Abstract
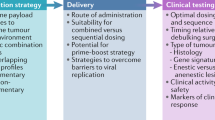
Oncolytic viruses (OVs) represent a novel class of cancer immunotherapy agents that preferentially infect and kill cancer cells and promote protective antitumor immunity. Furthermore, OVs can be used in combination with established or upcoming immunotherapeutic agents, especially immune checkpoint inhibitors, to efficiently target a wide range of malignancies. The development of OV-based therapy involves three major steps before clinical evaluation: design, production and preclinical testing. OVs can be designed as natural or engineered strains and subsequently selected for their ability to kill a broad spectrum of cancer cells rather than normal, healthy cells. OV selection is further influenced by multiple factors, such as the availability of a specific viral platform, cancer cell permissivity, the need for genetic engineering to render the virus non-pathogenic and/or more effective and logistical considerations around the use of OVs within the laboratory or clinical setting. Selected OVs are then produced and tested for their anticancer potential by using syngeneic, xenograft or humanized preclinical models wherein immunocompromised and immunocompetent setups are used to elucidate their direct oncolytic ability as well as indirect immunotherapeutic potential in vivo. Finally, OVs demonstrating the desired anticancer potential progress toward translation in patients with cancer. This tutorial provides guidelines for the design, production and preclinical testing of OVs, emphasizing considerations specific to OV technology that determine their clinical utility as cancer immunotherapy agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gujar, S., Bell, J. & Diallo, J.-S. SnapShot: cancer immunotherapy with oncolytic viruses. Cell 176, 1240–1240.e1 (2019).
Russell, S. J., Bell, J. C., Engeland, C. E. & McFadden, G. Advances in oncolytic virotherapy. Commun. Med. 2, 33 (2022).
Melcher, A., Harrington, K. & Vile, R. Oncolytic virotherapy as immunotherapy. Science 374, 1325–1326 (2021).
Lawler, S. E., Speranza, M.-C., Cho, C.-F. & Chiocca, E. A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 3, 841–849 (2017).
Shalhout, S. Z., Miller, D. M., Emerick, K. S. & Kaufman, H. L. Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol. 20, 160–177 (2023).
Azad, T. et al. Synthetic virology approaches to improve the safety and efficacy of oncolytic virus therapies. Nat. Commun. 14, 3035 (2023).
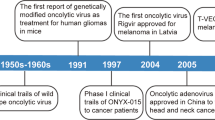
Bluming, A. Z. & Ziegler, J. L. Regression of Burkitt’s lymphoma in association with measles infection. Lancet 2, 105–106 (1971).
Pasquinucci, G. Possible effect of measles on leukaemia. Lancet 1, 136 (1971).
Zygiert, Z. Hodgkin’s disease: remissions after measles. Lancet 1, 593 (1971).
Gujar, S. A., Marcato, P., Pan, D. & Lee, P. W. K. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol. Cancer Ther. 9, 2924–2933 (2010).
Gujar, S. et al. Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation. Mol. Ther. 21, 338–347 (2013).
Tseha, S. T. Role of adenoviruses in cancer therapy. Front. Oncol. 12, 772659 (2022).
Pol, J., Kroemer, G. & Galluzzi, L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology 5, e1115641 (2016).
Frampton, J. E. Teserpaturev/G47Δ: first approval. BioDrugs 36, 667–672 (2022).
Chaurasiya, S., Fong, Y. & Warner, S. G. Oncolytic virotherapy for cancer: clinical experience. Biomedicines 9, 419 (2021).
Harrington, K., Freeman, D. J., Kelly, B., Harper, J. & Soria, J.-C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 18, 689–706 (2019).
Twumasi-Boateng, K., Pettigrew, J. L., Kwok, Y. Y. E., Bell, J. C. & Nelson, B. H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 18, 419–432 (2018).
Boorjian, S. A. et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22, 107–117 (2021).
Zhang, S. & Rabkin, S. D. The discovery and development of oncolytic viruses: are they the future of cancer immunotherapy? Expert Opin. Drug Discov. 16, 391–410 (2021).
Pol, J. et al. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology 3, e28694 (2014).
Pol, J. et al. Trial watch–oncolytic viruses and cancer therapy. Oncoimmunology 5, e1117740 (2016).
Pol, J. G. et al. Trial watch: oncolytic viro-immunotherapy of hematologic and solid tumors. Oncoimmunology 7, e1503032 (2018).
Marchini, A., Bonifati, S., Scott, E. M., Angelova, A. L. & Rommelaere, J. Oncolytic parvoviruses: from basic virology to clinical applications. Virol. J. 12, 6 (2015).
Haller, S. L., Peng, C., McFadden, G. & Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 21, 15–40 (2014).
Hendrickson, R. C., Wang, C., Hatcher, E. L. & Lefkowitz, E. J. Orthopoxvirus genome evolution: the role of gene loss. Viruses 2, 1933–1967 (2010).
Moussatche, N. & Condit, R. C. Fine structure of the vaccinia virion determined by controlled degradation and immunolocalization. Virology 475, 204–218 (2015).
Gujar, S., Pol, J. G., Kim, Y., Lee, P. W. & Kroemer, G. Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 39, 209–221 (2018).
Melcher, A., Parato, K., Rooney, C. M. & Bell, J. C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 19, 1008–1016 (2011).
Annels, N. E. et al. Oncolytic immunotherapy for bladder cancer using coxsackie A21 virus. Mol. Ther. Oncolytics 9, 1–12 (2018).
Thompson, E. M. et al. Poliovirus receptor (CD155) expression in pediatric brain tumors mediates oncolysis of medulloblastoma and pleomorphic xanthoastrocytoma. J. Neuropathol. Exp. Neurol. 77, 696–702 (2018).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015).
Tseng, J.-C., Granot, T., DiGiacomo, V., Levin, B. & Meruelo, D. Enhanced specific delivery and targeting of oncolytic Sindbis viral vectors by modulating vascular leakiness in tumor. Cancer Gene Ther. 17, 244–255 (2010).
Hess, M. et al. Bacterial glucuronidase as general marker for oncolytic virotherapy or other biological therapies. J. Transl. Med. 9, 172 (2011).
Pol, J. G. et al. Maraba virus as a potent oncolytic vaccine vector. Mol. Ther. 22, 420–429 (2014).
Schreiber, L.-M., Urbiola, C., Erlmann, P. & Wollmann, G. In vivo bioimaging for monitoring intratumoral virus activity. Methods Mol. Biol. 2058, 237–248 (2020).
Kuruppu, D. et al. Oncolytic HSV1 targets different growth phases of breast cancer leptomeningeal metastases. Cancer Gene Ther. 30, 833–844 (2023).
Miller, A. & Russell, S. J. The use of the NIS reporter gene for optimizing oncolytic virotherapy. Expert Opin. Biol. Ther. 16, 15–32 (2016).
Robertson, M. G. et al. Cancer imaging and therapy utilizing a novel NIS-expressing adenovirus: the role of adenovirus death protein deletion. Mol. Ther. Oncolytics 20, 659–668 (2021).
Chen, L. et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics 11, 6668–6681 (2021).
Lin, D., Shen, Y. & Liang, T. Oncolytic virotherapy: basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 8, 1–29 (2023).
Neault, S. et al. Robust envelope exchange platform for oncolytic measles virus. J. Virol. Methods 302, 114487 (2022).
Stepanenko, A. A. et al. Superior infectivity of the fiber chimeric oncolytic adenoviruses Ad5/35 and Ad5/3 over Ad5-delta-24-RGD in primary glioma cultures. Mol. Ther. Oncolytics 24, 230–248 (2022).
Baker, A. T. et al. The fiber knob protein of human adenovirus type 49 mediates highly efficient and promiscuous infection of cancer cell lines using a novel cell entry mechanism. J. Virol. 95, e01849-20 (2021).
Kleinlützum, D. et al. Enhancing the oncolytic activity of CD133-targeted measles virus: receptor extension or chimerism with vesicular stomatitis virus are most effective. Front. Oncol. 7, 127 (2017).
Menotti, L. & Avitabile, E. Herpes simplex virus oncolytic immunovirotherapy: the blossoming branch of multimodal therapy. Int. J. Mol. Sci. 21, 8310 (2020).
Wu, P.-H., Opadele, A. E., Onodera, Y. & Nam, J.-M. Targeting integrins in cancer nanomedicine: applications in cancer diagnosis and therapy. Cancers 11, 1783 (2019).
Bates, E. A. et al. Development of a low-seroprevalence, αvβ6 integrin-selective virotherapy based on human adenovirus type 10. Mol. Ther. Oncolytics 25, 43–56 (2022).
Parato, K. A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 20, 749–758 (2012).
Paraskevakou, G. et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol. Ther. 15, 677–686 (2007).
Allen, C. et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 66, 11840–11850 (2006).
Gao, Y. & Bergman, I. Vesicular stomatitis virus (VSV) G glycoprotein can be modified to create a Her2/neu-targeted VSV that eliminates large implanted mammary tumors. J. Virol. 97, e0037223 (2023).
Lauer, U. M. & Beil, J. Oncolytic viruses: challenges and considerations in an evolving clinical landscape. Future Oncol. https://doi.org/10.2217/fon-2022-0440 (2022).
Macedo, N., Miller, D. M., Haq, R. & Kaufman, H. L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 8, e001486 (2020).
Harrington, K. J. et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin. Cancer Res. 26, 5153–5161 (2020).
Stojdl, D. F. et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6, 821–825 (2000).
Stojdl, D. F. et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4, 263–275 (2003).
Ho, T. Y., Mealiea, D., Okamoto, L., Stojdl, D. F. & McCart, J. A. Deletion of immunomodulatory genes as a novel approach to oncolytic vaccinia virus development. Mol. Ther. Oncolytics 22, 85–97 (2021).
Cristi, F., Gutiérrez, T., Hitt, M. M. & Shmulevitz, M. Genetic modifications that expand oncolytic virus potency. Front. Mol. Biosci. 9, 831091 (2022).
Muster, T. et al. Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int. J. Cancer 110, 15–21 (2004).
Hong, B., Sahu, U., Mullarkey, M. P. & Kaur, B. Replication and spread of oncolytic herpes simplex virus in solid tumors. Viruses 14, 118 (2022).
Ramachandran, M. et al. Safe and effective treatment of experimental neuroblastoma and glioblastoma using systemically delivered triple microRNA-detargeted oncolytic semliki forest virus. Clin. Cancer Res. 23, 1519–1530 (2017).
Peter, M. & Kühnel, F. Oncolytic adenovirus in cancer immunotherapy. Cancers 12, 3354 (2020).
Gonzalez-Pastor, R., Goedegebuure, P. S. & Curiel, D. T. Understanding and addressing barriers to successful adenovirus-based virotherapy for ovarian cancer. Cancer Gene Ther. 28, 375–389 (2021).
Raimondi, G. et al. Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses. EBioMedicine 56, 102786 (2020).
Fiorini, E. & Corbo, V. Oncolytic virotherapy meets the human organoid technology for pancreatic cancers. EBioMedicine 57, 102828 (2020).
Diallo, J.-S., Roy, D., Abdelbary, H., De Silva, N. & Bell, J. C. Ex vivo infection of live tissue with oncolytic viruses. J. Vis. Exp. 2011, 2854 (2011).
