Abstract
CytoSorb is a hemoadsorptive column used to remove high concentrations of proinflammatory cytokines in septic shock. Data on CytoSorb application in acute-on-chronic liver failure (ACLF) is lacking. This retrospective observational study analyzed 21 ACLF patients admitted to ICUs at the Vienna General Hospital who received CytoSorb adsorber therapy between 2017 and 2023. Median ICU length of stay was 8 days (IQR: 3–13), the ICU survival rate was 23.8% (n = 5). Significant decreases in bilirubin (median peak: 20.7 mg/dL to median post-treatment: 10.8 mg/dL; − 47.8%; p < 0.001), procalcitonin (1.34 to 0.74 pg/mL; − 44.6%; p < 0.001), interleukin-6 (385 to 131 ng/mL; − 66.0%; p = 0.0182)—but also of platelets (72 to 31 G/L; − 56.9%; p = 0.0014) and fibrinogen (230 to 154 mg/dL; − 33.0%; p = 0.0297) were detected. ICU survivors had a trend towards a stronger relative decrease in bilirubin (− 76.1% vs. − 48.2%), procalcitonin (− 90.6% vs. − 23.5%), and IL-6 (− 54.6% vs. − 17.8%) upon CytoSorb treatment. Moreover, no serious CytoSorb-attributed complications were detected. In conclusion, use of CytoSorb adsorber in ACLF patients results in a significant decrease in bilirubin and proinflammatory cytokines, while platelets and fibrinogen were also lowered. Prospective trials are warranted to investigate the impact of CytoSorb on clinical outcomes of ACLF patients with high proinflammatory cytokine levels.
Similar content being viewed by others
Introduction
Acute-on-chronic liver failure (ACLF) is a syndrome that may occur in patients with cirrhosis experiencing acute decompensation and is characterized by the development of hepatic and/or extra-hepatic organ failure(s), resulting in excessive short-term mortality1. It has been demonstrated that systemic inflammation is the hallmark of ACLF driving disease development and progression1,2,3,4,5,6,7,8.
The underlying mechanisms leading to systemic inflammation in ACLF are only partly understood and depend on the specific precipitating event, which may be identified in up to 60% of cases7. Inflammation is associated with the release of damage-associated (DAMPs) or pathogen-associated molecular patterns (PAMPs), resulting in oxidative stress triggering immune-mediated tissue damage, mitochondrial dysfunction, renal hypoperfusion, and subsequently, single or multiple organ failures3,9. In line, Monteiro and colleagues recently demonstrated the impact of the inflammasome, as assessed by the pro-inflammatory cytokines interleukin (IL)-1α and IL-1β, on ACLF outcome10. Therefore, amelioration of systemic inflammation, e.g., by clearance of pro-inflammatory cytokines, may be an important therapeutic goal in ACLF patients in order to promote liver regeneration or as a bridging therapy to liver transplantation5,11,12.
The CytoSorb adsorber is an extracorporeal blood purification tool (hemoadsorber) that may be used together with continuous dialysis to remove excessive inflammatory mediators (i.e., cytokines), as well as to lower elevated bilirubin and myoglobin levels13. Regarding liver-related indications, there is some data on the use of CytoSorb in patients with isolated hyperbilirubinemia and case series on liver failure14. However, there is no data on the use of CytoSorb in the specific setting of ACLF. Previously, extracorporeal liver support devices failed to improve the prognosis of patients with ACLF15. Extracorporeal treatment with large-volume plasma exchange—in order to eliminate pro-inflammatory cytokines—is explored as a promising treatment option in ACLF16. Moreover, a recently published randomized, controlled clinical trial detected a significant decrease in biomarkers involved in the pathophysiological process of ACLF in patients treated with the liver dialysis system DIALIVE compared to patients with standard medical treatment. Patients treated with the DIALIVE device showed an improvement in CLIF-C OF and CLIF-C ACLF scores but no significant difference in 28-day mortality17. The results of a phase III study investigating the efficacy of plasma exchange in ACLF patients are still pending (APACHE, NCT03702920)18.
Given the unmet clinical need for supportive treatments in patients with ACLF, we aimed to investigate the impact of CytoSorb treatment on liver and extra-hepatic organ function and to evaluate potential complications occurring during this treatment in critically ill ACLF patients.
Methods
Study design and setting
We conducted a retrospective observational study of patients with ACLF admitted to the ICU at a large tertiary center (Vienna General Hospital). Importantly, we did not include patients with acute liver failure (ALF) without pre-existing liver disease. All adult (18 years and older) patients with ACLF receiving treatment with CytoSorb adsorber between January 1st, 2017, and January 1st, 2023, were included in this study. The diagnosis of ACLF was defined according to the European Association for the Study of the Liver (EASL) CLIF criteria19. The observation period started from ICU admission (baseline) until ICU discharge or death. We evaluated the effects of CytoSorb hemoadsorption on the clinical course, carefully screened for potential complications, and analyzed laboratory parameters directly prior to CytoSorb application, after 24 h, and at the end of CytoSorb treatment (or last available in case of death).
