1016/j.cvfa.2019.11.005 (2020).
Wu, Y. et al. Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan sheep in Gansu, China. Infect. Genet. Evol. 64, 46–51. https://doi.org/10.1016/j.meegid.2018.06.012 (2018).
Yang, X.-Y., Gong, Q.-L., Zhao, B., Cai, Y.-N. & Zhao, Q. Prevalence of Cryptosporidium infection in sheep and goat flocks in china during 2010–2019: A systematic review and meta-analysis. Vector Borne Zoonotic Dis. 21, 692–706. https://doi.org/10.1089/vbz.2020.2713 (2021).
Wang, P. et al. Genotyping of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi from sheep and goats in China. BMC Vet. Res. 18, 361. https://doi.org/10.1186/s12917-022-03447-6 (2022).
Lang, J. et al. Molecular characterization and prevalence of Cryptosporidium spp. in sheep and goats in western Inner Mongolia, China. Parasitol. Res. 122, 537–545. https://doi.org/10.1007/s00436-022-07756-5 (2023).
Hassan, E. M. et al. A review of Cryptosporidium spp. and their detection in water. Water Sci. Technol. 83, 1–25. https://doi.org/10.2166/wst.2020.515 (2021).
Nolan, M. J. et al. Molecular-based investigation of Cryptosporidium and Giardia from animals in water catchments in southeastern Australia. Water Res. 47, 1726–1740. https://doi.org/10.1016/j.watres.2012.12.027 (2013).
Efstratiou, A., Ongerth, J. & Karanis, P. Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res. 123, 96–112. https://doi.org/10.1016/j.watres.2012.12.027 (2017).
Castro-Hermida, J. A. et al. Contribution of treated wastewater to the contamination of recreational river areas with Cryptosporidium spp. and Giardia duodenalis. Water Res. 42, 3528–3538. https://doi.org/10.1016/j.watres.2008.05.001 (2008).
Zahedi, A. et al. Cryptosporidium and Giardia in dam water on sheep farms—An important source of transmission?. Vet. Parasitol. 288, 109281. https://doi.org/10.1016/j.vetpar.2020.109281 (2020).
Bordes, L. et al. Asymptomatic Cryptosporidium infections in ewes and lambs are a source of environmental contamination with zoonotic genotypes of Cryptosporidium parvum. Parasite. 27, 57. https://doi.org/10.1051/parasite/2020054 (2020).
Rio-Aige, K. et al. The breast milk immunoglobulinome. Nutrients. 13, 1810. https://doi.org/10.3390/nu13061810 (2021).
Fazio, E., Ferlazzo, A., Cravana, C. & Medica, P. Effects of weaning on total and free iodothyronines in lambs. Vet. Q. 35, 16–20. https://doi.org/10.1080/01652176.2014.989624 (2015).
Li, X., Atwill, E. R., Dunbar, L. A. & Tate, K. W. Effect of daily temperature fluctuation during the cool season on the infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 76, 989–993. https://doi.org/10.1128/AEM.02103-09 (2010).
Erickson, M. C. & Ortega, Y. R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 69, 2786–2808. https://doi.org/10.4315/0362-028x-69.11.2786 (2006).
Utaaker, K. S., Skjerve, E. & Robertson, L. J. Keeping it cool: Survival of Giardia cysts and Cryptosporidium oocysts on lettuce leaves. Int. J. Food Microbiol. 255, 51–57. https://doi.org/10.1016/j.ijfoodmicro.2017.05.009 (2017).
Li, N. et al. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg. Infect. Dis. 20, 217–224. https://doi.org/10.3201/eid2002.121797 (2014).
Koehler, A. V., Haydon, S. R., Jex, A. R. & Gasser, R. B. Is Cryptosporidium from the common wombat (Vombatus ursinus) a new species and distinct from Cryptosporidium ubiquitum?. Infect. Genet. Evol. 44, 28–33. https://doi.org/10.1016/j.meegid.2016.06.028 (2016).
