Abstract
Gut microbiota manipulation may reverse metabolic abnormalities in obesity. Our previous studies demonstrated that inulin supplementation significantly promoted Bifidobacterium and fat-free mass in obese children. We aimed to study gut-muscle axis from inulin supplementation in these children. In clinical phase, the plasma samples from 46 participants aged 7–15 years, were analyzed for muscle biomarkers before and after 6-month inulin supplementation. In parallel, the plausible mechanism of muscle production via gut-muscle axis was examined using macrophage cell line. Bifidobacterium was cultured in semi-refined medium with inulin used in the clinical phase. Cell-free supernatant was collected and used in lipopolysaccharide (LPS)-induced macrophage cell line to determine inflammatory and anti-inflammatory gene expression. In clinical phase, IL-15 and creatinine/cystatin C ratio significantly increased from baseline to the 6th month. In vitro study showed that metabolites derived from Bifidobacterium capable of utilizing inulin contained the abundance of SCFAs. In the presence of LPS, treatment from Bifidobacterium + inulin downregulated TNF-α, IL-6, IL-1β, and iNOS, but upregulated FIZZ-1 and TGF-β expression. Inulin supplementation promoted the muscle biomarkers in agreement with fat-free mass gain, elucidating by Bifidobacterium metabolites derived from inulin digestion showed in vitro anti-inflammatory activity and decreased systemic pro-inflammation, thus promoting muscle production via gut-muscle axis response.
Clinical Trial Registry number: NCT03968003.
Similar content being viewed by others
Introduction
Obesity is widely recognized as one of the leading causes of non-communicable diseases such as diabetes mellitus, cardiovascular diseases, non-alcoholic fatty liver disease (NAFLD), cancer, and impairment of immunity1. The global prevalence of childhood overweight and obesity stood at 6.7% in 2010 and exhibited a trajectory toward reaching 9.1% by 20202. Obese children's behaviors have an impact on their eating styles, such as an increased enjoyment of food, and they tend to prefer low-fiber diets and high sugary foods like sweetened drinks, such as fruit juices with varying glycemic load, contributing to obesity3,4. Therefore, providing suitable dietary management for children with obesity is crucial.
Presently, obesity is considered a chronic inflammatory condition with several supporting hypotheses. Lipopolysaccharide (LPS), a membrane component of gram-negative bacteria, is supposed to be one of the triggers in pathogenesis. LPS translocation across the intestinal barrier triggers inflammatory responses. LPS binds to plasma LPS-binding proteins, thereby triggering toll-like receptor 4 (TLR4) activation on the surface of macrophages within adipose tissue. This initiation stimulates the genes encoding nuclear factor-κB (NF-κB), which subsequently induce the production of inflammatory cytokines. Furthermore, LPS is involved in the inflammasome pathway, leading to the induction of interleukin-1β (IL-1β). Together, these processes contribute to the development of chronic, low-grade inflammation, a condition strongly linked to obesity and metabolic syndrome5. High-fat diets have also been observed to increase LPS absorption in the intestine, which is relevant as children with obesity often consume such high-fat diets, thereby increasing their risk of metabolic complications. Additionally, dysbiosis of the gut microbiota can disrupt intestinal permeability by reducing beneficial bacteria, like Bifidobacterium1,6,7. All these factors aggravate gram-negative bacteria which induce absorption of LPS to systemic circulation, resulting in the chronic inflammation in obesity.
To improve the intestinal microbial ecosystem, prebiotics, non-digestible polysaccharides have been studied and shown promising results in promoting a healthy balance of gut microbiota when supplemented in obese children1,8. Inulin, a fructan-type polysaccharide, is renowned as an evidence-based prebiotic. Several studies found inulin supplementation in individuals with obesity promoted the growth of Bifidobacterium spp. in the intestine9,10, decreases in body weight and body mass index (BMI)11, decreases in percent body and trunk fat10, an improvement in satiety and reductions in postprandial glucose and insulin levels12.
Recently, we conducted a randomized, double-blinded placebo-controlled study of inulin supplementation extracted from Thai Jerusalem artichoke using our patented technology in obese children (Patent no. 15858, Inventor: Chonnikant Visuthranukul and Supakarn Chamni, Chulalongkorn University and National Science and Technology Development Agency, Thailand). Of one hundred and fifty-five participants; interestingly, we found a significant increase in fat-free mass and Bifidobacterium only in the inulin group13. Improving the intestinal microbial ecosystem is believed to reduce pro-inflammation10 while enhance anti-inflammatory activity. It was already known that Bifidobacterium utilizes inulin to yield substrates essential for other short-chain fatty acid (SCFA)-producing bacteria, thereby contributing to the production of SCFAs14. Therefore, we hypothesized that metabolites-derived from Bifidobacterium would exhibit anti-inflammatory activity leading to myogenesis by increasing mitochondrial biogenesis, capillary density, glycogen storage, and protein synthesis in muscle mass15. Based on our previous finding in fat-free mass gain13, there has not been research done in the aspect of gut-muscle axis response in the pediatric obesity. To further underpin this knowledge, we aimed to study gut-muscle axis from inulin supplementation on muscle mass in this population.