In addition, we included a control group comprising 10 patients with ACLF who were all treated at the ICU during the respective time period 2017 to 2023 and received hemodialysis without the inclusion of the CytoSorb adsorber.
Hemoadsorption with CytoSorb adsorber
All included ACLF patients fulfilled an indication (i.e., acute kidney injury, hyperammonemia, and fluid- and acid–base disturbances) for hemodialysis during their respective ICU stay. The decision for additional CytoSorb adsorber application was made by the treating physicians. Thereby, CytoSorb adsorber was always used in addition to standard intensive care treatment of ACLF patients according to current EASL guidelines19. CytoSorb adsorber was always used with continuous venovenous hemodialysis (CVVHD; MultiFiltrate, Fresenius Medical Care) in prefilter position and changed after 8–24 h. Anticoagulation during extracorporeal blood circulation was primarily conducted with citrate. In case of suspected citrate accumulation, the anticoagulation regimen was switched to antithrombin III supplementation. According to our local standard operating procedures, citrate accumulation is rigorously monitored at least 3 times daily in patients with liver failure using the total calcium to ionized calcium ratio20. Dialysis with low-molecular-weight heparin was not applied in our patient cohort, nor was dialysis without anticoagulation21.
Laboratory parameters including liver chemistry [bilirubin, aspartate transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (aP), gamma-glutamyl transferase (GGT), and ammonia], inflammation parameters [procalcitonin (PCT), interleukin-6 (IL-6), C-reactive protein (CRP)], coagulation parameters [fibrinogen, international normalized ratio (INR)], white blood count (WBC) and platelets were collected in all patients and analyzed prior to CytoSorb application, 24 h after the commencement of CytoSorb therapy and after discontinuation of CytoSorb therapy (or prior to death). Overall CytoSorb treatment time and all CytoSorb adsorber changes were documented. For the evaluation of potential adverse events or complications directly associated with CytoSorb therapy, the patient's condition and laboratory changes were closely monitored during hemoadsorption in standardized time intervals.
Data collection
Data were extracted from electronic patient charts (IntelliSpace Critical Care and Anesthesia, Philips, Amsterdam, Netherlands) that are routinely used at all ICUs at the Vienna General Hospital. The system enables prospective and digital documentation of crucial patient data, including patient characteristics (age, gender, height, weight, BMI, vital signs and comorbidities/underlying disease), laboratory tests (blood chemistry, global tests of coagulation and blood cell count) and complete information about ICU-specific parameters like fluid balances, medication, nutrition and extracorporeal life support (mechanical ventilation, renal replacement therapy and ECMO). In order to quantify the severity of critical illness and extent of organ dysfunction for patients admitted to the ICU, we calculated the following scores within the first 24 h upon ICU admission: simplified acute physiology score (SAPS II)22 and sequential organ failure assessment score (SOFA)23. In addition, CLIF-C-ACLF and CLIF-C-OF scores were calculated as ACLF-specific prognostic scores24,25. Child–Pugh-Score (CPS) was collected using laboratory data and clinical parameters at ICU admission26,27.
Statistical analysis
Descriptive statistical analysis was used to provide a demographic overview of our patient cohort. Continuous variables were reported as mean ± standard deviation or median (interquartile range), while categorical variables were reported as numbers (relative proportions, %). Differences in laboratory parameters before and after CytoSorb adsorber application were compared using the Wilcoxon test for non-parametric variables. All statistical analyses were performed using IBM SPSS Statistics 27 (IBM, New York, NY, USA) and GraphPad Prism 8 (GraphPad Software, CA, USA).
Ethical approval and informed consent
The study was conducted according to the guidelines of the Declaration of Helsinki28, and approved by the local Ethics Committee of the Medical University of Vienna (Ethics committee number: 1924/2020). Given the retrospective design of the study, informed consent requirement was waived by the ethics committee of the Medical University of Vienna.
Results
Patient characteristics
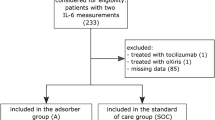
During the study period, 21 patients with ACLF were admitted to the ICU and received CytoSorb adsorber therapy (Supplemental Figure S1). The baseline characteristics of our patient population are depicted in Table 1. The individual characteristics of each patient are shown in Table 2. The median age was 50 years (IQR: 35–58), and most patients were male (n = 18; 86%). SOFA and SAPS II scores at ICU admission were 16 (IQR: 13–19) and 59 (IQR: 53–69), respectively. The most common underlying etiology of cirrhosis was alcohol-related liver disease (ALD; n = 11), followed by primary sclerosing cholangitis (PSC, n = 4), re-cirrhosis after LTX (n = 2), autoimmune hepatitis (AIH; n = 1), porphyria (n = 1), chronic hepatitis B (n = 1) and secondary sclerosing cholangitis (n = 1). At the time of ICU admission, most patients presented with CPS C (n = 16), while 5 patients showed CPS B. The median CPS score was 12 (IQR: 10–14). Infections (n = 12) and gastrointestinal bleeding events (n = 8) were the main precipitating events for the development of ACLF. The median number of organ failures at ICU admission was 4 (IQR: 4–6), corresponding to a median CLIF-C ACLF and CLIF-C OF Score of 67 (IQR: 57–76) and 15 (IQR: 14–18), respectively. We reported a median ICU length of stay (LOS) of 8 days (IQR: 3–13) and an ICU survival rate of 23.8% (n = 5). Additionally, we detected a 1-month survival of 23.8% (n = 5) and a 3-month survival of 19% (n = 4). Based on the CLIF-C-ACLF score, a predicted 1-month mortality of 82% and a predicted 3-month mortality of 92.9% were determined. Detailed information on the individual patients’ courses during CytoSorb adsorber application is shown in Fig. 1 and Supplemental Figure S2. The temporal relationship between CVVHD, CytoSorb application, outcome, and anticoagulation is depicted in Supplemental Table S1.