Workenhe, S. T. & Mossman, K. L. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol. Ther. 22, 251–256 (2014).
Gujar, S., Pol, J. G. & Kroemer, G. Heating it up: oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies. Oncoimmunology 7, e1442169 (2018).
Galluzzi, L. et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 8, e000337 (2020).
Brown, M. Engaging pattern recognition receptors in solid tumors to generate systemic antitumor immunity. Cancer Treat. Res. 183, 91–129 (2022).
Hoden, B., DeRubeis, D., Martinez-Moczygemba, M., Ramos, K. S. & Zhang, D. Understanding the role of Toll-like receptors in lung cancer immunity and immunotherapy. Front. Immunol. 13, 1033483 (2022).
Li, D. & Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 6, 291 (2021).
Humeau, J., Le Naour, J., Galluzzi, L., Kroemer, G. & Pol, J. G. Trial watch: intratumoral immunotherapy. Oncoimmunology 10, 1984677 (2021).
Kepp, O. et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 3, e955691 (2014).
Kim, Y. et al. Dendritic cells in oncolytic virus-based anti-cancer therapy. Viruses 7, 6506–6525 (2015).
Zafar, S. et al. CD40L coding oncolytic adenovirus allows long-term survival of humanized mice receiving dendritic cell therapy. Oncoimmunology 7, e1490856 (2018).
Xu, Q. et al. Evaluation of Newcastle disease virus mediated dendritic cell activation and cross-priming tumor-specific immune responses ex vivo. Int. J. Cancer 146, 531–541 (2020).
Humeau, J., Lévesque, S., Kroemer, G. & Pol, J. G. Gold standard assessment of immunogenic cell death in oncological mouse models. Methods Mol. Biol. 1884, 297–315 (2019).
Deng, L. et al. Oncolytic therapy with vaccinia virus carrying IL-24 for hepatocellular carcinoma. Virol. J. 19, 44 (2022).
Oh, E., Hong, J., Kwon, O.-J. & Yun, C.-O. A hypoxia- and telomerase-responsive oncolytic adenovirus expressing secretable trimeric TRAIL triggers tumour-specific apoptosis and promotes viral dispersion in TRAIL-resistant glioblastoma. Sci. Rep. 8, 1420 (2018).
Bressy, C., Hastie, E. & Grdzelishvili, V. Z. Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Mol. Ther. Oncolytics 5, 20–40 (2017).
Yamada, S. et al. Oncolytic herpes simplex virus expressing yeast cytosine deaminase: relationship between viral replication, transgene expression, prodrug bioactivation. Cancer Gene Ther. 19, 160–170 (2012).
Zhang, J., Ding, M., Xu, K., Mao, L. & Zheng, J. shRNA-armed conditionally replicative adenoviruses: a promising approach for cancer therapy. Oncotarget 7, 29824–29834 (2016).
Atherton, M. J. et al. Customized viral immunotherapy for HPV-associated cancer. Cancer Immunol. Res. 5, 847–859 (2017).
Chen, T. et al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J. Immunother. Cancer 9, e001647 (2021).
Zhu, Z. et al. Improving cancer immunotherapy by rationally combining oncolytic virus with modulators targeting key signaling pathways. Mol. Cancer 21, 196 (2022).
Zhao, Y. et al. Oncolytic adenovirus: prospects for cancer immunotherapy. Front. Microbiol. 12, 707290 (2021).
Heidbuechel, J. P. W. & Engeland, C. E. Oncolytic viruses encoding bispecific T cell engagers: a blueprint for emerging immunovirotherapies. J. Hematol. Oncol. 14, 63 (2021).
Bridle, B. W. et al. HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol. Ther. 21, 887–894 (2013).
Bridle, B. W. et al. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8+ T-cell responses to anticancer vaccines. Oncoimmunology 2, e26013 (2013).
Bridle, B. W. et al. Privileged antigen presentation in splenic B cell follicles maximizes T cell responses in prime-boost vaccination. J. Immunol. 196, 4587–4595 (2016).
Pol, J., Le Bœuf, F. & Diallo, J.-S. Genetic, immunological, and pharmacological strategies to generate improved oncolytic viruses. Med. Sci. (Paris) 29, 165–173 (2013).
Pol, J. G. et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. Oncoimmunology 8, e1512329 (2019).
Pol, J. G. et al. Development and applications of oncolytic Maraba virus vaccines. Oncolytic Virother. 7, 117–128 (2018).
Pol, J. G. et al. Enhanced immunotherapeutic profile of oncolytic virus-based cancer vaccination using cyclophosphamide preconditioning. J. Immunother. Cancer 8, e000981 (2020).
Pol, J. G., Bridle, B. W. & Lichty, B. D. Detection of tumor antigen-specific T-cell responses after oncolytic vaccination. Methods Mol. Biol. 2058, 191–211 (2020).
Pol, J. G., Workenhe, S. T., Konda, P., Gujar, S. & Kroemer, G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. 56, 4–27 (2020).
Zhang, L. et al. Delivery of viral-vectored vaccines by B cells represents a novel strategy to accelerate CD8+ T-cell recall responses. Blood 121, 2432–2439 (2013).
Pol, J. G., Rességuier, J. & Lichty, B. D. Oncolytic viruses: a step into cancer immunotherapy. Virus Adapt. Treat. 4, 1–21 (2011).
Naumenko, V. A., Stepanenko, A. A., Lipatova, A. V., Vishnevskiy, D. A. & Chekhonin, V. P. Infection of non-cancer cells: a barrier or support for oncolytic virotherapy? Mol. Ther. Oncolytics 24, 663–682 (2022).
Toro Bejarano, M. & Merchan, J. R. Targeting tumor vasculature through oncolytic virotherapy: recent advances. Oncolytic Virother. 4, 169–181 (2015).
Carew, J. S. et al. Oncolytic reovirus inhibits angiogenesis through induction of CXCL10/IP-10 and abrogation of HIF activity in soft tissue sarcomas. Oncotarget 8, 86769–86783 (2017).
Nair, M. et al. Enhancing antitumor efficacy of heavily vascularized tumors by RAMBO virus through decreased tumor endothelial cell activation. Cancers 12, 1040 (2020).
Hou, W., Chen, H., Rojas, J., Sampath, P. & Thorne, S. H. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int. J. Cancer 135, 1238–1246 (2014).
Hardcastle, J. et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol. Ther. 18, 285–294 (2010).
Zhang, Z. et al. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol. Ther. 11, 553–562 (2005).
Raja, J., Ludwig, J. M., Gettinger, S. N., Schalper, K. A. & Kim, H. S. Oncolytic virus immunotherapy: future prospects for oncology. J. Immunother. Cancer 6, 140 (2018).
Evgin, L. et al. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol. Ther. 23, 1066–1076 (2015).
Doronin, K. et al. Coagulation factor X activates innate immunity to human species C adenovirus. Science 338, 795–798 (2012).
Minuk, G. Y., Paul, R. W. & Lee, P. W. The prevalence of antibodies to reovirus type 3 in adults with idiopathic cholestatic liver disease. J. Med. Virol. 16, 55–60 (1985).
Russell, S. J. & Peng, K. W. Measles virus for cancer therapy. Curr. Top. Microbiol. Immunol. 330, 213–241 (2009).
Yu, B. et al. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J. Med. Virol. 84, 1408–1414 (2012).
Maroun, J. et al. Designing and building oncolytic viruses. Future Virol. 12, 193–213 (2017).
Atasheva, S. et al. Systemic cancer therapy with engineered adenovirus that evades innate immunity. Sci. Transl. Med. 12, eabc6659 (2020).
Lee, N. et al. Generation of novel oncolytic vaccinia virus with improved intravenous efficacy through protection against complement-mediated lysis and evasion of neutralization by vaccinia virus-specific antibodies. J. Immunother. Cancer 11, e006024 (2023).
Heiniö, C. et al. TNFa and IL2 encoding oncolytic adenovirus activates pathogen and danger-associated immunological signaling. Cells 9, 798 (2020).
Hemminki, O. et al. Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget 6, 4467–4481 (2015).
Short, J. J. et al. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol. Cancer Ther. 9, 2536–2544 (2010).
Muharemagic, D. et al. Aptamer-facilitated protection of oncolytic virus from neutralizing antibodies. Mol. Ther. Nucleic Acids 3, e167 (2014).
Castleton, A. et al. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood 123, 1327–1335 (2014).
Kaczorowski, A. et al. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget 7, 9046–9059 (2016).
Melen, G. J. et al. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 371, 161–170 (2016).
Dey, M. et al. Intranasal oncolytic virotherapy with CXCR4-enhanced stem cells extends survival in mouse model of glioma. Stem Cell Rep. 7, 471–482 (2016).
Keshavarz, M. et al. Oncolytic virus delivery modulated immune responses toward cancer therapy: challenges and perspectives. Int. Immunopharmacol. 108, 108882 (2022).
Thambi, T., Hong, J., Yoon, A.-R. & Yun, C.-O. Challenges and progress toward tumor-targeted therapy by systemic delivery of polymer-complexed oncolytic adenoviruses. Cancer Gene Ther. 29, 1321–1331 (2022).
Shin, D. H. et al. Current strategies to circumvent the antiviral immunity to optimize cancer virotherapy. J. Immunother. Cancer 9, e002086 (2021).
Munguia, A., Ota, T., Miest, T. & Russell, S. J. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 15, 797–806 (2008).
Peng, K.-W. et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 20, 255–261 (2013).
Fulci, G. et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl Acad. Sci. USA 103, 12873–12878 (2006).
Liu, Y.-P. et al. Polyinosinic acid decreases sequestration and improves systemic therapy of measles virus. Cancer Gene Ther. 19, 202–211 (2012).
Koski, A. et al. Systemic adenoviral gene delivery to orthotopic murine breast tumors with ablation of coagulation factors, thrombocytes and Kupffer cells. J. Gene Med. 11, 966–977 (2009).
Shashkova, E. V., Doronin, K., Senac, J. S. & Barry, M. A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 68, 5896–5904 (2008).
Ikeda, K. et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 5, 881–887 (1999).
Niemann, J. et al. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy. Nat. Commun. 10, 3236 (2019).
Wein, L. M., Wu, J. T. & Kirn, D. H. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 63, 1317–1324 (2003).
Lopez, M. V. et al. Tumor associated stromal cells play a critical role on the outcome of the oncolytic efficacy of conditionally replicative adenoviruses. PLoS ONE 4, e5119 (2009).
Li, L., Liu, S., Han, D., Tang, B. & Ma, J. Delivery and biosafety of oncolytic virotherapy. Front. Oncol. 10, 475 (2020).
Zhou, P. et al. Intratumoral delivery of a novel oncolytic adenovirus encoding human antibody against PD-1 elicits enhanced antitumor efficacy. Mol. Ther. Oncolytics 25, 236–248 (2022).
Knapp, J. P., Kakish, J. E., Bridle, B. W. & Speicher, D. J. Tumor temperature: friend or foe of virus-based cancer immunotherapy. Biomedicines 10, 2024 (2022).