Materials and methods
Subjects
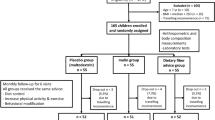
This present study used the plasma and serum samples from the previously published randomized double-blinded placebo-controlled trial which conducted from August 2017 to July 2020 at the King Chulalongkorn Memorial Hospital (KCMH), Thailand13. The Institutional Review Board of the Faculty of Medicine, Chulalongkorn University approved the study protocol (IRB No. 240/60). All experiments were performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and their legal guardians prior to study enrollment. This trial was registered at clinicaltrials.gov (NCT03968003). Thai children aged 7 to 15 years with obesity as a BMI more than 2 standard deviations (SDs) above median as per the WHO growth reference16 were recruited as mentioned in the previous study13.
Study design
The detailed study design has been published elsewhere13. In brief, Thai children aged 7–15 years with obesity were randomly assigned to inulin extracted from Jerusalem artichoke by our patented technique (intervention), maltodextrin (placebo), and dietary fiber advice groups. All participants received monthly follow-up with the same standard advice of dietary intake and exercise for 6 months. The details and flow diagram of the study are reported elsewhere13.
The in vivo biomarkers of muscle building
The stored plasma and serum samples from the randomised controlled trial (RCT) of inulin supplementation in obese children were analyzed for biomarkers of muscle building at the 1st and 6th visits. Plasma and serum samples were stored at − 80 °C until analysis. For the present study, 56 plasma/serum samples (n = 46 from the inulin group, n = 5 from each placebo and dietary fiber advice group) were analyzed for myokine and creatinine/cystatin C ratio to support the results of muscle building from the previous study13. Myokine, interleukin-15 (IL-15), was analyzed by enzyme-linked immunosorbent assay (ELISA). The quantification of IL-15, using the respective Human IL-15 ELISA kit (ab218266, Abcam, UK), was conducted in accordance with the prescribed manufacturer's protocols. Briefly, 50 µL of the sample was introduced into each well, followed by the addition of 50 µL of antibody, and incubation at room temperature for one hour. Subsequently, the sample mixture underwent three wash cycles using 350 µL of 1X wash buffer per cycle prior to the introduction of 100 µL of TMB development solution. The reaction proceeded at room temperature for 10 min and was subsequently terminated by the addition of 100 µL of stop solution. Measurement of the absorbance was carried out at a wavelength of 450 nm using a microplate reader. Furthermore, the concentration of creatinine in serum was measured using an enzymatic method on the Alinity C analyzers (Abbott Laboratories, USA), and that of cystatin C, N Latex Cystatin C, is an in vitro diagnostics kit containing reagents for the quantitative determination of cystatin C in human serum by means of particle-enhanced immunonephelometry using the BN Systems (SIEMENS, USA) with blood specimens stored at − 80 °C from the baseline and final visit. The samples were automatically diluted 1:100 with N Diluent and then were measured within four hours. If the results obtained were outside the measuring range, the assay was repeated using a higher or lower dilution of the sample.
The in vitro experiments
In parallel, the plausible mechanism of muscle mass building in the obese participants via gut-muscle axis was examined for in vitro phase. To test an influence of the metabolite from Bifidobacterium with or without inulin against macrophages, the condition media from Bifidobacterium (Bifidobacterium condition media; Bifido) with or without inulin were tested in a macrophage cell line (RAW264.7).