Individual patients' courses during CytoSorb adsorber application. The patient numbers refer to Table 2. CytoSorb adsorber changes were highlighted with red dots. The evolution of ACLF Grade is represented by bars of different colors as indicated.
All but one patient (95.2%) required vasopressor (i.e., noradrenaline) therapy during ICU stay. A detailed description of noradrenaline doses during CytoSorb treatment is depicted in Supplemental Table S2. In 10 patients, an increase in noradrenalin dose was observed 6 h after the start of CytoSorb treatment. After discontinuation of CytoSorb therapy, 6 patients (28.6%) were resolved from vasopressors. In addition, mechanical invasive ventilation (MIV) was conducted in 18 patients with a median length of MIV of 4 days (IQR: 2–8).
Differences in baseline characteristics between survivors and non-survivors are shown in Table 1. Survivors were younger (47 vs. 51 years), showed a lower CLIF-C-ACLF score at ICU admission (60 vs. 68.5), and had a longer ICU LOS (9 vs. 5.5 days).
Changes in laboratory parameters
The median number of CytoSorb applications per patient was 4 (IQR: 2.5–8), with a median CytoSorb therapy duration of 64 h (IQR: 42.5–130). We found a significant decrease in bilirubin levels during and after CytoSorb therapy (median peak: 20.7 mg/dL to median post-treatment: 10.8 mg/dL; – 47.8%; p < 0.001; Fig. 2). Interestingly, these changes were already observed after 24 h of CytoSorb treatment (median peak: 20.7 mg/dL to median 24 h-treatment: 13.8 mg/dL; – 33.3%; p < 0.001; Fig. 2a). Except for a significant decrease in GGT levels after 24 h (median peak: 47 U/L to median post 24 h-treatment: 32 U/L; – 31.9%; p = 0.0018), we did not find a clear trend for the remaining liver chemistries.
Evolution of liver chemistries in ACLF patients prior to and after CytoSorb adsorber application. Black dots and error bars indicate the median and interquartile range of bilirubin, AST, ALT, aP, GGT, and ammonia levels prior, 24 h after the commencement of CytoSorb adsorber therapy (a), and after CytoSorb adsorber therapy (b). The red and blue dots represent the individual patients' values prior to and 24 h after the commencement of CytoSorb adsorber therapy (a) and after CytoSorb adsorber therapy (b) from left to right. Patients with decreased or unchanged liver chemistries are shown in blue, while those with increased liver chemistries are shown in red. Data of ammonia was missing in one patient. aP alkalic phosphatase, ALT alanine aminotransferase, AST aspartate transferase, GGT gamma-glutamyl transferase, mg/dl milligram per deciliter, U/L units per liter, µmol/l micromole per liter.
WBC, CRP, PCT, and IL-6 were monitored as surrogate parameters for systemic inflammation. While CytoSorb therapy did not affect WBC and CRP levels, we observed a significant decline in PCT levels (median peak: 1.34 ng/mL to median 24 h-treatment: 1.09 ng/mL; – 18.4%; p < 0.001) 24 h after first CytoSorb adsorber therapy (Fig. 3a). There was also a relevant but non-significant trend regarding the decline in IL-6 levels after 24 h of CytoSorb therapy (median peak: 385 pg/mL to median 24 h-treatment: 327 pg/mL; – 15.2%; p = 0.0599). Importantly, after total CytoSorb therapy, both PCT (median peak: 1.34 ng/mL to median post-treatment: 0.74 ng/mL; – 44.9%; p < 0.001) and IL-6 (median peak: 385 pg/mL to median post-treatment: 131 ng/mL; – 66.1%; p = 0.0182) showed a significant decrease (Fig. 3b).
Evolution of inflammatory parameters in ACLF patients before and after CytoSorb adsorber application. Black dots and error bars indicate the median and interquartile range of CRP, PCT, IL-6, and leukocyte levels prior to and 24 h after the commencement of CytoSorb adsorber therapy (a) and after total CytoSorb adsorber therapy (b). The red and blue dots represent the individual patients' values prior to and 24 h after the commencement of CytoSorb adsorber therapy (a) and after total CytoSorb adsorber therapy (b) from left to right. Patients with decreased or unchanged inflammatory parameters are shown in blue, while patients with increased inflammatory parameters are shown in red. Data of PCT and IL-6 was missing in 3 patients. CRP C-reactive protein, IL-6 interleukin 6, mg/dl milligram per deciliter, ng/mL nanogram per milliliter, pg/mL picogram per milliliter, PCT procalcitonin.