Bilbao, R. et al. A blood-tumor barrier limits gene transfer to experimental liver cancer: the effect of vasoactive compounds. Gene Ther. 7, 1824–1832 (2000).
Rust, N. M. et al. Bradykinin enhances Sindbis virus infection in human brain microvascular endothelial cells. Virology 422, 81–91 (2012).
Jaime-Ramirez, A. C. et al. Reolysin and histone deacetylase inhibition in the treatment of head and neck squamous cell carcinoma. Mol. Ther. Oncolytics 5, 87–96 (2017).
Springfeld, C. et al. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 66, 7694–7700 (2006).
Ebert, O. et al. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 64, 3265–3270 (2004).
Zhang, J., Frolov, I. & Russell, S. J. Gene therapy for malignant glioma using Sindbis vectors expressing a fusogenic membrane glycoprotein. J. Gene Med. 6, 1082–1091 (2004).
Le Boeuf, F. et al. Reovirus FAST protein enhances vesicular stomatitis virus oncolytic virotherapy in primary and metastatic tumor models. Mol. Ther. Oncolytics 6, 80–89 (2017).
Morodomi, Y. et al. BioKnife, a uPA activity-dependent oncolytic Sendai virus, eliminates pleural spread of malignant mesothelioma via simultaneous stimulation of uPA expression. Mol. Ther. 20, 769–777 (2012).
Jung, K. H. et al. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 396, 155–166 (2017).
Oh, E., Choi, I.-K., Hong, J. & Yun, C.-O. Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model. Oncotarget 8, 4730–4746 (2017).
Yang, Y. et al. Systemic delivery of an oncolytic adenovirus expressing decorin for the treatment of breast cancer bone metastases. Hum. Gene Ther. 26, 813–825 (2015).
Yumul, R. et al. Epithelial junction opener improves oncolytic adenovirus therapy in mouse tumor models. Hum. Gene Ther. 27, 325–337 (2016).
Tedcastle, A., Illingworth, S., Brown, A., Seymour, L. W. & Fisher, K. D. Actin-resistant DNAse I expression from oncolytic adenovirus enadenotucirev enhances its intratumoral spread and reduces tumor growth. Mol. Ther. 24, 796–804 (2016).
Sette, P. et al. GBM-targeted oHSV armed with matrix metalloproteinase 9 enhances anti-tumor activity and animal survival. Mol. Ther. Oncolytics 15, 214–222 (2019).
Ungerechts, G. et al. Moving oncolytic viruses into the clinic: clinical-grade production, purification, and characterization of diverse oncolytic viruses. Mol. Ther. Methods Clin. Dev. 3, 16018 (2016).
Harrington, K. J. Oncolytic viruses: methods and protocols. Br. J. Cancer 108, 735 (2013).
Kirn, D. H., Liu, T.-C. & Thorne, S. H. (eds) Oncolytic Viruses: Methods and Protocols (Humana Press, 2012).
Abdelmageed, A. A. & Ferran, M. C. The propagation, quantification, and storage of vesicular stomatitis virus. Curr. Protoc. Microbiol. 58, e110 (2020).
Nakamura, T. et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 23, 209–214 (2005).
Schneider, U., von Messling, V., Devaux, P. & Cattaneo, R. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 76, 7460–7467 (2002).
Dybas, J. M. et al. Adenovirus remodeling of the host proteome and host factors associated with viral genomes. mSystems https://doi.org/10.1128/mSystems.00468-21 (2021).
Gueret, V., Negrete-Virgen, J. A., Lyddiatt, A. & Al-Rubeai, M. Rapid titration of adenoviral infectivity by flow cytometry in batch culture of infected HEK293 cells. Cytotechnology 38, 87–97 (2002).
Mendoza, E. J., Manguiat, K., Wood, H. & Drebot, M. Two detailed plaque assay protocols for the quantification of infectious SARS‐CoV‐2. Curr. Protoc. Microbiol. 57, ecpmc105 (2020).
James, K. T. et al. Novel high-throughput approach for purification of infectious virions. Sci. Rep. 6, 36826 (2016).
Shmulevitz, M. & Lee, P. W. K. in Oncolytic Viruses: Methods and Protocols (eds Kirn, D. H., Liu, T.-C. & Thorne, S. H.) 163–176 (Humana Press, 2012).
Langfield, K. K., Walker, H. J., Gregory, L. C. & Federspiel, M. J. Manufacture of measles viruses. Methods Mol. Biol. 737, 345–366 (2011).
Shmulevitz, M., Gujar, S. A., Ahn, D.-G., Mohamed, A. & Lee, P. W. K. Reovirus variants with mutations in genome segments S1 and L2 exhibit enhanced virion infectivity and superior oncolysis. J. Virol. 86, 7403–7413 (2012).
Peck, K. M. & Lauring, A. S. Complexities of viral mutation rates. J. Virol. 92, e01031-17 (2018).
Howley, P. M. Fields Virology: Fundamentals. (Wolters Kluwer Medical, 2023).
Cacciabue, M., Currá, A. & Gismondi, M. I. ViralPlaque: a Fiji macro for automated assessment of viral plaque statistics. PeerJ 7, e7729 (2019).
Liu, T. et al. Rapid and stain-free quantification of viral plaque via lens-free holography and deep learning. Nat. Biomed. Eng. 7, 1040–1052 (2023).
Dulbecco, R. & Vogt, M. Some problems of animal virology as studied by the plaque technique. Cold Spring Harb. Symp. Quant. Biol. 18, 273–279 (1953).
Baer, A. & Kehn-Hall, K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J. Vis. Exp. 2014, e52065 (2014).
McSharry, J. J. Uses of flow cytometry in virology. Clin. Microbiol. Rev. 7, 576–604 (1994).
Tang, V. A. et al. Single-particle characterization of oncolytic vaccinia virus by flow virometry. Vaccine 34, 5082–5089 (2016).
Roingeard, P., Raynal, P.-I., Eymieux, S. & Blanchard, E. Virus detection by transmission electron microscopy: still useful for diagnosis and a plus for biosafety. Rev. Med. Virol. 29, e2019 (2019).
Lee, P. & Gujar, S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat. Rev. Urol. 15, 235–250 (2018).
Petkov, C. I. et al. Unified ethical principles and an animal research ‘Helsinki’ declaration as foundations for international collaboration. Curr. Res. Neurobiol. 3, 100060 (2022).
Waehler, R., Russell, S. J. & Curiel, D. T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8, 573–587 (2007).
Russell, L. et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat. Commun. 9, 5006 (2018).
Farrera-Sal, M., Moya-Borrego, L., Bazan-Peregrino, M. & Alemany, R. Evolving status of clinical immunotherapy with oncolytic adenovirus. Clin. Cancer Res. 27, 2979–2988 (2021).
Suksanpaisan, L. et al. Preclinical development of oncolytic immunovirotherapy for treatment of HPVPOS cancers. Mol. Ther. Oncolytics 10, 1–13 (2018).
Evgin, L. et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 14, eabn2231 (2022).
Loken, S. D. et al. Morbidity in immunosuppressed (SCID/NOD) mice treated with reovirus (Dearing 3) as an anti-cancer biotherapeutic. Cancer Biol. Ther. 3, 734–738 (2004).
Gujar, S. A., Pan, D., Marcato, P., Garant, K. A. & Lee, P. W. Oncolytic virus-initiated protective immunity against prostate cancer. Mol. Ther. 19, 797–804 (2011).
Mosely, S. I. S. et al. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. Cancer Immunol. Res. 5, 29–41 (2017).
Prestwich, R. J. et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin. Cancer Res. 14, 7358–7366 (2008).
Nelson, A., Gebremeskel, S., Lichty, B. D. & Johnston, B. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J. Immunother. Cancer 10, e003923 (2022).
Kwan, A. et al. Macrophages mediate the antitumor effects of the oncolytic virus HSV1716 in mammary tumors. Mol. Cancer Ther. 20, 589–601 (2021).
Karandikar, S. H. et al. New epitopes in ovalbumin provide insights for cancer neoepitopes. JCI Insight 5, e127882 (2019).
Wang, D. et al. Ultralow-dose binary oncolytic/helper-dependent adenovirus promotes antitumor activity in preclinical and clinical studies. Sci. Adv. 9, eade6790 (2023).
Allen, T. M. et al. Humanized immune system mouse models: progress, challenges and opportunities. Nat. Immunol. 20, 770–774 (2019).
McKenna, M. K., Rosewell-Shaw, A. & Suzuki, M. Modeling the efficacy of oncolytic adenoviruses in vitro and in vivo: current and future perspectives. Cancers 12, 619 (2020).
Shenderov, E. et al. Generation and characterization of HLA-A2 transgenic mice expressing the human TCR 1G4 specific for the HLA-A2 restricted NY-ESO-1157-165 tumor-specific peptide. J. Immunother. Cancer 9, e002544 (2021).
Fulci, G. et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 67, 9398–9406 (2007).
Kurozumi, K. et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J. Natl Cancer Inst. 99, 1768–1781 (2007).
Sánchez, D., Cesarman-Maus, G., Amador-Molina, A. & Lizano, M. Oncolytic viruses for canine cancer treatment. Cancers 10, 404 (2018).
Martín-Carrasco, C. et al. Safety and efficacy of an oncolytic adenovirus as an immunotherapy for canine cancer patients. Vet. Sci. 9, 327 (2022).
Gentschev, I. et al. Oncolytic virotherapy of canine and feline cancer. Viruses 6, 2122–2137 (2014).
Hummel, J. et al. Maraba virus-vectored cancer vaccines represent a safe and novel therapeutic option for cats. Sci. Rep. 7, 15738 (2017).
Jogler, C. et al. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J. Virol. 80, 3549–3558 (2006).
Cervera-Carrascon, V. et al. Comparison of clinically relevant oncolytic virus platforms for enhancing T cell therapy of solid tumors. Mol. Ther. Oncolytics 17, 47–60 (2020).
Steel, J. C. et al. Immunocompetent syngeneic cotton rat tumor models for the assessment of replication-competent oncolytic adenovirus. Virology 369, 131–142 (2007).
Zhang, H. et al. Naturally existing oncolytic virus M1 is nonpathogenic for the nonhuman primates after multiple rounds of repeated intravenous injections. Hum. Gene Ther. 27, 700–711 (2016).
Cassady, K. A. et al. Pre-clinical assessment of C134, a chimeric oncolytic herpes simplex virus, in mice and non-human primates. Mol. Ther. Oncolytics 5, 1–10 (2017).
Samson, A. et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 10, eaam7577 (2018).
Clements, D. R., Kim, Y., Gujar, S. A. & Lee, P. W. All that glitters is not gold: the need to consider desirable and undesirable immune aspects of oncolytic virus therapy. Oncoimmunology 5, e1057674 (2016).
Clements, D. R. et al. Newly recruited CD11b+, GR-1+, Ly6Chigh myeloid cells augment tumor-associated immunosuppression immediately following the therapeutic administration of oncolytic reovirus. J. Immunol. 194, 4397–4412 (2015).
Zhang, L. et al. Robust oncolytic virotherapy induces tumor lysis syndrome and associated toxicities in the MPC-11 plasmacytoma model. Mol. Ther. 24, 2109–2117 (2016).