In Bifido preparation, Bifidobacterium longum HFDGO02 was cultured in SM medium using either glucose or inulin as the carbon source. Briefly, SM medium, glucose, and inulin were separately autoclaved. The medium containing either 1% (W/V) glucose or inulin was prepared and used for bacterial culture at 37 °C under an anaerobic condition for 48 h17. The supernatants were then collected by centrifugation and filtered (0.22-µm membrane filter) (Minisart; Sartorius Stedim Biotech GmbH, Göttingen, Germany), and 500 µl of the preparation was concentrated by speed vacuum drying at 40 °C for 3 h (Savant Instruments, Farmingdale, NY). The cell-free concentrated pellets were resuspended in an equal volume of DMEM or further diluted by DMEM into 50 and 25% dilution of the preparation and stored at − 20 °C until use. Then, murine macrophages (RAW264.7; ATCC-TIB-71) (Manassas, VA, USA) were cultured Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum and 1% Penicillin–Streptomycin at 37 °C under 5% CO2 before use. After that, the macrophages at 1 × 105 cells/well in 6-well plates were incubated for 24 h with several conditions (DMEM, inulin, Bifido, or Bifido with inulin) with or without LPS, using the LPS from Escherichia coli 026:B6 (Sigma-Aldrich, St. Louis, MO, USA) at 100 ng/ml. Notably, the dose of LPS to activate macrophages used here was followed our previous protocol18,19,20. After that, the expressions of several markers were measured by real time-qualitative polymerase chain reaction (RT-qPCR) using the primer presented in Table 1 with an established protocol. The RNA was extracted from the cells with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) together with RNeasy Mini Kit (Qiagen, Hilden, Germany) as 1 mg of total RNA was used for cDNA synthesis with iScriptreverse transcription supermix (Bio‐Rad, Hercules, CA, USA). Quantitative real‐time PCR was performed on a QuantStudio 5 real‐time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using SsoAdvanced Universal SYBR Green Supermix (Bio‐Rad, Hercules, CA, USA). Expression values were normalized to Beta‐actin (β‐actin) as an endogenous housekeeping gene and the fold change was calculated by the ∆∆Ct method. Additionally, supernatant cytokines of macrophage pro-inflammatory activity, including tumor necrosis factor–α (TNF-α), IL-6, and IL-1β, alone with anti-inflammatory function, including transforming growth factor-β (TGF-β) and IL-10, were also evaluated by ELISA assay followed the manufacturer’s protocols (R&D Systems, Inc., Minneapolis, MN, USA).
Macrophage cell energy status (the extracellular flux analysis)
The extracellular flux analysis using Seahorse XFp Analyzers (Agilent, Santa Clara, CA, USA) with oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) representing mitochondrial function (respiration) and glycolysis activity, respectively, were determined in the 24 h-activated cells. Briefly, the stimulated macrophages at 1 × 105 cells/well were incubated by Seahorse media (DMEM complemented with glucose, pyruvate, and l-glutamine) (Agilent, 103575-100) for 1 h before activation by different metabolic interference compounds, including oligomycin, carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP) and rotenone/antimycin A, for OCR evaluation. In parallel, glycolysis stress tests were performed using glucose, oligomycin, and 2-Deoxy-d-glucose (2-DG) for ECAR measurement. The data were analysed by Seahorse Wave 2.6 software based on the following equations: (i) maximal respiration = OCR between FCCP and rotenone/antimycin A - OCR after rotenone/antimycin A; (ii) maximal glycolysis (glycolysis capacity) = ECAR between oligomycin and 2-DG - ECAR after 2-DG.
Statistical analysis
Wilcoxon signed-rank test was used to evaluate the difference in the change of variable outcomes between baseline and the 6th month, which included muscle biomarkers, IL-15 and creatinine cystatin C ratio, in clinical phase. The gene expressions of inflammatory and anti-inflammatory markers in macrophage cell lines, presenting with or without LPS, among the Bifidobacterium and inulin controls, and treated with Bifidobacterium + inulin were evaluated using One-way ANOVA with Tukey analysis for in vitro phase. The alpha level of 0.05 was considered statistically significant for all analyses. All analyses were conducted using SPSS version 28.0 (SPSS Inc., Armonk, NY, USA) and GraphPad Prism version 9.0 (GraphPad Software, Boston, MA, USA).
Results
The change in biomarkers of muscle building in clinical study
The stored plasma and serum samples from the children with obesity who received inulin supplementation (mean age: 10.3 ± 2.1 years, 54% male), placebo, and dietary fiber advice were analyzed. As mentioned above, in our previously published RCT of supplementation with inulin on body composition, we found that inulin supplementation increased fat-free mass in children with obesity13.
IL-15, a myokine linked to muscle building, significantly increased in the inulin group (p < 0.0001, 95% CI 10.9–18.6). Moreover, within group analysis showed that (p = 0.0066, 95% CI 0.005–0.05) (Fig. 1a,b). The changes of IL-15 and creatinine/cytatin C ratio in the placebo and dietary fiber advice groups were not observed.
Change of IL-15 and serum creatinine/cystatin C ratio in the inulin group. Within group analysis showed significant increased IL-15 and creatinine/cystatin C ratio from the baseline only in the inulin group using Wilcoxon signed-rank test (p < 0.0001, 95% CI 10.9–18.6, and p = 0.0066, 95% CI 0.005–0.05, respectively) (n = 46). IL interleukin.