In addition, the 5 ICU survivors tended to have a stronger initial decrease in bilirubin (– 76.1% vs. – 48.2%), procalcitonin (– 90.6% vs. – 23.5%), and IL-6 (– 54.6.3% vs. – 17.8%) upon CytoSorb treatment compared to non-survivors.
In order to analyze if the aforementioned changes in laboratory parameters were achieved by the addition of CytoSorb adsorber to CVVHD and not by conventional hemodialysis alone, we also evaluated laboratory changes in patients with ACLF and CVVHD without inclusion of the CytoSorb adsorber. The basic characteristics of this control group are depicted in Supplemental Table S3–S4. As seen in Supplemental Table S5, CVVHD without CytoSorb had no significant impact on the investigated laboratory parameters. In the control group, we detected a noticeable but not statistically significant decrease in median ammonia levels after 24 h and at CVVHD discontinuation.
Feasibility, safety, and complications of CytoSorb therapy in ACLF patients
We found a significant decrease in platelet counts during and after CytoSorb therapy (median peak: 72 G/L to median post-treatment: 31 G/L; – 56.9%; p = 0.0014; Fig. 4). These changes could already be detected 24 h after the start of CytoSorb treatment (median pre-treatment: 72 G/L to median 24 h-treatment: 44 G/L; – 38.9%; p = 0.043). Concerning global coagulation tests, we observed a significant increase in INR after 24 h of CytoSorb therapy (median pre-treatment: 2.5 to median 24-treatment: 3.2; 28%; p = 0.0215). In addition, fibrinogen levels showed a significant decrease during the course of CytoSorb therapy (median pre-treatment: 230 mg/dL to median post-treatment: 154 mg/dL; 33%; p = 0.0297). We did not observe any bleeding or other complications (i.e., allergic reactions) attributed to treatment with the CytoSorb adsorber in our ACLF patient cohort.
Evolution of coagulation parameters in ACLF patients prior to and after CytoSorb adsorber application. Black dots and error bars indicate the median and interquartile range of platelet count, INR, and fibrinogen levels prior, 24 h after the commencement of CytoSorb adsorber therapy (a) and after total CytoSorb adsorber therapy (b). The red and blue dots represent the individual patients' values prior to and 24 h after the commencement of CytoSorb adsorber therapy (a) and after total CytoSorb adsorber therapy (b) from left to right. Patients with a decrease in or unchanged platelet count are shown in blue, while patients whose platelet count remained unchanged or increased are shown in red. G/L Giga per liter, INR international normalized ratio, mg/dl milligram per deciliter.
In all patients, the CytoSorb adsorber was combined with CVVHD using regional citrate anticoagulation by local standards. In our ACLF cohort, citrate accumulation was suspected in 6 (28.6%) patients, and in all of these cases, citrate anticoagulation was replaced by substituting antithrombin III (Fig. 1 and Supplemental Table S1)21. In these patients, antithrombin III anticoagulation was continued for the remaining CVVHD circuits.
Discussion
Here we provide first observational data on the use of the CytoSorb adsorber as supportive therapy in ACLF patients. Importantly, we detected a significant reduction of bilirubin after 24 h and at the end of CytoSorb application in all ACLF patients. In addition, CytoSorb therapy also led to a significant decrease in PCT and IL-6 levels after CytoSorb application, indicating a potential benefit by ameliorating the systemic proinflammatory state in ACLF. We also found a significant decrease in platelets and alterations in the plasmatic coagulation, but did not detect bleeding and other complications directly attributed to CytoSorb use.
The integration of CytoSorb in an extracorporeal circuit to modulate hyperinflammation (‘cytokine storm’) in sepsis represents a novel therapeutic approach first introduced in 200829,30,31. CytoSorb treatment has been approved in Europe since 2011 and is authorized for use as an additional treatment for all indications associated with elevated cytokine levels. In animal studies, CytoSorb treatment was able to reduce inflammation-related molecules such as IL-6, IL-10, and TNF- α32,33. Brouwer et al. demonstrated reduced 28-day mortality in septic patients treated with CytoSorb31. Supporting data for the use of CytoSorb in the setting of ACLF are derived from patients with ALF (i.e., notably a different setting) occurring in patients with sepsis and systemic inflammation. Importantly, systemic inflammation is a key risk factor for the development and progression of ACLF6,8. Therapy with CytoSorb adsorber in ACLF patients is used with the rationale to ameliorate a hyperinflammatory state and thereby promote liver regeneration5,11,12. While we did not find any clear trend regarding CRP and WBC during and after CytoSorb treatment, we detected a significant decline in PCT and IL-6 levels13. Importantly, the declines in PCT and IL-6 levels were already evident as early as 24 h after the start of CytoSorb application, i.e., in a critical early phase of ACLF. This finding argues for a direct effect of the CytoSorb adsorber on these proinflammatory parameters. However, in our study, the reported decrease in IL-6 was rather small compared to in vitro data34. An explanation for the discrepancy between in vitro experiments and clinical application might be the constant hyperinflammatory response during ACLF, especially at the beginning of the disease course. In this regard, the observed small decrease of IL-6 by CytoSorb therapy might indicate a successful dampening of hyperinflammation that would have otherwise happened. Similar effects could already be seen in a study investigating the effects of CytoSorb hemoperfusion on systemic inflammation in humans in vivo35.