Zhang, L. et al. Safety studies in tumor and non-tumor-bearing mice in support of clinical trials using oncolytic VSV-IFNβ-NIS. Hum. Gene Ther. Clin. Dev. 27, 111–122 (2016).
Wang, Y. et al. Preclinical safety evaluation of oncolytic herpes simplex virus type 2. Hum. Gene Ther. 30, 651–660 (2019).
Abdullahi, S. et al. A novel chimeric oncolytic virus vector for improved safety and efficacy in hepatocellular carcinoma. J. Virol. 92, e01386-18 (2018).
Kurzrock, R. et al. Moving beyond 3+3: The future of clinical trial design. Am. Soc. Clin. Oncol. Educ. Book 41, e133–e144 (2021).
Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J. & Schiebinger, L. Sex and gender analysis improves science and engineering. Nature 575, 137–146 (2019).
White, J., Tannenbaum, C., Klinge, I., Schiebinger, L. & Clayton, J. The integration of sex and gender considerations into biomedical research: lessons from international funding agencies. J. Clin. Endocrinol. Metab. 106, 3034–3048 (2021).
Canadian Institutes of Health Research. How to integrate sex and gender into research CIHR https://cihr-irsc.gc.ca/e/50836.html (2018).
Jang, S. R. et al. Association between sex and immune checkpoint inhibitor outcomes for patients with melanoma. JAMA Netw. Open 4, e2136823 (2021).
Carrera, C., Potrony, M. & Puig, S. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 19, e375 (2018).
Madala, S. et al. Gender differences and their effects on survival outcomes in lung cancer patients treated with PD-1/PD-L1 checkpoint inhibitors: a systematic review and meta-analysis. Clin. Oncol. 34, 799–809 (2022).
Russell, L., Peng, K. W., Russell, S. J. & Diaz, R. M. Oncolytic viruses: priming time for cancer immunotherapy. BioDrugs 33, 485–501 (2019).
Kim, Y. et al. Immune checkpoint blockade augments changes within oncolytic virus-induced cancer MHC-I peptidome, creating novel antitumor CD8 T cell reactivities. Mol. Cell. Proteom. 21, 100182 (2022).
Roy, D. G. et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination. Nat. Commun. 12, 2626 (2021).
Ding, J. et al. Pre-existing HSV-1 immunity enhances anticancer efficacy of a novel immune-stimulating oncolytic virus. Viruses 14, 2327 (2022).
Tähtinen, S. et al. Exploiting preexisting immunity to enhance oncolytic cancer immunotherapy. Cancer Res. 80, 2575–2585 (2020).
Bourgeois-Daigneault, M.-C. et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 10, eaao1641 (2018).
Murphy, J. P. et al. Therapy-induced MHC I ligands shape neo-antitumor CD8 T cell responses during oncolytic virus-based cancer immunotherapy. J. Proteome Res. 18, 2666–2675 (2019).
Altman, J. D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Koutsakos, M. et al. SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity 56, 879–892.e4 (2023).
Pai, C.-C. S. et al. Clonal deletion of tumor-specific T cells by interferon-γ confers therapeutic resistance to combination immune checkpoint blockade. Immunity 50, 477–492.e8 (2019).
Zhang, Y. et al. Enhancing CD8+ T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell 32, 377–391.e9 (2017).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Argüello, R. J. et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32, 1063–1075.e7 (2020).
Sharma, P. et al. The next decade of immune checkpoint therapy. Cancer Discov. 11, 838–857 (2021).
Chesney, J. A. et al. Randomized, double-blind, placebo-controlled, global phase III trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J. Clin. Oncol. 41, 528–540 (2023).
Chesney, J. A. et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 11, e006270 (2023).
Zhang, H. et al. Oncolytic adenoviruses synergistically enhance anti-PD-L1 and anti-CTLA-4 immunotherapy by modulating the tumour microenvironment in a 4T1 orthotopic mouse model. Cancer Gene Ther. 29, 456–465 (2022).
Gujar, S. A. et al. Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms. Br. J. Cancer 110, 83–93 (2014).
Ju, F. et al. Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade. J. Immunother. Cancer 10, e004762 (2022).
du Sert, N. P. et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411 (2020).
Zafar, S. et al. Oncolytic adenovirus type 3 coding for CD40L facilitates dendritic cell therapy of prostate cancer in humanized mice and patient samples. Hum. Gene Ther. 32, 192–202 (2021).
Zafar, S. et al. Intravenously usable fully serotype 3 oncolytic adenovirus coding for CD40L as an enabler of dendritic cell therapy. Oncoimmunology 6, e1265717 (2017).
Trinh, H. V. et al. Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor CD46 triggers infection. J. Virol. 86, 1623–1637 (2012).
Hemminki, O. et al. Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol. Ther. 20, 1821–1830 (2012).
Agrelli, A., de Moura, R. R., Crovella, S. & Brandão, L. A. C. ZIKA virus entry mechanisms in human cells. Infect. Genet. Evol. 69, 22–29 (2019).
Hemminki, O. et al. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 18, 288–296 (2011).
Arnberg, N. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol. Sci. 33, 442–448 (2012).
Gerber-Tichet Dienst, E. & Kremer, E. J. Adenovirus receptors on antigen-presenting cells of the skin. Biol. Cell 114, 297–308 (2022).
Chen, C. Y. et al. Species D adenoviruses as oncolytics against B-cell cancers. Clin. Cancer Res. 17, 6712–6722 (2011).
Wu, H. & Mei, Y.-F. An oncolytic adenovirus 11p vector expressing adenovirus death protein in the E1 region showed significant apoptosis and tumour-killing ability in metastatic prostate cells. Oncotarget 10, 1957–1974 (2019).
Dyer, A. et al. Oncolytic group B adenovirus enadenotucirev mediates non-apoptotic cell death with membrane disruption and release of inflammatory mediators. Mol. Ther. Oncolytics 4, 18–30 (2017).
Silver, J. & Mei, Y.-F. Transduction and oncolytic profile of a potent replication-competent adenovirus 11p vector (RCAd11pGFP) in colon carcinoma cells. PLoS ONE 6, e17532 (2011).
Ono, R., Takayama, K., Sakurai, F. & Mizuguchi, H. Efficient antitumor effects of a novel oncolytic adenovirus fully composed of species B adenovirus serotype 35. Mol. Ther. Oncolytics 20, 399–409 (2021).
Doerner, J. et al. Novel group C oncolytic adenoviruses carrying a miRNA inhibitor demonstrate enhanced oncolytic activity in vitro and in vivo. Mol. Cancer Ther. 21, 460–470 (2022).
Zheng, H. et al. Combination IFNβ and membrane-stable CD40L maximize tumor dendritic cell activation and lymph node trafficking to elicit systemic T-cell immunity. Cancer Immunol. Res. 11, 466–485 (2023).
Gállego Pérez-Larraya, J. et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 386, 2471–2481 (2022).
Kazemi Shariat Panahi, H. et al. Oncolytic viruses as a promising therapeutic strategy against the detrimental health impacts of air pollution: the case of glioblastoma multiforme. Semin. Cancer Biol. 86, 1122–1142 (2022).
Zhang, J. et al. Efficacy and safety of recombinant human adenovirus type 5 (H101) in persistent, recurrent, or metastatic gynecologic malignancies: a retrospective study. Front. Oncol. 12, 877155 (2022).
Garcia-Carbonero, R. et al. Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors. J. Immunother. Cancer 10, e003255 (2022).
Kesari, S. et al. BETA PRIME: phase I study of AdAPT-001 as monotherapy and combined with a checkpoint inhibitor in superficially accessible, treatment-refractory solid tumors. Future Oncol. 18, 3245–3254 (2022).
Larson, C., Oronsky, B. & Reid, T. AdAPT-001, an oncolytic adenovirus armed with a TGF-β trap, overcomes in vivo resistance to PD-L1-immunotherapy. Am. J. Cancer Res. 12, 3141–3147 (2022).
Yun, C.-O., Hong, J. & Yoon, A.-R. Current clinical landscape of oncolytic viruses as novel cancer immunotherapeutic and recent preclinical advancements. Front. Immunol. 13, 953410 (2022).
Ylösmäki, E. et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol. Ther. Oncolytics 20, 459–469 (2021).
Gao, J., Zhang, W. & Ehrhardt, A. Expanding the spectrum of adenoviral vectors for cancer therapy. Cancers 12, 1139 (2020).
Zhang, Z. et al. A tumor-targeted replicating oncolytic adenovirus Ad-TD-nsIL12 as a promising therapeutic agent for human esophageal squamous cell carcinoma. Cells 9, 2438 (2020).
Cook, M. & Chauhan, A. Clinical application of oncolytic viruses: a systematic review. Int. J. Mol. Sci. 21, 7505 (2020).
Garcia-Moure, M. et al. The oncolytic adenovirus VCN-01 promotes anti-tumor effect in primitive neuroectodermal tumor models. Sci. Rep. 9, 14368 (2019).
García, M. et al. A phase 1 trial of oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum. Gene Ther. 30, 352–364 (2019).
Garcia-Moure, M., Martinez-Vélez, N., Patiño-García, A. & Alonso, M. M. Oncolytic adenoviruses as a therapeutic approach for osteosarcoma: a new hope. J. Bone Oncol. 9, 41–47 (2017).
Dong, W. et al. ORCA-010, a novel potency-enhanced oncolytic adenovirus, exerts strong antitumor activity in preclinical models. Hum. Gene Ther. 25, 897–904 (2014).
Farzad, L. et al. Combinatorial treatment with oncolytic adenovirus and helper-dependent adenovirus augments adenoviral cancer gene therapy. Mol. Ther. Oncolytics 1, 14008 (2014).
Nasu, Y. et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol. Ther. 15, 834–840 (2007).
Ries, S. & Korn, W. M. ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus. Br. J. Cancer 86, 5–11 (2002).
Osipov, I. D. et al. Development of oncolytic vectors based on human adenovirus type 6 for cancer treatment. Viruses 15, 182 (2023).
Persson, B. D. et al. Human species D adenovirus hexon capsid protein mediates cell entry through a direct interaction with CD46. Proc. Natl Acad. Sci. USA 118, e2020732118 (2021).
Bullard, B. L., Corder, B. N. & Weaver, E. A. Species D adenoviruses as oncolytic viral vectors. Viruses 12, 1399 (2020).
Zhu, Z. et al. Zika virus targets glioblastoma stem cells through a SOX2-integrin αvβ5 Axis. Cell Stem Cell 26, 187–204.e10 (2020).
Oei, S. L. & Schad, F. Are aspects of integrative concepts helpful to improve pancreatic cancer therapy? Cancers 15, 1116 (2023).
Cui, C. et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. J. Immunother. Cancer 10, e004307 (2022).
Miller, K. E. et al. Immune activity and response differences of oncolytic viral therapy in recurrent glioblastoma: gene expression analyses of a phase IB study. Clin. Cancer Res. 28, 498–506 (2022).
Haines, B. B. et al. ONCR-177, an oncolytic HSV-1 designed to potently activate systemic antitumor immunity. Cancer Immunol. Res. 9, 291–308 (2021).