The change in pro-inflammatory and anti-inflammatory macrophage responses in cell line study
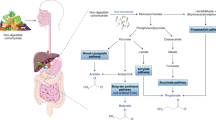
Our in vitro phase demonstrated that metabolites derived from Bifidobacterium capable of utilizing inulin contained the abundance of short chain fatty acids (SCFAs) (Supplement Fig. 1). In the presence of LPS, treatment of the cell-free supernatant from Bifidobacterium + inulin significantly downregulated pro-inflammatory genes, including TNF-α and IL-6 expression compared to the media control, Bifidobacterium alone, inulin alone, and the LPS (p < 0.05) (Fig. 2a,b). Downregulation of IL-1β and inducible nitric oxide synthase (iNOS) after Bifidobacterium + inulin administration was also significantly different from the LPS (p < 0.05) (Fig. 2c,d). Although inulin and Bifidobacterium supernatant alone reduced supernatant TNF-α and IL-6 compared to LPS control, Bifidobacterium + inulin further decreased both cytokines (p < 0.05) (Fig. 2e,f). On the other hand, only Bifidobacterium + inulin but not each factor in separation attenuated LPS-activated IL-1β in macrophages (p < 0.05) (Fig. 2g). For the anti-inflammatory responses, treatment from Bifidobacterium + inulin significantly upregulated FIZZ-1 and TGF-β expression compared to the LPS (p < 0.05) (Fig. 3a,b). No significant upregulation of,Arginase-1, and IL-10 expression (Fig. 3c,d) and alteration of supernatant anti-inflammatory cytokines (TGF-β and IL-10) (Fig. 3e,f) was observed after Bifidobacterium + inulin administration compared with LPS control. Dose effect of Bifidobacterium longum fermentative inulin (Bifido in Inulin or Bifidobacterium + inulin) for macrophage responses was performed using no dilution, 50% dilution, and 25% dilution by culture media (DMEM). Compared to LPS group, treatment with Bifidobacterium + inulin without dilution significantly downregulated gene expression of TNF-α, IL-6, IL-1β, and iNOS and reduced production of supernatant cytokines (TNF-α, IL-6, and IL-1β), while the dilution attenuated only IL-6 expression and supernatant IL-6 (Fig. 4a–g). The signal pathway diagram of the relationship between inflammation and microbiota-muscle axis was illustrated in Supplement Fig. 2.
Pro-inflammatory macrophage responses. In the presence of LPS, treatment of the cell-free supernatant from Bifidobacterium + inulin significantly decreased expression of TNF-α, IL-6, IL-1β, and iNOS compared to the inulin and Bifidobacterium controls, and the LPS (a–d) (p < 0.05). Supernatant pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) was also significantly lower than LPS control (e–g) using One-way ANOVA with Tukey analysis (p < 0.05). IL interleukin, iNOS inducible nitric oxide synthase, LPS lipopolysaccharide, TNF tumor necrosis factor.
Anti-inflammatory macrophage responses. Treatment from Bifidobacterium + inulin significantly upregulated gene expression of FIZZ-1 and TGF-β, but not Arg-1 and IL-10 compared to the LPS (a–d). No alteration of supernatant anti-inflammatory cytokines (TGF-β and IL-10) was observed after Bifidobacterium + inulin administration compared with LPS control (e,f) using One-way ANOVA with Tukey analysis (p < 0.05). Arg, arginase, IL interleukin, TGF transforming growth factor.
Dose effect of Bifidobacterium longum fermentative inulin (Bifido in Inulin or Bifidobacterium + inulin) for macrophage responses using no dilution, 50% dilution, and 25% dilution by culture media (DMEM). Compared to LPS group, treatment with Bifidobacterium + inulin without dilution significantly downregulated gene expression of TNF-α, IL-6, IL-1β, and iNOS and also reduced production of supernatant cytokines (TNF-α, IL-6, and IL-1β), while the dilution attenuated only IL-6 expression and supernatant IL-6 (a–g) as calculated by One-way ANOVA with Tukey analysis (p < 0.05). IL interleukin, iNOS inducible nitric oxide synthase, LPS lipopolysaccharide, TNF tumor necrosis factor.
The change in cell energy status in cell line study
Because of a possible relationship between cell energy status and macrophage functions, mitochondrial and glycolysis activities were determined through oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), respectively (Fig. 5a,b). For mitochondrial activities, the OCR of macrophages was decreased by all activations; however, LPS and LPS plus inulin demonstrated the most severe reduction of mitochondrial functions (maximal respiration) (Fig. 5c). The mitochondrial activities were improved with the presence of Bifidobacterium supernatant regardless of inulin (Fig. 5c), implying some beneficial molecules from the probiotics. For glycolysis status, only LPS and LPS plus inulin elevated glycolysis (Fig. 5d) which might be correlated with LPS-induced pro-inflammatory macrophage activity (Fig. 2). Nevertheless, the LPS-enhanced maximal glycolysis was reduced into the control regular state with incubation by supernatant of Bifidobacterium, alone or with inulin (Fig. 5d). In comparison with the control, the LPS-induced glycolytic stress effect in Bifidobacterium and Bifidobacterium + inulin supernatants was not observed (Fig. 5d), suggesting the influence of some bifidobacterial molecules on glycolysis.