We also found a significant decrease in bilirubin levels during hemoadsorption, confirming data from other studies analyzing the effects of CytoSorb in different settings14,36,37,38,39. Again, these effects could already be achieved as early as 24 h after the commencement of CytoSorb therapy. According to recent data, the impact of CytoSorb treatment on bilirubin removal is even more effective compared to MARS13,40. However, the removal of further clinically relevant metabolites accumulating during liver failure by the CytoSorb adsorber is still unclear and under debate. Effects on hyperammonemia have been reported mainly in ex vivo studies40,41. However, recent data from one clinical report indicate that CytoSorb does not directly affect ammonia levels, and the elimination may be mainly achieved through simultaneously applied hemodialysis42. Interestingly, we found a more pronounced decrease in median ammonia levels in the control group, which only received CVVHD. However, the sample size was small, and the results were not statistically significant. It is yet not clear how CytoSorb application influences ammonia levels in patients with ACLF. Recent data suggests that the decrease in ammonia levels is a primary effect of hemodialysis, which is a perquisition for CytoSorb use43. However, we do not think CytoSorb negatively affects serum ammonia levels. Therefore, the observed difference in the clearance of ammonia levels between the CytoSorb group and the control group is likely due to the small sample size in both subgroups. Further studies are needed to investigate the impact of CytoSorb therapy on hyperammonemia and encephalopathy during liver failure. Nevertheless, our findings suggest that hemoadsorption with CytoSorb might support blood detoxification during ACLF.
Besides standard medical treatment, there is considerable interest in extracorporeal liver support systems for patients with liver failure15,44. Artificial liver support devices, such as MARS, Prometheus, and single-pass albumin dialysis (SPAD), led to an amelioration of laboratory parameters in ACLF. However, no consistent improvement in survival was found compared to standard medical treatment15,44. A prospective, randomized controlled trial reported no benefit in laboratory chemistries and outcome in ACLF patients treated with ELAD45. Potential positive effects were reported in ALF patients with high volume plasma exchange, while a survival benefit in ACLF patients is debated controversially15.
There is a lack of data on the efficacy of CytoSorb adsorber in the specific setting of ACLF. In a recent retrospective study of a small patient cohort with liver failure (ALF and ACLF) by Popescu et al.40, CytoSorb therapy was found to be equally or even more effective in rebalancing liver functional tests in patients with liver failure compared to MARS. However, there were no differences in patient outcome40. Nevertheless, CytoSorb adsorber is a rather cheap, easy-to-use, and readily available blood purification tool installed in conventional hemodialysis, which could also be used in smaller centers, in contrast to artificial liver support devices, that often require significant expertise for their application38.
The application of CytoSorb adsorber is generally associated with a low risk of complications46. In our cohort of ACLF patients, platelets were significantly decreased, as reported in other studies using renal replacement therapy with CytoSorb adsorber40,47. Moreover, we detected changes in plasmatic coagulation, including a significant decrease in fibrinogen levels and an increase in INR after the commencement of CytoSorb that may, however, also be due to liver disease progression. We did not detect bleeding complications or other adverse effects directly associated with the use of CytoSorb adsorber therapy. In 10 patients CytoSorb application was accompanied by increased vasopressor requirements shortly after commencement of adsorber therapy. This observation is best explained by progressive multiorgan failure and refractory shock in ACLF grade 3 rather than a negative impact of CytoSorb adsorber on hemodynamics itself.
Due to an imbalance of coagulation and risk of bleeding, regional citrate dialysis was used in all our patients, and a switch to antithrombin III supplementation due to citrate accumulation was only rarely necessary. Nevertheless, citrate accumulation was detected in 6 patients. In our experience, the risk for citrate accumulation is highest during the first cycle of CVVHD when the patient is still in an unstable condition. In this situation, the already limited liver function is further compromised by circulatory/septic shock, resulting in the inability to metabolize citrate and ultimately leading to citrate accumulation20. In this regard, citrate accumulation might also represent a negative prognostic marker for outcome in ACLF patients48.