Chiocca, E. A., Nakashima, H., Kasai, K., Fernandez, S. A. & Oglesbee, M. Preclinical toxicology of rQNestin34.5v.2: an oncolytic herpes virus with transcriptional regulation of the ICP34.5 neurovirulence gene. Mol. Ther. Methods Clin. Dev. 17, 871–893 (2020).
Zhang, K.-X. et al. Intravesical treatment of advanced urothelial bladder cancers with oncolytic HSV-1 co-regulated by differentially expressed microRNAs. Gene Ther. 23, 460–468 (2016).
Fujiwara, S. et al. Carrier cell-based delivery of replication-competent HSV-1 mutants enhances antitumor effect for ovarian cancer. Cancer Gene Ther. 18, 77–86 (2011).
Lee, C. Y. F., Rennie, P. S. & Jia, W. W. G. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin. Cancer Res. 15, 5126–5135 (2009).
Agelidis, A. M. & Shukla, D. Cell entry mechanisms of HSV: what we have learned in recent years. Future Virol. 10, 1145–1154 (2015).
Madavaraju, K., Koganti, R., Volety, I., Yadavalli, T. & Shukla, D. Herpes simplex virus cell entry mechanisms: an update. Front. Cell. Infect. Microbiol. 10, 617578 (2020).
Zhang, B. et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J. Immunother. Cancer 9, e002224 (2021).
Luo, S. et al. Contribution of N-linked glycans on HSV-2 gB to cell-cell fusion and viral entry. Virology 483, 72–82 (2015).
Fu, X., Tao, L. & Zhang, X. An oncolytic virus derived from type 2 herpes simplex virus has potent therapeutic effect against metastatic ovarian cancer. Cancer Gene Ther. 14, 480–487 (2007).
Qiu, W., Ding, X., Li, S., He, Y. & Zhu, L. Oncolytic bovine herpesvirus 1 inhibits human lung adenocarcinoma A549 cell proliferation and tumor growth by inducing DNA damage. Int. J. Mol. Sci. 22, 8582 (2021).
Cuddington, B. P., Dyer, A. L., Workenhe, S. T. & Mossman, K. L. Oncolytic bovine herpesvirus type 1 infects and kills breast tumor cells and breast cancer-initiating cells irrespective of tumor subtype. Cancer Gene Ther. 20, 282–289 (2013).
Pastenkos, G., Lee, B., Pritchard, S. M. & Nicola, A. V. Bovine herpesvirus 1 entry by a low-pH endosomal pathway. J. Virol. 92, e00839-18 (2018).
Chai, C. et al. The effects of oncolytic pseudorabies virus vaccine strain inhibited the growth of colorectal cancer HCT-8 cells in vitro and in vivo. Animals 12, 2416 (2022).
Shiau, A.-L. et al. Development of a conditionally replicating pseudorabies virus for HER-2/neu-overexpressing bladder cancer therapy. Mol. Ther. 15, 131–138 (2007).
Li, A. et al. Structural basis of nectin-1 recognition by pseudorabies virus glycoprotein D. PLoS Pathog. 13, e1006314 (2017).
Aligholipour Farzani, T. et al. Assessment of replication of bovine herpesvirus type 4 in human glioblastoma and breast cancer cells as a potential oncolytic virus. Virus Genes 57, 31–39 (2021).
Redaelli, M. et al. Herpes simplex virus type 1 thymidine kinase-armed bovine herpesvirus type 4-based vector displays enhanced oncolytic properties in immunocompetent orthotopic syngenic mouse and rat glioma models. Neuro Oncol. 14, 288–301 (2012).
Gillet, L., Dewals, B., Farnir, F., de Leval, L. & Vanderplasschen, A. Bovine herpesvirus 4 induces apoptosis of human carcinoma cell lines in vitro and in vivo. Cancer Res. 65, 9463–9472 (2005).
Macnab, S. A. et al. Herpesvirus saimiri-mediated delivery of the adenomatous polyposis coli tumour suppressor gene reduces proliferation of colorectal cancer cells. Int. J. Oncol. 39, 1173–1181 (2011).
Stevenson, A. J. et al. Specific oncolytic activity of herpesvirus saimiri in pancreatic cancer cells. Br. J. Cancer 83, 329–332 (2000).
Ferreira, T. et al. Oncolytic H-1 parvovirus hijacks galectin-1 to enter cancer cells. Viruses 14, 1018 (2022).
Kulkarni, A. et al. Oncolytic H-1 parvovirus binds to sialic acid on laminins for cell attachment and entry. Nat. Commun. 12, 3834 (2021).
Marchini, A., Daeffler, L., Pozdeev, V. I., Angelova, A. & Rommelaere, J. Immune conversion of tumor microenvironment by oncolytic viruses: the protoparvovirus H-1PV case study. Front. Immunol. 10, 1848 (2019).
Lacroix, J. et al. Preclinical testing of an oncolytic parvovirus in Ewing sarcoma: protoparvovirus H-1 induces apoptosis and lytic infection in vitro but fails to improve survival in vivo. Viruses 10, 302 (2018).
Geletneky, K. et al. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol. Ther. 25, 2620–2634 (2017).
Angelova, A. L., Witzens-Harig, M., Galabov, A. S. & Rommelaere, J. The oncolytic virotherapy era in cancer management: prospects of applying H-1 parvovirus to treat blood and solid cancers. Front. Oncol. 7, 93 (2017).
Angelova, A. L., Geletneky, K., Nüesch, J. P. F. & Rommelaere, J. Tumor selectivity of oncolytic parvoviruses: from in vitro and animal models to cancer patients. Front. Bioeng. Biotechnol. 3, 55 (2015).
Heinrich, B., Goepfert, K., Delic, M., Galle, P. R. & Moehler, M. Influence of the oncolytic parvovirus H-1, CTLA-4 antibody tremelimumab and cytostatic drugs on the human immune system in a human in vitro model of colorectal cancer cells. Onco Targets Ther. 6, 1119–1127 (2013).
Li, J. et al. Synergistic combination of valproic acid and oncolytic parvovirus H-1PV as a potential therapy against cervical and pancreatic carcinomas. EMBO Mol. Med. 5, 1537–1555 (2013).
Paglino, J. C., Ozduman, K. & van den Pol, A. N. LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells. J. Virol. 86, 7280–7291 (2012).
Angelova, A. L. et al. Oncolytic rat parvovirus H-1PV, a candidate for the treatment of human lymphoma: in vitro and in vivo studies. Mol. Ther. 17, 1164–1172 (2009).
Grekova, S. P., Raykov, Z., Zawatzky, R., Rommelaere, J. & Koch, U. Activation of a glioma-specific immune response by oncolytic parvovirus Minute Virus of Mice infection. Cancer Gene Ther. 19, 468–475 (2012).
Halder, S. et al. Profiling of glycan receptors for minute virus of mice in permissive cell lines towards understanding the mechanism of cell recognition. PLoS ONE 9, e86909 (2014).
Ricordel, M. et al. Cowpox virus: a new and armed oncolytic poxvirus. Mol. Ther. Oncolytics 7, 1–11 (2017).
Chung, C. S., Hsiao, J. C., Chang, Y. S. & Chang, W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72, 1577–1585 (1998).
Howard, A. R., Senkevich, T. G. & Moss, B. Vaccinia virus A26 and A27 proteins form a stable complex tethered to mature virions by association with the A17 transmembrane protein. J. Virol. 82, 12384–12391 (2008).
Cousin, S. et al. Phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced breast cancer. Exp. Hematol. Oncol. 11, 104 (2022).
Samson, A. et al. Neoadjuvant intravenous oncolytic vaccinia virus therapy promotes anticancer immunity in patients. Cancer Immunol. Res. 10, 745–756 (2022).
Zhang, Z. et al. CF33-hNIS-antiPDL1 virus primes pancreatic ductal adenocarcinoma for enhanced anti-PD-L1 therapy. Cancer Gene Ther. 29, 722–733 (2022).
Zuo, S. et al. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J. Immunother. Cancer 9, e002843 (2021).
Kloker, L. D. et al. Oncolytic vaccinia virus GLV-1h68 exhibits profound antitumoral activities in cell lines originating from neuroendocrine neoplasms. BMC Cancer 20, 628 (2020).
O’Leary, M. P. et al. Novel oncolytic chimeric orthopoxvirus causes regression of pancreatic cancer xenografts and exhibits abscopal effect at a single low dose. J. Transl. Med. 16, 110 (2018).
Peng, J. et al. Synergistic suppression effect on tumor growth of acute myeloid leukemia by combining cytarabine with an engineered oncolytic vaccinia virus. Onco Targets Ther. 11, 6887–6900 (2018).
Futami, M. et al. Efficacy and safety of doubly-regulated vaccinia virus in a mouse xenograft model of multiple myeloma. Mol. Ther. Oncolytics 6, 57–68 (2017).
Lv, C., Su, Q., Liang, Y., Hu, J. & Yuan, S. Oncolytic vaccine virus harbouring the IL-24 gene suppresses the growth of lung cancer by inducing apoptosis. Biochem. Biophys. Res. Commun. 476, 21–28 (2016).
Kim, M. Replicating poxviruses for human cancer therapy. J. Microbiol. 53, 209–218 (2015).
Guse, K., Cerullo, V. & Hemminki, A. Oncolytic vaccinia virus for the treatment of cancer. Expert Opin. Biol. Ther. 11, 595–608 (2011).
Thorne, S. H. Immunotherapeutic potential of oncolytic vaccinia virus. Immunol. Res. 50, 286–293 (2011).
Brun, J. et al. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol. Ther. 18, 1440–1449 (2010).
Luteijn, R. D. et al. A genome-wide haploid genetic screen identifies heparan sulfate-associated genes and the macropinocytosis modulator TMED10 as factors supporting vaccinia virus infection. J. Virol. 93, e02160-18 (2019).
Bengali, Z., Townsley, A. C. & Moss, B. Vaccinia virus strain differences in cell attachment and entry. Virology 389, 132–140 (2009).
Ricordel, M. et al. Oncolytic properties of non-vaccinia poxviruses. Oncotarget 9, 35891–35906 (2018).
Evgin, L. et al. Potent oncolytic activity of raccoonpox virus in the absence of natural pathogenicity. Mol. Ther. 18, 896–902 (2010).
Rintoul, J. L. et al. ORFV: a novel oncolytic and immune stimulating parapoxvirus therapeutic. Mol. Ther. 20, 1148–1157 (2012).
von Buttlar, H., Siegemund, S., Büttner, M. & Alber, G. Identification of Toll-like receptor 9 as parapoxvirus ovis-sensing receptor in plasmacytoid dendritic cells. PLoS ONE 9, e106188 (2014).
Mahar, R. et al. Metabolic signatures associated with oncolytic myxoma viral infections. Sci. Rep. 12, 12599 (2022).
Christie, J. D. et al. Systemic delivery of TNF-armed myxoma virus plus immune checkpoint inhibitor eliminates lung metastatic mouse osteosarcoma. Mol. Ther. Oncolytics 22, 539–554 (2021).
Rahman, M. M. & McFadden, G. Oncolytic virotherapy with myxoma virus. J. Clin. Med. 9, 171 (2020).