Cell energy status. All activations reduced mitochondrial activities as indicated by maximal respiration (most prominence in LPS and LPS plus inulin), while only LPS and LPS plus inulin elevated glycolysis (p < 0.05). One-way ANOVA with Tukey analysis was used to evaluate parametric variables. Macrophages, RAW264.7 murine cell line; Oligomycin, an inhibitor against mitochondrial ATP synthesis; Antimycin A, a respiratory complex III inhibitor; Rotenone, a respiratory complex I inhibitor; 2-DG (2-Deoxy- d-glucose), a glycolysis inhibitor.
Discussion
Obesity is a worldwide health problem and links with dysbiosis of the gut microbiota1,7. Pediatric obesity is associated with compromised muscular capacity and fitness21. Sarcopenic obesity is an arising issue in this population characterized by a significant decline in muscle mass alongside excessive fat accumulation22. We studied the benefits of the inulin extracted from Thai Jerusalem artichoke on children with obesity and found a significant increase in fat-free mass after a 6-month period of inulin supplementation13. In the present study, we examined the mechanism underlying gut-muscle axis from inulin supplementation on muscle mass in children with obesity. The in vivo study found a significant increase in IL-15 and creatinine/cystatin C ratio in the inulin supplementation group. For the in vitro study of inflammatory and anti-inflammatory gene expression, a significant downregulation of TNF, IL-6, IL-1β, and iNOS expression in the treatment with Bifidobacterium plus inulin was observed, whereas a significant upregulation of FIZZ-1 and TGF-β as the anti-inflammatory gene expressions was found. Furthermore, there were no significant differences in cellular glycolytic capacity between the treatment with Bifidobacterium plus inulin and controls.
Several studies in adults showed that supplementation with prebiotics, probiotics, and synbiotics improved muscle mass and strength23,24,25,26,27. Some studies were conducted on animal models to examine muscle mass and strength, but they did not specifically explore muscular biomarkers28,29. As far as we are aware, there has not been research done in children regarding the effect of prebiotics on muscular biomarkers via gut-muscle axis; therefore, our study might be the first to support this hypothesis. IL-15 was proposed as a contraction-induced myokine acting on local skeletal muscle to improve energy metabolism30. O'Leary et al. found that IL-15 played a role in myogenesis and protected muscles from degradation in isolated human myogenic cultures31. Another study revealed that exercise leads to higher serum IL-15, contributing to improved physical fitness32. In addition, IL-15 has been investigated for its role in indicating cancer-induced cachexia and its potential to prevent sepsis-induced muscle wasting33,34. Serum creatinine/cystatin C ratio was also offered as a biomarker of skeletal muscle status35. Basically, creatinine and cystatin C are used for estimating glomerular filtration. In contrast to creatinine, which is solely a product of muscle catabolism, cystatin C is produced from all nucleated cells and remains unaffected by muscle mass. Kashani et al. firstly investigated the benefits of serum creatinine to cystatin C ratio, so called the sarcopenia index, for assessing muscle mass36. Studies have highlighted the potential of this ratio in predicting severity, muscle mass, muscle strength, and muscle-adjusted visceral fat mass in non-alcoholic fatty liver disease patients37,38. In the case of the elderly, the researchers found the usefulness of creatinine/cystatin C ratio in assessing sarcopenia in terms of muscle mass and strength39. Therefore, based on the previous literatures, all the proxies of myogenesis from our study indicated inulin-induced muscle mass production and corresponded with increased fat-free mass in the children with obesity.
Systemic inflammation contributes to organ damage, including skeletal muscle loss, thereby increasing the risk of sarcopenia in children with obesity22. The study by Kalinkovich et al. revealed that a muscle mass reduction was caused by adipose tissue inflammation along with decreased uptake of fatty acids which led to an increased deposition of lipids within skeletal muscle tissue. This, in turn, could result in a decline in skeletal muscle mass40. Another previous study illustrated that change in the composition of gut microbiota, resulting in heightened absorption of bacterial products like LPS, could induce chronic inflammation and muscle production through elevated levels of inflammatory cytokines such as IL-6 and TNF-α, and then TNF-α triggers the activation of NF-κB and muscle ring finger-1, which in turn promotes increased ubiquitination of muscle proteins. This cascade subsequently leads to the breakdown of actin and myosin myofilaments, culminating in a reduction of muscle mass41. Additionally, there are other potential pathways, such as the production of mediators by the gut microbiota, including SCFAs, which play a role in positively influencing skeletal muscle production. The administration of butyrate, recognized for its anti-inflammatory and muscle-building properties attributed to its ability to inhibit the enzyme histone deacetylase, facilitates improved muscle building42. Our in vitro study showed that metabolites produced by Bifidobacterium, which can utilize inulin, were rich in SCFAs, and for in vitro study in macrophage cell lines, treatment with Bifidobacterium together with inulin significantly downregulated the key inflammatory gene expression, TNF-α and IL-6, and significantly decreased the expression of M1 macrophage markers, IL-1β and iNOS. These findings align with a study by Nicolucci et al. which reported a significant reduction in IL-6 with inulin supplementation compared to the control group. However, contrary to our results, they found no significant difference in lean mass gain in the inulin group10. Kelishadi et al. found a significant reduction of TNF-α and IL-6 in the synbiotic supplementation group43. The finding corresponded to our study reflecting the potential of rebalancing the gut microbiota dysbiosis leading to decreased inflammation. Interestingly, Bifidobacterium in DMEM (the glucose containing culture media) demonstrated a less potent anti-inflammation than Bifidobacterium with inulin (Fig. 2e–g) suggesting an impact of the different metabolites secreted from the probiotics after an incubation by glucose and inulin. Indeed, inulin stimulates the growth and activity of lactic acid bacteria as a bacterial nutrient source resulting in production of SCFAs, a beneficial factor for enterocyte to strengthen gut permeability44. More mechanistic studies are interesting.