The ICU survival of our study population was rather low, with 23.8%. However, our patients were admitted to the ICU with advanced ACLF as indicated by high CLIF-C ACLF scores and CLIF-OF scores at ICU admission corresponding to a predicted 1-month and 3-month mortality of 82% and 92.9%, respectively. 95.2% of our patients had an ACLF grade 3 before CytoSorb application. In our small study population, we observed comparable numbers with a 1-month mortality of 76.2% and 3-month mortality of 81%. Similar outcome data concerning ACLF patients was reported in the CANONIC study (28-day = 76.7%; 90-day mortality = 79.1%)1. Overall, in patients with advanced ACLF stages, treatment with CytoSorb adsorber might be less effective as organ failures may no longer be reversible. Therefore, close monitoring of organ function and, consequently, the earlier application of CytoSorb adsorber in ACLF patients might represent a treatment strategy to prevent disease progression to multiorgan failure (i.e., bridge to recovery) or to enable liver transplantation5,49,50. Indeed, liver transplantation for ACLF patients has shown very promising results, with a 1-year probability of survival after liver transplantation of 81%50. Unfortunately, liver transplantation for ACLF patients is not established at our center. Therefore, none of our patients was even evaluated for eligibility for liver transplantation.
The present study has limitations: First, in our single center study, we only report the data of a rather small cohort, including 21 ACLF patients treated with CytoSorb adsorber. However, to our knowledge, this is the first study to date that investigates the effects of hemoadsorption with CytoSorb in the specific setting of ACLF—while others have included patients with ALF in other settings15,40. Second, due to the retrospective design, we had some missing laboratory values, especially for ammonia, but also for PCT and IL-6, which may therefore introduce a bias in the interpretation of the results. Third, we acknowledge that in addition to CytoSorb therapy, patients always received standard medical treatment, including hemodialysis, according to the EASL guidelines19. Therefore, it is unclear to what extent the laboratory changes may be allocated to hemoadsorption or standard medical treatment. However, we found significant changes in a couple of laboratory parameters 24 h after the commencement of CytoSorb treatment, suggesting a direct effect of hemoadsorption. Fourth, due to the small number of patients in the control group, we were not able to perform a propensity matched comparison. Future multicenter prospective trials are required to provide a more comprehensive comparison regarding the efficacy of CVVHD + CytoSorb therapy and CVVHD alone in patients with ACLF. Nevertheless, we here present a comprehensive summary of our single center experience using CytoSorb adsorber in ACLF patients.
Conclusions
Our study supports the use of CytoSorb adsorber as a feasible and easy-to-use blood purification tool with few complications in patients with ACLF. CytoSorb treatment led to a significant decrease in important surrogate markers of systemic inflammation and supported blood detoxification by removing bilirubin in ACLF patients. Larger randomized controlled trials are warranted to further investigate the clinical value of CytoSorb therapy in ACLF patients.
Data availability
Data are available from the corresponding author upon reasonable request.
Abbreviations
- ACLF:
-
Acute-on-chronic liver failure
- ALD:
-
Alcoholic liver disease
- ALF:
-
Acute liver failure
- ALT:
-
Alanine aminotransferase
- aP:
-
Alkaline phosphatase
- AIH:
-
Autoimmune hepatitis
- AST:
-
Aspartate transferase
- CRP:
-
C-reactive protein
- CVVHD:
-
Continuous venovenous hemodialysis
- CPS:
-
Child–Pugh-score
- DAMP:
-
Damage-associated molecular patterns
- EASL:
-
European Association for the Study of the Liver
- GGT:
-
Gamma-glutamyl transferase
- ICU:
-
Intensive Care Unit
- IL-6:
-
Interleukin-6
- INR:
-
International normalized ratio
- IQR:
-
Interquartile range
- LOS:
-
Length of stay
- LTX:
-
Liver transplantation
- MCI:
-
Myocardial infarction
- MIV:
-
Mechanical invasive ventilation
- MOF:
-
Multi organ failure
- PAMP:
-
Pathogen-associated molecular patterns
- PCT:
-
Procalcitonin
- Pt.:
-
Patient
- PSC:
-
Primary sclerosing cholangitis
- RC:
-
Re-cirrhosis
- SAPS II:
-
Simplified acute physiology score
- SOFA:
-
Sequential organ failure assessment score
- VP:
-
Vasopressor
- WBC:
-
White blood count
References
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144, 1426–1437, 1437.e1421–1429. https://doi.org/10.1053/j.gastro.2013.02.042 (2013).
Arroyo, V. et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 74, 670–685. https://doi.org/10.1016/j.jhep.2020.11.048 (2021).
Engelmann, C., Clària, J., Szabo, G., Bosch, J. & Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 75(Suppl 1), S49-s66. https://doi.org/10.1016/j.jhep.2021.01.002 (2021).
Arroyo, V., Moreau, R., Jalan, R. & Ginès, P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 62, S131-143. https://doi.org/10.1016/j.jhep.2014.11.045 (2015).
Trebicka, J., Sundaram, V., Moreau, R., Jalan, R. & Arroyo, V. Liver transplantation for acute-on-chronic liver failure: Science or fiction?. Liver Transpl. 26, 906–915. https://doi.org/10.1002/lt.25788 (2020).
Balcar, L. et al. Patterns of acute decompensation in hospitalized patients with cirrhosis and course of acute-on-chronic liver failure. United Eur. Gastroenterol. J. 9, 427–437. https://doi.org/10.1002/ueg2.12089 (2021).
Trebicka, J. et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J. Hepatol. 74, 1097–1108. https://doi.org/10.1016/j.jhep.2020.11.019 (2021).