Najarro, P. et al. Yaba-like disease virus chemokine receptor 7L, a CCR8 orthologue. J. Gen. Virol. 87, 809–816 (2006).
Würdinger, T. et al. Targeting non-human coronaviruses to human cancer cells using a bispecific single-chain antibody. Gene Ther. 12, 1394–1404 (2005).
Tresnan, D. B., Levis, R. & Holmes, K. V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 70, 8669–8674 (1996).
Cook, S., Castillo, D., Williams, S., Haake, C. & Murphy, B. Serotype I and II feline coronavirus replication and gene expression patterns of feline cells—building a better understanding of serotype I FIPV biology. Viruses 14, 1356 (2022).
Verheije, M. H. et al. Coronavirus genetically redirected to the epidermal growth factor receptor exhibits effective antitumor activity against a malignant glioblastoma. J. Virol. 83, 7507–7516 (2009).
Würdinger, T. et al. Soluble receptor-mediated targeting of mouse hepatitis coronavirus to the human epidermal growth factor receptor. J. Virol. 79, 15314–15322 (2005).
Taguchi, F. & Hirai-Yuki, A. Mouse hepatitis virus receptor as a determinant of the mouse susceptibility to MHV infection. Front. Microbiol. 3, 68 (2012).
Chen, L. et al. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol. Ther. Oncolytics 24, 522–534 (2022).
Nair, S. et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight 6, e144619 (2021).
Lei, G. et al. Therapeutic efficacy of an oncolytic influenza virus carrying an antibody against programmed cell death 1 in hepatocellular carcinoma. Hum. Gene Ther. 33, 309–317 (2022).
Yang, H. et al. Oncolytic activity of a chimeric influenza A virus carrying a human CTLA4 antibody in hepatocellular carcinoma. Front. Oncol. 12, 875525 (2022).
Sitnik, S., Masemann, D., Leite Dantas, R., Wixler, V. & Ludwig, S. PD-1 IC inhibition synergistically improves influenza A virus-mediated oncolysis of metastatic pulmonary melanoma. Mol. Ther. Oncolytics 17, 190–204 (2020).
Penghui, Y. et al. Oncolytic activity of a novel influenza A virus carrying granulocyte-macrophage colony-stimulating factor in hepatocellular carcinoma. Hum. Gene Ther. 30, 330–338 (2019).
Masemann, D. et al. Oncolytic influenza virus infection restores immunocompetence of lung tumor-associated alveolar macrophages. Oncoimmunology 7, e1423171 (2018).
Kuznetsova, I. et al. Targeting an oncolytic influenza A virus to tumor tissue by elastase. Mol. Ther. Oncolytics 7, 37–44 (2017).
Kasloff, S. B. et al. Oncolytic activity of avian influenza virus in human pancreatic ductal adenocarcinoma cell lines. J. Virol. 88, 9321–9334 (2014).
Sturlan, S. et al. Endogenous expression of proteases in colon cancer cells facilitate influenza A viruses mediated oncolysis. Cancer Biol. Ther. 10, 592–599 (2010).
Sempere Borau, M. & Stertz, S. Entry of influenza A virus into host cells — recent progress and remaining challenges. Curr. Opin. Virol. 48, 23–29 (2021).
Huang, F. et al. Development of molecular mechanisms and their application on oncolytic Newcastle disease virus in cancer therapy. Front. Mol. Biosci. 9, 889403 (2022).
Liu, T. et al. Optimization of oncolytic effect of Newcastle disease virus Clone30 by selecting sensitive tumor host and constructing more oncolytic viruses. Gene Ther. 28, 697–717 (2021).
Burman, B., Pesci, G. & Zamarin, D. Newcastle disease virus at the forefront of cancer immunotherapy. Cancers 12, 3552 (2020).
Vijayakumar, G., McCroskery, S. & Palese, P. Engineering Newcastle disease virus as an oncolytic vector for intratumoral delivery of immune checkpoint inhibitors and immunocytokines. J. Virol. 94, e01677-19 (2020).
Burke, S. et al. Oncolytic Newcastle disease virus activation of the innate immune response and priming of antitumor adaptive responses in vitro. Cancer Immunol. Immunother. 69, 1015–1027 (2020).
Schirrmacher, V. Fifty years of clinical application of Newcastle disease virus: time to celebrate! Biomedicines 4, 16 (2016).
Matveeva, O. V., Guo, Z. S., Shabalina, S. A. & Chumakov, P. M. Oncolysis by paramyxoviruses: multiple mechanisms contribute to therapeutic efficiency. Mol. Ther. Oncolytics 2, 15011 (2015).
Csatary, L. K. et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neurooncol. 67, 83–93 (2004).
Phuangsab, A., Lorence, R. M., Reichard, K. W., Peeples, M. E. & Walter, R. J. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 172, 27–36 (2001).
Reichard, K. W. et al. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 52, 448–453 (1992).
Sánchez-Felipe, L., Villar, E. & Muñoz-Barroso, I. Entry of Newcastle Disease Virus into the host cell: role of acidic pH and endocytosis. Biochim. Biophys. Acta 1838, 300–309 (2014).
Armando, F. et al. Intratumoral canine distemper virus infection inhibits tumor growth by modulation of the tumor microenvironment in a murine xenograft model of canine histiocytic sarcoma. Int. J. Mol. Sci. 22, 3578 (2021).
Li, P. et al. Oncolytic activity of canine distemper virus in canine mammary tubular adenocarcinoma cells. Vet. Comp. Oncol. 17, 174–183 (2019).
Zhao, J. & Ren, Y. Multiple receptors involved in invasion and neuropathogenicity of canine distemper virus: a review. Viruses 14, 1520 (2022).
Suter, S. E. et al. In vitro canine distemper virus infection of canine lymphoid cells: a prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 11, 1579–1587 (2005).
Naseer, F. et al. Formulation for the targeted delivery of a vaccine strain of oncolytic measles virus (OMV) in hyaluronic acid coated thiolated chitosan as a green nanoformulation for the treatment of prostate cancer: a viro-immunotherapeutic approach. Int. J. Nanomed. 18, 185–205 (2023).
Engeland, C. E. & Ungerechts, G. Measles virus as an oncolytic immunotherapy. Cancers 13, 544 (2021).
Pidelaserra-Martí, G. & Engeland, C. E. Mechanisms of measles virus oncolytic immunotherapy. Cytokine Growth Factor Rev. 56, 28–38 (2020).
Leber, M. F. et al. Engineering and combining oncolytic measles virus for cancer therapy. Cytokine Growth Factor Rev. 56, 39–48 (2020).
Maurer, S., Salih, H. R., Smirnow, I., Lauer, U. M. & Berchtold, S. Suicide gene‑armed measles vaccine virus for the treatment of AML. Int. J. Oncol. 55, 347–358 (2019).
Allagui, F. et al. Modulation of the type I interferon response defines the sensitivity of human melanoma cells to oncolytic measles virus. Curr. Gene Ther. 16, 419–428 (2017).
Patel, M. R. et al. Measles vaccine strains for virotherapy of non-small-cell lung carcinoma. J. Thorac. Oncol. 9, 1101–1110 (2014).
Msaouel, P. et al. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 69, 82–91 (2009).
Gonçalves-Carneiro, D., McKeating, J. A. & Bailey, D. The measles virus receptor SLAMF1 can mediate particle endocytosis. J. Virol. 91, e02255-16 (2017).
Dhiman, N., Jacobson, R. M. & Poland, G. A. Measles virus receptors: SLAM and CD46. Rev. Med. Virol. 14, 217–229 (2004).
Tanaka, Y. et al. Sentinel lymph node-targeted therapy by oncolytic Sendai virus suppresses micrometastasis of head and neck squamous cell carcinoma in an orthotopic nude mouse model. Mol. Cancer Ther. 18, 1430–1438 (2019).
Ilyinskaya, G. V., Mukhina, E. V., Soboleva, A. V., Matveeva, O. V. & Chumakov, P. M. Oncolytic Sendai virus therapy of canine mast cell tumors (a pilot study). Front. Vet. Sci. 5, 116 (2018).
Saga, K. & Kaneda, Y. Oncolytic Sendai virus-based virotherapy for cancer: recent advances. Oncolytic Virother. 4, 141–147 (2015).
Bossow, S. et al. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Ther. 18, 598–608 (2011).
Kinoh, H. et al. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Ther. 16, 392–403 (2009).
Kinoh, H. et al. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases. Gene Ther. 11, 1137–1145 (2004).
Suzuki, Y., Suzuki, T. & Matsumoto, M. Isolation and characterization of receptor sialoglycoprotein for hemagglutinating virus of Japan (Sendai virus) from bovine erythrocyte membrane. J. Biochem. 93, 1621–1633 (1983).
Oku, N., Nojima, S. & Inoue, K. Studies on the interaction of Sendai virus with liposomal membranes. Sendai virus-induced agglutination of liposomes containing glycophorin. Biochim. Biophys. Acta 646, 36–42 (1981).
Müthing, J. Influenza A and Sendai viruses preferentially bind to fucosylated gangliosides with linear poly-N-acetyllactosaminyl chains from human granulocytes. Carbohydr. Res. 290, 217–224 (1996).
Holmgren, J., Svennerholm, L., Elwing, H., Fredman, P. & Strannegård, O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc. Natl Acad. Sci. USA 77, 1947–1950 (1980).
Suzuki, Y., Suzuki, T., Matsunaga, M. & Matsumoto, M. Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. J. Biochem. 97, 1189–1199 (1985).
Suzuki, T. et al. Receptor specificities of human respiroviruses. J. Virol. 75, 4604–4613 (2001).
Behrens, M. D. et al. Oncolytic Urabe mumps virus: a promising virotherapy for triple-negative breast cancer. Mol. Ther. Oncolytics 27, 239–255 (2022).
Son, H. A. et al. Combination of vaccine-strain measles and mumps viruses enhances oncolytic activity against human solid malignancies. Cancer Invest. 36, 106–117 (2018).
Ammayappan, A., Russell, S. J. & Federspiel, M. J. Recombinant mumps virus as a cancer therapeutic agent. Mol. Ther. Oncolytics 3, 16019 (2016).
Gainey, M. D., Manuse, M. J. & Parks, G. D. A hyperfusogenic F protein enhances the oncolytic potency of a paramyxovirus simian virus 5 P/V mutant without compromising sensitivity to type I interferon. J. Virol. 82, 9369–9380 (2008).
Wansley, E. K. & Parks, G. D. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 76, 10109–10121 (2002).
Bose, S. et al. Structure and mutagenesis of the parainfluenza virus 5 hemagglutinin-neuraminidase stalk domain reveals a four-helix bundle and the role of the stalk in fusion promotion. J. Virol. 85, 12855–12866 (2011).
Penza, V., Maroun, J. W., Nace, R. A., Schulze, A. J. & Russell, S. J. Polycytidine tract deletion from microRNA-detargeted oncolytic Mengovirus optimizes the therapeutic index in a murine multiple myeloma model. Mol. Ther. Oncolytics 28, 15–30 (2023).
Suryawanshi, Y. R., Nace, R. A., Russell, S. J. & Schulze, A. J. MicroRNA-detargeting proves more effective than leader gene deletion for improving safety of oncolytic Mengovirus in a nude mouse model. Mol. Ther. Oncolytics 23, 1–13 (2021).