Although we found Bifidobacterium could be an important link to promote muscle mass gain via gut-muscle axis, this is not limited only to this bacterium. Previous study reported that Bacteroides was positively associated with parameter of muscle function41, while a decrease in the genus Akkermansia was found in the gut microbiota of sarcopenia patients with cirrhosis45.
Dysbiosis of the intestinal microbiota impairs the gut barrier, enabling toxic substances derived from pathogenic bacteria, such as LPS, to enter the bloodstream. Activation of TLR4 by LPS can increase the levels of NF-κB and proinflammatory cytokines, including IL-6 and TNF-α46,47. Additionally, an initiation of inflammatory cascade in obesity via M1 macrophage that contributes to increased M1 macrophage markers, IL-1β and iNOS, leading to increased systemic inflammation and then affects muscle mass production15,48. Without LPS, Bifidobacterium condition media (with or without inulin) mildly upregulated expression of IL-1β and iNOS indicated some degree of inflammatory responses, perhaps due to the pathogen-associated molecular patterns (PAMPs) of bacteria. On the other hand, with LPS, the Bifidobacterium condition media downregulated these genes supporting the possible secretion of anti-inflammatory molecules. In the future, the separation of the anti-inflammatory fraction from Bifidobacterium culture media might be interesting. However, inulin did not show inflammatory response because Bifidobacterium combined with inulin without LPS was not different from the Bifidobacterium culture media alone. Moreover, inulin itself might have an anti-inflammatory response because inulin alone with LPS downregulated IL-1β and iNOS (the genes of M1-pro-inflammatory polarization)49 and upregulated FIZZ-1 and TGF-β, the anti-inflammatory M2 macrophage polarization genes50. Perhaps, inulin ameliorates LPS-TLR4 on macrophages by inhibiting their adhesion, leading to reduced inflammation49 and promotes M2 macrophage polarization. In addition, SCFAs, induced by inulin might attenuate inflammation through increased mitochondrial function and muscle building51 as indicated by increased fat-free mass and muscle biomarkers in our previous RCT13 and this present study. Moreover, LPS reduced mitochondrial function and elevated glycolysis (extracellular flux analysis) supported the association between energy status and macrophage polarization52 which was normalized by Bifidobacterium condition media. Because the cell energy status using Bifidobacterium with or without inulin was not different, inulin did not have an additional impact on cell energy status that was already improved by Bifidobacterium. Probiotic-induced anti-inflammatory macrophages might partly attenuate obesity-induced systemic inflammation through the strengthening of gut permeability18,19,20.
Indeed, there are several evidences of probiotic-induced anti-inflammation. For instance, Lactobacillus acidophilus was shown to counter inflammation through TGF-β1 signalling after Salmonella infection53. Fujii et al. investigated the anti-inflammatory properties of Bifidobacterium breve supplementation in preterm infants, which resulted in an observable augmentation in serum TGF-β1 levels54. Taken together, these might support our results of the anti-inflammatory role of prebiotics and probiotics, as we observed a trend towards elevated the gene expression of anti-inflammatory biomarkers, FIZZ-1 and TGF-β, in the current study. Furthermore, prebiotics like inulin might stimulate anti-inflammatory responses through alternative pathways. The incorporation of additional anti-inflammatory markers, such as peroxisome proliferator-activated receptor gamma, could yield supplementary evidence and further strengthen the findings55.
To the best of our knowledge, this is the first study regarding the gut-muscle axis in children with obesity. The research was meticulously designed to elucidate the mechanisms underlying prebiotic-induced muscle building. All the results in myogenesis were the novel findings which were not found in the previous studies of prebiotics in obese children. Another strength is the method used in the study. We performed a clinical study according to our previous RCT study and the study was done in human subjects enhancing the potential for future practical applications.
In conclusion, the supplementation of inulin significantly promoted the biomarkers of muscle building in agreement with fat-free mass gain in children with obesity. This could be explained by Bifidobacterium metabolites derived from inulin digestion exhibited in vitro anti-inflammatory activity. Those metabolites may decrease systemic pro-inflammation, thus promoting muscle production in children with obesity via gut-muscle axis response. To bolster these results, future studies incorporating inulin alongside multi-strain probiotics may uncover further insights into the interaction between gut microbiota and muscle health in children with obesity.