Trebicka, J. et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 73, 842–854. https://doi.org/10.1016/j.jhep.2020.06.013 (2020).
Arroyo, V., Moreau, R. & Jalan, R. Acute-on-chronic liver failure. N Engl. J. Med. 382, 2137–2145. https://doi.org/10.1056/NEJMra1914900 (2020).
Monteiro, S. et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 70, 379–387. https://doi.org/10.1136/gutjnl-2019-320170 (2021).
Bernal, W., Karvellas, C., Saliba, F., Saner, F. H. & Meersseman, P. Intensive care management of acute-on-chronic liver failure. J. Hepatol. 75(Suppl 1), S163-s177. https://doi.org/10.1016/j.jhep.2020.10.024 (2021).
Caraceni, P., Abraldes, J. G., Ginès, P., Newsome, P. N. & Sarin, S. K. The search for disease-modifying agents in decompensated cirrhosis: From drug repurposing to drug discovery. J. Hepatol. 75(Suppl 1), S118-s134. https://doi.org/10.1016/j.jhep.2021.01.024 (2021).
Dominik, A. & Stange, J. Similarities, differences, and potential synergies in the mechanism of action of albumin dialysis using the MARS albumin dialysis device and the CytoSorb hemoperfusion device in the treatment of liver failure. Blood Purif. 50, 119–128. https://doi.org/10.1159/000508810 (2021).
Ocskay, K. et al. Hemoadsorption in “liver indication’-analysis of 109 patients” data from the CytoSorb international registry. J. Clin. Med. 10, 5182. https://doi.org/10.3390/jcm10215182 (2021).
Ocskay, K. et al. Uncertainty in the impact of liver support systems in acute-on-chronic liver failure: A systematic review and network meta-analysis. Ann. Intensive Care 11, 10. https://doi.org/10.1186/s13613-020-00795-0 (2021).
Maiwall, R. et al. Therapeutic plasma-exchange improves systemic inflammation and survival in acute-on-chronic liver failure: A propensity-score matched study from AARC. Liver Int. 41, 1083–1096. https://doi.org/10.1111/liv.14806 (2021).
Agarwal, B. et al. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on- chronic liver failure. J. Hepatol. 79, 79–92. https://doi.org/10.1016/j.jhep.2023.03.013 (2023).
Stahl, K., Bode, C. & David, S. Bridging patients with acute-on-chronic liver failure for transplantation: plasma exchange to stabilize multiorgan failure?. Intensive Care Med. 49, 890–891. https://doi.org/10.1007/s00134-023-07092-x (2023).
European Association for the Study of the Liver. Electronic address, e. e. e. & European Association for the Study of the, L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 69, 406–460. https://doi.org/10.1016/j.jhep.2018.03.024 (2018).
Schneider, A. G., Journois, D. & Rimmele, T. Complications of regional citrate anticoagulation: Accumulation or overload?. Crit. Care 21, 281. https://doi.org/10.1186/s13054-017-1880-1 (2017).
Brunner, R., Leiss, W., Madl, C., Druml, W. & Holzinger, U. Single-dose application of antithrombin as a potential alternative anticoagulant during continuous renal replacement therapy in critically ill patients with advanced liver cirrhosis: A retrospective data analysis. Anesth. Analg. 116, 527–532. https://doi.org/10.1213/ANE.0b013e31827ced39 (2013).
Le Gall, J. R., Lemeshow, S. & Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270, 2957–2963. https://doi.org/10.1001/jama.270.24.2957 (1993).
Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22, 707–710. https://doi.org/10.1007/BF01709751 (1996).
Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 61, 1038–1047. https://doi.org/10.1016/j.jhep.2014.06.012 (2014).
Lee, M. et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: A retrospective analysis. Liver Int. 35, 46–57. https://doi.org/10.1111/liv.12683 (2015).
Child, C. G. & Turcotte, J. G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1, 1–85 (1964).
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60, 646–649. https://doi.org/10.1002/bjs.1800600817 (1973).
World Medical, A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. https://doi.org/10.1001/jama.2013.281053 (2013).
Paul, R. et al. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb(®)) in patients with sepsis and septic shock. World J. Crit. Care Med. 10, 22–34. https://doi.org/10.5492/wjccm.v10.i1.22 (2021).
Zuccari, S. et al. Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with CytoSorb. Blood Purif 49, 107–113. https://doi.org/10.1159/000502540 (2020).
Brouwer, W. P., Duran, S., Kuijper, M. & Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 23, 317. https://doi.org/10.1186/s13054-019-2588-1 (2019).
Kellum, J. A., Song, M. & Venkataraman, R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit. Care Med. 32, 801–805. https://doi.org/10.1097/01.ccm.0000114997.39857.69 (2004).
Peng, Z. Y., Carter, M. J. & Kellum, J. A. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit. Care Med. 36, 1573–1577. https://doi.org/10.1097/CCM.0b013e318170b9a7 (2008).
Malard, B., Lambert, C. & Kellum, J. A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 6, 12. https://doi.org/10.1186/s40635-018-0177-2 (2018).