Maroun, J. W., Penza, V., Weiskittel, T. M., Schulze, A. J. & Russell, S. J. Collateral lethal effects of complementary oncolytic viruses. Mol. Ther. Oncolytics 18, 236–246 (2020).
Ruiz, A. J., Hadac, E. M., Nace, R. A. & Russell, S. J. MicroRNA-detargeted mengovirus for oncolytic virotherapy. J. Virol. 90, 4078–4092 (2016).
Roos, F. C. et al. Oncolytic targeting of renal cell carcinoma via encephalomyocarditis virus. EMBO Mol. Med. 2, 275–288 (2010).
Hammoumi, S., Guy, M., Eloit, M. & Bakkali-Kassimi, L. Encephalomyocarditis virus may use different pathways to initiate infection of primary human cardiomyocytes. Arch. Virol. 157, 43–52 (2012).
Smyth, M., Symonds, A., Brazinova, S. & Martin, J. Bovine enterovirus as an oncolytic virus: foetal calf serum facilitates its infection of human cells. Int. J. Mol. Med. 10, 49–53 (2002).
Hodes, M. E., Morgan, S., Hubbard, J. D., Yu, P. L. & Lukemeyer, J. W. Tissue culture and animal studies with an oncolytic bovine enterovirus (bovine enterovirus 1). Cancer Res. 33, 2408–2414 (1973).
Taylor, M. W., Cordell, B., Souhrada, M. & Prather, S. Viruses as an aid to cancer therapy: regression of solid and ascites tumors in rodents after treatment with bovine enterovirus. Proc. Natl Acad. Sci. USA 68, 836–840 (1971).
Au, G. G., Beagley, L. G., Haley, E. S., Barry, R. D. & Shafren, D. R. Oncolysis of malignant human melanoma tumors by Coxsackieviruses A13, A15 and A18. Virol. J. 8, 22 (2011).
Rossmann, M. G. Viral cell recognition and entry. Protein Sci. 3, 1712–1725 (1994).
Xiao, C. et al. Interaction of coxsackievirus A21 with its cellular receptor, ICAM-1. J. Virol. 75, 2444–2451 (2001).
Rudin, C. M. et al. Phase 1, open-label, dose-escalation study on the safety, pharmacokinetics, and preliminary efficacy of intravenous Coxsackievirus A21 (V937), with or without pembrolizumab, in patients with advanced solid tumors. J. Immunother. Cancer 11, e005007 (2023).
Andtbacka, R. H. I. et al. Clinical responses of oncolytic Coxsackievirus A21 (V937) in patients with unresectable melanoma. J. Clin. Oncol. 39, 3829–3838 (2021).
Bradley, S. et al. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 3, 47–55 (2014).
Bahreyni, A. et al. A new miRNA-modified coxsackievirus B3 inhibits triple negative breast cancer growth with improved safety profile in immunocompetent mice. Cancer Lett. 548, 215849 (2022).
Geisler, A., Hazini, A., Heimann, L., Kurreck, J. & Fechner, H. Coxsackievirus B3—its potential as an oncolytic virus. Viruses 13, 718 (2021).
Miyamoto, S. et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 72, 2609–2621 (2012).
Beasley, G. M. et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 9, e002203 (2021).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018).
Walton, R. W., Brown, M. C., Sacco, M. T. & Gromeier, M. Engineered oncolytic poliovirus PVSRIPO subverts MDA5-dependent innate immune responses in cancer cells. J. Virol. 92, e00879-18 (2018).
Holl, E. K. et al. Recombinant oncolytic poliovirus, PVSRIPO, has potent cytotoxic and innate inflammatory effects, mediating therapy in human breast and prostate cancer xenograft models. Oncotarget 7, 79828–79841 (2016).
Atsumi, S. et al. Oncolytic virotherapy for human bone and soft tissue sarcomas using live attenuated poliovirus. Int. J. Oncol. 41, 893–902 (2012).
Haley, E. S., Au, G. G., Carlton, B. R., Barry, R. D. & Shafren, D. R. Regional administration of oncolytic Echovirus 1 as a novel therapy for the peritoneal dissemination of gastric cancer. J. Mol. Med. 87, 385–399 (2009).
Shafren, D. R., Sylvester, D., Johansson, E. S., Campbell, I. G. & Barry, R. D. Oncolysis of human ovarian cancers by echovirus type 1. Int. J. Cancer 115, 320–328 (2005).
He, Y. et al. Structure of decay-accelerating factor bound to echovirus 7: a virus-receptor complex. Proc. Natl Acad. Sci. USA 99, 10325–10329 (2002).
Hietanen, E., Koivu, M. K. A. & Susi, P. Cytolytic properties and genome analysis of Rigvir® oncolytic virotherapy virus and other echovirus 7 isolates. Viruses 14, 525 (2022).
Ismailov, Z. et al. A case of stage IV chromophobe renal cell carcinoma treated with the oncolytic ECHO-7 virus, Rigvir®. Am. J. Case Rep. 20, 48–52 (2019).
Alberts, P., Tilgase, A., Rasa, A., Bandere, K. & Venskus, D. The advent of oncolytic virotherapy in oncology: the Rigvir® story. Eur. J. Pharmacol. 837, 117–126 (2018).
Kim, C. & Bergelson, J. M. Echovirus 7 entry into polarized intestinal epithelial cells requires clathrin and Rab7. mBio 3, e00304-11 (2012).
Israelsson, S., Jonsson, N., Gullberg, M. & Lindberg, A. M. Cytolytic replication of echoviruses in colon cancer cell lines. Virol. J. 8, 473 (2011).
Li, J. et al. Pathological characteristics of Echovirus 30 infection in a mouse model. J. Virol. 96, e0012922 (2022).
Li, J., Zhang, Y., Qu, Z., Ding, R. & Yin, X. ABCD3 is a prognostic biomarker for glioma and associated with immune infiltration: a study based on oncolysis of gliomas. Front. Cell. Infect. Microbiol. 12, 956801 (2022).
Zhang, X. et al. Enterovirus A71 oncolysis of malignant gliomas. Mol. Ther. 28, 1533–1546 (2020).
Kobayashi, K. & Koike, S. Adaptation and virulence of enterovirus-A71. Viruses 13, 1661 (2021).
Kobayashi, K. & Koike, S. Cellular receptors for enterovirus A71. J. Biomed. Sci. 27, 23 (2020).
Luo, D. et al. Senecavirus A as an oncolytic virus: prospects, challenges and development directions. Front. Oncol. 12, 839536 (2022).
Schenk, E. L. et al. A randomized double-blind phase II study of the Seneca Valley virus (NTX-010) versus placebo for patients with extensive-stage SCLC (ES SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J. Thorac. Oncol. 15, 110–119 (2020).
Burke, M. J. Oncolytic Seneca Valley virus: past perspectives and future directions. Oncolytic Virother. 5, 81–89 (2016).
Reddy, P. S. et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J. Natl Cancer Inst. 99, 1623–1633 (2007).
Cao, L. et al. Seneca Valley virus attachment and uncoating mediated by its receptor anthrax toxin receptor 1. Proc. Natl Acad. Sci. USA 115, 13087–13092 (2018).
Hou, L. et al. Seneca Valley virus enters PK-15 cells via caveolae-mediated endocytosis and macropinocytosis dependent on low-pH, dynamin, Rab5, and Rab7. J. Virol. 96, e0144622 (2022).
Li, J. K.-K. Oncolytic bluetongue viruses: promise, progress, and perspectives. Front. Microbiol. 2, 46 (2011).
Hu, J. et al. Selective in vitro cytotoxic effect of human cancer cells by bluetongue virus-10. Acta Oncol. 47, 124–134 (2008).
Roy, P. Bluetongue virus assembly and exit pathways. Adv. Virus Res. 108, 249–273 (2020).
Hassan, S. S. & Roy, P. Expression and functional characterization of bluetongue virus VP2 protein: role in cell entry. J. Virol. 73, 9832–9842 (1999).
Bhattacharya, B. & Roy, P. Role of lipids on entry and exit of bluetongue virus, a complex non-enveloped virus. Viruses 2, 1218–1235 (2010).
Müller, L., Berkeley, R., Barr, T., Ilett, E. & Errington-Mais, F. Past, present and future of oncolytic reovirus. Cancers 12, 3219 (2020).
Mahalingam, D. et al. A phase II study of pelareorep (REOLYSIN®) in combination with gemcitabine for patients with advanced pancreatic adenocarcinoma. Cancers 10, 160 (2018).
Noonan, A. M. et al. Randomized phase 2 trial of the oncolytic virus pelareorep (Reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 24, 1150–1158 (2016).
Danthi, P., Holm, G. H., Stehle, T. & Dermody, T. S. Reovirus receptors, cell entry, and proapoptotic signaling. Adv. Exp. Med. Biol. 790, 42–71 (2013).
Kushiya, H. et al. Retroviral replicating vector Toca 511 (Vocimagene Amiretrorepvec) for prodrug activator gene therapy of lung cancer. Cancers 14, 5820 (2022).
Collins, S. A., Shah, A. H., Ostertag, D., Kasahara, N. & Jolly, D. J. Clinical development of retroviral replicating vector Toca 511 for gene therapy of cancer. Expert Opin. Biol. Ther. 21, 1199–1214 (2021).
Lee, E. S., Jin, S. Y., Kang, B. K. & Jung, Y.-T. Construction of replication-competent oncolytic retroviral vectors expressing R peptide-truncated 10A1 envelope glycoprotein. J. Virol. Methods 268, 32–36 (2019).
O Bryan, S. M. & Mathis, J. M. Oncolytic virotherapy for breast cancer treatment. Curr. Gene Ther. 18, 192–205 (2018).
Cloughesy, T. F. et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 8, 341ra75 (2016).
Rein, A. Murine leukemia viruses: objects and organisms. Adv. Virol. 2011, 403419 (2011).
Budzik, K. M., Nace, R. A., Ikeda, Y. & Russell, S. J. Evaluation of the stability and intratumoral delivery of foreign transgenes encoded by an oncolytic Foamy Virus vector. Cancer Gene Ther. 29, 1240–1251 (2022).
Budzik, K. M., Nace, R. A., Ikeda, Y. & Russell, S. J. Oncolytic Foamy Virus—generation and properties of a nonpathogenic replicating retroviral vector system that targets chronically proliferating cancer cells. J. Virol. 95, e00015-21 (2021).
Berka, U., Hamann, M. V. & Lindemann, D. Early events in foamy virus-host interaction and intracellular trafficking. Viruses 5, 1055–1074 (2013).
McGray, A. J. R. et al. Oncolytic Maraba virus armed with tumor antigen boosts vaccine priming and reveals diverse therapeutic response patterns when combined with checkpoint blockade in ovarian cancer. J. Immunother. Cancer 7, 189 (2019).
Tong, J. G. et al. Evidence for differential viral oncolytic efficacy in an in vitro model of epithelial ovarian cancer metastasis. Mol. Ther. Oncolytics 2, 15013 (2015).
Tong, J. G. et al. Spatial and temporal epithelial ovarian cancer cell heterogeneity impacts Maraba virus oncolytic potential. BMC Cancer 17, 594 (2017).