Data availability
Data described in the manuscript will be made available upon request pending application and approval from the corresponding author.
References
Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 73(1), 147–162 (2016).
de Onis, M., Blössner, M. & Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 92(5), 1257–1264 (2010).
Panichsillaphakit, E. et al. Children’s eating behavior questionnaire correlated with body compositions of Thai children and adolescents with obesity: A pilot study. J. Nutr. Metab. 2021, 6496134 (2021).
Visuthranukul, C. et al. Glycemic index and glycemic load of common fruit juices in Thailand. J. Health Popul. Nutr. 41(1), 5 (2022).
Cavalcante-Silva, L. H. et al. Obesity-driven gut microbiota inflammatory pathways to metabolic syndrome. Front. Physiol. 6, 341 (2015).
Jialal, I., Kaur, H. & Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 99(1), 39–48 (2014).
Saad, M. J., Santos, A. & Prada, P. O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 31(4), 283–293 (2016).
Verdam, F. J. et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 21(12), E607–E615 (2013).
Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 5(4), 1417–1435 (2013).
Nicolucci, A. C. et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 153(3), 711–722 (2017).
Liber, A. & Szajewska, H. Effects of inulin-type fructans on appetite, energy intake, and body weight in children and adults: Systematic review of randomized controlled trials. Ann. Nutr. Metab. 63(1–2), 42–54 (2013).
Kellow, N. J., Coughlan, M. T. & Reid, C. M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 111(7), 1147–1161 (2014).
Visuthranukul, C. et al. Effects of inulin supplementation on body composition and metabolic outcomes in children with obesity. Sci. Rep. 12(1), 13014 (2022).
Louis, P. & Flint, H. J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294(1), 1–8 (2009).
Przewłócka, K. et al. Gut-muscle axisexists and may affect skeletal muscle adaptation to training. Nutrients 12(5), 1451 (2020).
World Health Organization. Obesity and Overweight. http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Rossi, M. et al. Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 71(10), 6150–6158 (2005).
Udompornpitak, K. et al. Obesity exacerbates lupus activity in Fc gamma receptor IIb deficient lupus mice partly through saturated fatty acid-induced gut barrier defect and systemic inflammation. J. Innate Immun. 15(1), 240–261 (2023).
Ondee, T. et al. High fructose causes more prominent liver steatohepatitis with leaky gut similar to high glucose administration in mice and attenuation by Lactiplantibacillus plantarum dfa1. Nutrients 15(6), 1462 (2023).
Ondee, T. et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 11(1), 6367 (2021).
Thivel, D. et al. Muscle strength and fitness in pediatric obesity: A systematic review from the european childhood obesity group. Obes. Facts 9(1), 52–63 (2016).
Zembura, M. & Matusik, P. Sarcopenic obesity in children and adolescents: A systematic review. Front. Endocrinol. (Lausanne) 13, 914740 (2022).
Giron, M. et al. Gut microbes and muscle function: Can probiotics make our muscles stronger?. J. Cachexia Sarcopenia Muscle 13(3), 1460–1476 (2022).
van Krimpen, S. J. et al. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review-gut permeability as potential treatment target. Nutrients 13(4), 1115 (2021).
Shing, C. M. et al. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 114(1), 93–103 (2014).
Buigues, C. et al. Effect of a prebiotic formulation on frailty syndrome: A randomized, double-blind clinical trial. Int. J. Mol. Sci. 17(6), 932 (2016).
Huang, W. C. et al. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients 11(11), 2836 (2019).
Ni, Y. et al. Lactobacillus and bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 63(22), e1900603 (2019).
Chen, L. H. et al. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging (Albany NY) 11(2), 756–770 (2019).
Nadeau, L. & Aguer, C. Interleukin-15 as a myokine: Mechanistic insight into its effect on skeletal muscle metabolism. Appl. Physiol. Nutr. Metab. 44(3), 229–238 (2019).
O’Leary, M. F. et al. IL-15 promotes human myogenesis and mitigates the detrimental effects of TNFα on myotube development. Sci. Rep. 7(1), 12997 (2017).
Rinnov, A. et al. Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine 45(2), 271–278 (2014).
Martínez-Hernández, P. L. et al. Serum interleukin-15 levels in cancer patients with cachexia. Oncol. Rep. 28(4), 1443–1452 (2012).
Kim, H. C., Cho, H. Y. & Hah, Y. S. Role of IL-15 in sepsis-induced skeletal muscle atrophy and proteolysis. Tuberc. Respir. Dis. (Seoul) 73(6), 312–319 (2012).
Zheng, W. H. et al. Serum creatinine/cystatin C ratio as a muscle mass evaluating tool and prognostic indicator for hospitalized patients: A meta-analysis. Front. Med. (Lausanne) 9, 1058464 (2022).