Huang, C. et al. Environmental transport of emerging human-pathogenic Cryptosporidium species and subtypes through combined sewer overflow and wastewater. Appl. Environ. Microbiol. 83, e00682-e717. https://doi.org/10.1128/AEM.00682-17 (2017).
Chen, J. et al. Genetic characterizations of Cryptosporidium spp. from pet rodents indicate high zoonotic potential of pathogens from chinchillas. One Health 13, 100269. https://doi.org/10.1016/j.onehlt.2021.100269 (2021).
Mi, R. et al. Prevalence and molecular characterization of Cryptosporidium in goats across four provincial level areas in China. PLoS One. 9, e111164. https://doi.org/10.1371/journal.pone.0111164 (2014).
Li, X., Vodovoza, T. & Atwill, E. R. Diverse genotypes of Cryptosporidium in sheep in California, USA. Pathogens. 11, 1023. https://doi.org/10.3390/pathogens11091023 (2022).
Sweeny, J. P. A. et al. Longitudinal investigation of protozoan parasites in meat lamb farms in southern Western Australia. Prev. Vet. Med. 101, 192–203. https://doi.org/10.1016/j.prevetmed.2011.05.016 (2011).
Fan, Y. et al. Subtyping Cryptosporidium xiaoi, a common pathogen in sheep and goats. Pathogens. 10(7), 800. https://doi.org/10.3390/pathogens10070800 (2021).
Dumaine, J. E., Tandel, J. & Striepen, B. Cryptosporidium parvum. Trends Parasitol. 36, 485–486. https://doi.org/10.1016/j.pt.2019.11.003 (2020).
Feng, Y., Ryan, U. M. & Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 34, 997–1011. https://doi.org/10.1016/j.pt.2018.07.009 (2018).
Jiang, Y. et al. Cryptosporidium andersoni as a novel predominant Cryptosporidium species in outpatients with diarrhea in Jiangsu Province, China. BMC Infect. Dis. 14, 555. https://doi.org/10.1186/s12879-014-0555-7 (2014).
Hussain, G., Roychoudhury, S., Singha, B. & Paul, J. Incidence of Cryptosporidium andersoni in diarrheal patients from southern Assam, India: A molecular approach. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1023–1032. https://doi.org/10.1007/s10096-016-2887-2 (2017).
Jiang, Y. et al. Molecular identification and genetic characteristics of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in human immunodeficiency virus/acquired immunodeficiency syndrome patients in Shanghai, China. Parasit. Vectors 16, 53. https://doi.org/10.1186/s13071-023-05666-8 (2023).
Ryan, U., Zahedi, A., Feng, Y. & Xiao, L. An update on zoonotic Cryptosporidium species and genotypes in humans. Animals (Basel). 11, 3307. https://doi.org/10.3390/ani11113307 (2021).
Alves, M. et al. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41, 2744–2747. https://doi.org/10.1128/JCM.41.6.2744-2747.2003 (2003).
Acknowledgements
We thank Suzanne Leech, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
This study was partly supported by the Earmarked Fund for China Modern Agro-industry Technology Research System (nycytx-38).
Author information
Authors and Affiliations
Contributions
Q.Z.: Software, investigation, resources, formal analysis, writing—original draft. M.Q.: Resources, investigation, formal analysis. B.J.: Investigation, formal analysis. F.J.: Investigation, formal analysis. P.G.: Software, formal analysis. C.L.: Resources, supervision, project administration. Y.Y.: Resources, supervision, project administration. Z.P.: Resources, supervision, project administration. C.N.: Resources, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Q., Qi, M., Jing, B. et al. Cryptosporidium spp. in large-scale sheep farms in China: prevalence and genetic diversity. Sci Rep 14, 11218 (2024). https://doi.org/10.1038/s41598-024-62110-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62110-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.