Jansen, A., Waalders, N. J. B., van Lier, D. P. T., Kox, M. & Pickkers, P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit. Care 27, 117. https://doi.org/10.1186/s13054-023-04391-z (2023).
Piwowarczyk, P. et al. Hemoadsorption in isolated conjugated hyperbilirubinemia after extracorporeal membrane oxygenation support Cholestasis of sepsis: A case report and review of the literature on differential causes of jaundice in ICU patient. Int. J. Artif. Organs 42, 263–268. https://doi.org/10.1177/0391398819834012 (2019).
Faltlhauser, A. & Kullmann, F. Use of hemoadsorption in a case of severe hepatic failure and hyperbilirubinemia. Blood Purif. 44, 98–99. https://doi.org/10.1159/000470826 (2017).
Scharf, C. et al. Successful elimination of bilirubin in critically ill patients with acute liver dysfunction using a cytokine adsorber and albumin dialysis: a pilot study. Sci. Rep. 11, 10190. https://doi.org/10.1038/s41598-021-89712-4 (2021).
Dhokia, V. D., Madhavan, D., Austin, A. & Morris, C. G. Novel use of Cytosorb haemadsorption to provide biochemical control in liver impairment. J. Intensive Care Soc. 20, 174–181. https://doi.org/10.1177/1751143718772789 (2019).
Popescu, M. et al. Artificial liver support with CytoSorb and MARS in liver failure: A retrospective propensity matched analysis. J. Clin. Med. 12, 2258. https://doi.org/10.3390/jcm12062258 (2023).
Tomescu, D., Popescu, M., David, C., Sima, R. & Dima, S. Haemoadsorption by CytoSorb(R) in patients with acute liver failure: A case series. Int. J. Artif. Organs 44, 560–564. https://doi.org/10.1177/0391398820981383 (2021).
Liebchen, U., Scharf, C., Zoller, M., Weinelt, F. & Kloft, C. No clinically relevant removal of meropenem by cytokine adsorber CytoSorb(®) in critically ill patients with sepsis or septic shock. Intensive Care Med. 47, 1332–1333. https://doi.org/10.1007/s00134-021-06487-y (2021).
Liebchen, U. et al. The cytokine adsorber Cytosorb(R) does not reduce ammonia concentrations in critically ill patients with liver failure. Intensive Care Med. 49, 360–362. https://doi.org/10.1007/s00134-023-06998-w (2023).
Abbas, N., Rajoriya, N., Elsharkawy, A. M. & Chauhan, A. Acute-on-chronic liver failure (ACLF) in 2022: Have novel treatment paradigms already arrived?. Expert Rev. Gastroenterol. Hepatol. 16, 639–652. https://doi.org/10.1080/17474124.2022.2097070 (2022).
Thompson, J. et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl. 24, 380–393. https://doi.org/10.1002/lt.24986 (2018).
Poli, E. C., Rimmele, T. & Schneider, A. G. Hemoadsorption with CytoSorb((R)). Intensive Care Med. 45, 236–239. https://doi.org/10.1007/s00134-018-5464-6 (2019).
Alharthy, A. et al. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artif. Organs 45, E101–E112. https://doi.org/10.1111/aor.13864 (2021).
Khadzhynov, D. et al. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J. Crit. Care 29, 265–271. https://doi.org/10.1016/j.jcrc.2013.10.015 (2014).
Li, X., Zhang, L., Pu, C. & Tang, S. Liver transplantation in Acute-on-Chronic liver failure: Timing of transplantation and selection of patient population. Front. Med. (Lausanne) 9, 1030336. https://doi.org/10.3389/fmed.2022.1030336 (2022).
Belli, L. S. et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J. Hepatol. 75, 610–622. https://doi.org/10.1016/j.jhep.2021.03.030 (2021).
Author information
Authors and Affiliations
Contributions
Concept of the study (P.H., B.S., T.R., C.Z., M.S-G.), data collection (P.H., M.R.-W., M.S., M.S-G.), statistical analysis (P.H., B.S., G.S., M.S-G.), drafting of the manuscript (P.H., B.S., L.B., T.R., M.S-G.,) and revision for important intellectual content as well as approval of the final manuscript (all authors).
Corresponding author
Ethics declarations
Competing interests
The authors have nothing to disclose regarding the work under consideration for publication. Conflicts of interests outside the submitted work: B.S. received travel support from AbbVie, Ipsen, and Gilead; G.S. received travel support from Gilead; T.R. received grant support from Abbvie, Boehringer-Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, Siemens and W. L Gore & Associates; speaking honoraria from Abbvie, Gilead, Intercept, Roche, MSD, W. L Gore & Associates; consulting/advisory board fee from Abbvie, Astra Zeneca, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Resolution Therapeutics, Siemens; and travel support from Abbvie, Boehringer-Ingelheim, Gilead and Roche.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haselwanter, P., Scheiner, B., Balcar, L. et al. Use of the CytoSorb adsorber in patients with acute-on-chronic liver failure. Sci Rep 14, 11309 (2024). https://doi.org/10.1038/s41598-024-61658-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61658-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.