Kashani, K. B. et al. Evaluating muscle mass by using markers of kidney function: Development of the sarcopenia index. Crit. Care Med. 45(1), e23–e29 (2017).
Li, S. et al. Serum creatinine-to-cystatin C ratio in the progression monitoring of non-alcoholic fatty liver disease. Front. Physiol. 12, 664100 (2021).
Mikami, K. et al. Association of serum creatinine-to-cystatin C ratio with skeletal muscle mass and strength in nonalcoholic fatty liver disease in the Iwaki Health Promotion Project. J. Clin. Biochem. Nutr. 70(3), 273–282 (2022).
Tabara, Y. et al. Creatinine-to-cystatin C ratio as a marker of skeletal muscle mass in older adults: J-SHIPP study. Clin. Nutr. 39(6), 1857–1862 (2020).
Kalinkovich, A. & Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 35, 200–221 (2017).
Ticinesi, A. et al. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and Sarcopenia. Nutrients 11(7), 1633 (2019).
Grosicki, G. J., Fielding, R. A. & Lustgarten, M. S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 102(4), 433–442 (2018).
Kelishadi, R. et al. A randomized triple-masked controlled trial on the effects of synbiotics on inflammation markers in overweight children. J. Pediatr. (Rio J) 90(2), 161–168 (2014).
Song, J. et al. Dietary inulin supplementation modulates short-chain fatty acid levels and cecum microbiota composition and function in chickens infected with Salmonella. Front. Microbiol. 11, 584380 (2020).
Ponziani, F. R. et al. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 41(6), 1320–1334 (2021).
Li, G., Jin, B. & Fan, Z. Mechanisms involved in gut microbiota regulation of skeletal muscle. Oxid. Med. Cell Longev. 2022, 2151191 (2022).
Prokopidis, K. et al. Mechanisms linking the gut-muscle axis with muscle protein metabolism and anabolic resistance: Implications for older adults at risk of sarcopenia. Front. Physiol. 12, 770455 (2021).
Fujisaka, S. et al. M2 macrophages in metabolism. Diabetol. Int. 7(4), 342–351 (2016).
Yang, X. et al. Inulin ameliorates alcoholic liver disease via suppressing LPS-TLR4-Mψ axis and modulating gut microbiota in mice. Alcohol Clin. Exp. Res. 43(3), 411–424 (2019).
Viola, A. et al. The metabolic signature of macrophage responses. Front. Immunol. 10, 1462 (2019).
Wang, Z. et al. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 78, 106062 (2020).
Binmama, S. et al. Beta-glucan from S. cerevisiae protected AOM-induced colon cancer in cGAS-deficient mice partly through Dectin-1-manipulated macrophage cell energy. Int. J. Mol. Sci. 23(18), 10951 (2022).
Huang, I. F. et al. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling. BMC Microbiol. 15, 203 (2015).
Fujii, T. et al. Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating Smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 43(1), 83–88 (2006).
Zenhom, M. et al. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J. Nutr. 141(5), 971–977 (2011).
Acknowledgements
The study was conducted by the Pediatric Nutrition Research Unit, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. We would like to thank Assoc Prof. Maneerat Chayanupatkul, MD. for supporting the research information and Nipa Chokesajjawatee for supporting the materials. The authors appreciate the effort and dedication of all the researchers, data collection team, enumerators, and all of those involved in this project. Asst Prof. Dr. Chonnikant Visuthranukul, MD, PhD and Eakkarin Mekangkul, MD have drawn the Supplement Fig. 2 using BioRender, URL: BioRender.com. The authors sincerely thank all the participants and their parents for their participation in this study.
Funding
This study was supported by the Fundamental Fund, Chulalongkorn University, Thailand [Grant no. CUFRB65_hea(34)_041_30_22].
Author information
Authors and Affiliations
Contributions
Authorships are based on fulfilment of the criteria recommended by the International Committee of Medical Journal Editors (ICMJE). C.V. was the principal investigator and responsible for the design and conduct of the study. C.V., A.L., S.T., E.M., and S.C. (Sirinuch Chomtho) jointly wrote the research article. C.V. was involved in the monitoring and acquisition of clinical data. C.V., A.L., and S.T. were involved in the monitoring and acquisition of the laboratory analysis. S.C. (Supakarn Chamni), E.M., and S.C. (Sirinuch Chomtho) were involved in acquisition and interpretation of data. C.V. and A.L. performed the statistical analysis, interpretation of the data, and made the figures. All authors critically reviewed the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Visuthranukul, C., Leelahavanichkul, A., Tepaamorndech, S. et al. Inulin supplementation exhibits increased muscle mass via gut-muscle axis in children with obesity: double evidence from clinical and in vitro studies. Sci Rep 14, 11181 (2024). https://doi.org/10.1038/s41598-024-61781-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61781-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.