Porosnicu, M., Quinson, A.-M., Crossley, K., Luecke, S. & Lauer, U. M. Phase I study of VSV-GP (BI 1831169) as monotherapy or combined with ezabenlimab in advanced and refractory solid tumors. Future Oncol. 18, 2627–2638 (2022).
Wedge, M.-E. et al. Virally programmed extracellular vesicles sensitize cancer cells to oncolytic virus and small molecule therapy. Nat. Commun. 13, 1898 (2022).
Zhang, Y. & Nagalo, B. M. Immunovirotherapy based on recombinant vesicular stomatitis virus: where are we? Front. Immunol. 13, 898631 (2022).
Innao, V., Rizzo, V., Allegra, A. G., Musolino, C. & Allegra, A. Oncolytic viruses and hematological malignancies: a new class of immunotherapy drugs. Curr. Oncol. 28, 159–183 (2020).
Nagalo, B. M. et al. Oncolytic virus with attributes of vesicular stomatitis virus and measles virus in hepatobiliary and pancreatic cancers. Mol. Ther. Oncolytics 18, 546–555 (2020).
Felt, S. A. & Grdzelishvili, V. Z. Recent advances in vesicular stomatitis virus-based oncolytic virotherapy: a 5-year update. J. Gen. Virol. 98, 2895–2911 (2017).
Nguyen, A. et al. HDACi delivery reprograms tumor-infiltrating myeloid cells to eliminate antigen-loss variants. Cell Rep. 24, 642–654 (2018).
Shen, W., Patnaik, M. M., Ruiz, A., Russell, S. J. & Peng, K.-W. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood 127, 1449–1458 (2016).
Naik, S., Nace, R., Barber, G. N. & Russell, S. J. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther. 19, 443–450 (2012).
Finkelshtein, D., Werman, A., Novick, D., Barak, S. & Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl Acad. Sci. USA 110, 7306–7311 (2013).
Lundstrom, K. Alphaviruses in immunotherapy and anticancer therapy. Biomedicines 10, 2263 (2022).
Muhuri, M. & Gao, G. Oncolytic virus Alphavirus M1: a new and promising weapon to fight cancer. Hum. Gene Ther. 32, 136–137 (2021).
Liang, J. et al. Inhibition of the mevalonate pathway enhances cancer cell oncolysis mediated by M1 virus. Nat. Commun. 9, 1524 (2018).
Lin, Y. et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl Acad. Sci. USA 111, E4504–E4512 (2014).
Wang, N. et al. Attenuation of Getah virus by a single amino acid substitution at residue 253 of the E2 protein that might be part of a new heparan sulfate binding site on alphaviruses. J. Virol. 96, e0175121 (2022).
Herrador-Cañete, G. et al. Galectin-3 inhibition boosts the therapeutic efficacy of Semliki Forest virus in pediatric osteosarcoma. Mol. Ther. Oncolytics 26, 246–264 (2022).
Martikainen, M. et al. IFN-I-tolerant oncolytic Semliki Forest virus in combination with anti-PD1 enhances T cell response against mouse glioma. Mol. Ther. Oncolytics 21, 37–46 (2021).
Quetglas, J. I. et al. Virotherapy with a Semliki Forest virus-based vector encoding IL12 synergizes with PD-1/PD-L1 blockade. Cancer Immunol. Res. 3, 449–454 (2015).
Ma, J. et al. Concurrent expression of HP-NAP enhances antitumor efficacy of oncolytic vaccinia virus but not for Semliki Forest virus. Mol. Ther. Oncolytics 21, 356–366 (2021).
Martikainen, M. et al. Oncolytic alphavirus SFV-VA7 efficiently eradicates subcutaneous and orthotopic human prostate tumours in mice. Br. J. Cancer 117, 51–55 (2017).
Ketola, A. et al. Oncolytic Semliki Forest virus vector as a novel candidate against unresectable osteosarcoma. Cancer Res. 68, 8342–8350 (2008).
Ylösmäki, E., Martikainen, M., Hinkkanen, A. & Saksela, K. Attenuation of Semliki Forest virus neurovirulence by microRNA-mediated detargeting. J. Virol. 87, 335–344 (2013).
Ruotsalainen, J. J. et al. Clonal variation in interferon response determines the outcome of oncolytic virotherapy in mouse CT26 colon carcinoma model. Gene Ther. 22, 65–75 (2015).
Vähä-Koskela, M. J. V. et al. Oncolytic capacity of attenuated replicative Semliki Forest virus in human melanoma xenografts in severe combined immunodeficient mice. Cancer Res. 66, 7185–7194 (2006).
Lu, Y. E., Cassese, T. & Kielian, M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 73, 4272–4278 (1999).
Schroeder, C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell. Biochem. 51, 77–108 (2010).
Clark, L. E. et al. VLDLR and ApoER2 are receptors for multiple alphaviruses. Nature 602, 475–480 (2022).
Zimmerman, O., Holmes, A. C., Kafai, N. M., Adams, L. J. & Diamond, M. S. Entry receptors—the gateway to alphavirus infection. J. Clin. Invest. 133, e165307 (2023).
Opp, S., Hurtado, A., Pampeno, C., Lin, Z. & Meruelo, D. Potent and targeted Sindbis virus platform for immunotherapy of ovarian cancer. Cells 12, 77 (2022).
Scherwitzl, I. et al. Sindbis virus with anti-OX40 overcomes the immunosuppressive tumor microenvironment of low-immunogenic tumors. Mol. Ther. Oncolytics 17, 431–447 (2020).
Scherwitzl, I. et al. Systemically administered Sindbis virus in combination with immune checkpoint blockade induces curative anti-tumor immunity. Mol. Ther. Oncolytics 9, 51–63 (2018).
Takenouchi, A. et al. Oncolytic viral therapy for neuroblastoma cells with Sindbis virus AR339 strain. Pediatr. Surg. Int. 31, 1151–1159 (2015).
Sargent, A. L. et al. Quantitatively assessing the respiratory burst in innate immune cells. Methods Mol. Biol. 2614, 47–70 (2023).
Xu, B. et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat. Commun. 12, 5908 (2021).
Boero, E. et al. Use of flow cytometry to evaluate phagocytosis of Staphylococcus aureus by human neutrophils. Front. Immunol. 12, 635825 (2021).
Li, X. et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology 11, 2118210 (2022).
Benencia, F. et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol. Ther. 12, 789–802 (2005).
Zhang, Y. et al. Attenuated, oncolytic, but not wild-type measles virus infection has pleiotropic effects on human neutrophil function. J. Immunol. 188, 1002–1010 (2012).
Hamdan, F. et al. Novel oncolytic adenovirus expressing enhanced cross-hybrid IgGA Fc PD-L1 inhibitor activates multiple immune effector populations leading to enhanced tumor killing in vitro, in vivo and with patient-derived tumor organoids. J. Immunother. Cancer 9, e003000 (2021).
Leung, E. Y. L. et al. NK cells augment oncolytic adenovirus cytotoxicity in ovarian cancer. Mol. Ther. Oncolytics 16, 289–301 (2020).
Floerchinger, A. et al. A vector-encoded bispecific killer engager to harness virus-activated NK cells as anti-tumor effectors. Cell Death Dis. 14, 104 (2023).
Ma, R. et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 81, 3635–3648 (2021).
Bhat, R., Dempe, S., Dinsart, C. & Rommelaere, J. Enhancement of NK cell antitumor responses using an oncolytic parvovirus. Int. J. Cancer 128, 908–919 (2011).
Svensson-Arvelund, J. et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity. Nat. Commun. 13, 7149 (2022).
Krug, A. et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21, 107–119 (2004).
Dalod, M. et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197, 885–898 (2003).
Cella, M., Facchetti, F., Lanzavecchia, A. & Colonna, M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1, 305–310 (2000).
Guillerme, J.-B. et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin. Cancer Res. 19, 1147–1158 (2013).
Boudreau, J. E. et al. Recombinant vesicular stomatitis virus transduction of dendritic cells enhances their ability to prime innate and adaptive antitumor immunity. Mol. Ther. 17, 1465–1472 (2009).
Gauvrit, A. et al. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 68, 4882–4892 (2008).
Ilett, E. J. et al. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin. Cancer Res. 17, 2767–2776 (2011).
Schierer, S. et al. Human dendritic cells efficiently phagocytose adenoviral oncolysate but require additional stimulation to mature. Int. J. Cancer 130, 1682–1694 (2012).
van den Bossche, W. B. L. et al. Oncolytic virotherapy in glioblastoma patients induces a tumor macrophage phenotypic shift leading to an altered glioblastoma microenvironment. Neuro Oncol. 20, 1494–1504 (2018).
Zhang, L. et al. Reshaping the immune microenvironment by oncolytic herpes simplex virus in murine pancreatic ductal adenocarcinoma. Mol. Ther. 29, 744–761 (2021).
Tan, Z. et al. Virotherapy-recruited PMN-MDSC infiltration of mesothelioma blocks antitumor CTL by IL-10-mediated dendritic cell suppression. Oncoimmunology 8, e1518672 (2019).
Finak, G. et al. Standardizing flow cytometry immunophenotyping analysis from the human immunophenotyping consortium. Sci. Rep. 6, 20686 (2016).
Woodruff, M. C. et al. Response under pressure: deploying emerging technologies to understand B-cell-mediated immunity in COVID-19. Nat. Methods 19, 387–391 (2022).
Izadi, N. & Hauk, P. J. Cellular assays to evaluate B-cell function. J. Immunol. Methods 512, 113395 (2023).
Ogbe, A. et al. T cell assays differentiate clinical and subclinical SARS-CoV-2 infections from cross-reactive antiviral responses. Nat. Commun. 12, 2055 (2021).
Ansari, A. et al. An efficient immunoassay for the B cell help function of SARS-CoV-2-specific memory CD4+ T cells. Cell Rep. Methods 2, 100224 (2022).
Huseni, M. A. et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med 4, 100878 (2023).
Nakajima, Y., Chamoto, K., Oura, T. & Honjo, T. Critical role of the CD44lowCD62Llow CD8+ T cell subset in restoring antitumor immunity in aged mice. Proc. Natl Acad. Sci. USA 118, e2103730118 (2021).
Sottile, R. et al. Human cytomegalovirus expands a CD8+ T cell population with loss of BCL11B expression and gain of NK cell identity. Sci. Immunol. 6, eabe6968 (2021).
Notarangelo, G. et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 377, 1519–1529 (2022).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Nelson, N. et al. A cell-engineered system to assess tumor cell sensitivity to CD8+ T cell-mediated cytotoxicity. Oncoimmunology 8, 1599635 (2019).
Zhu, J., Cao, J., Liesz, A. & Roth, S. A macrophage-T cell coculture model for severe tissue injury-induced T cell death. STAR Protoc. 2, 100983 (2021).
Acknowledgements
S.G. is supported by grants from the CIHR, the Canadian Cancer Society (CCS), the Canada Foundation for Innovation-John R. Evans Leaders Fund (CFI-JELF), Research Nova Scotia (RNS), the Cancer Research Society (CRS) and the Dalhousie Medical Research Foundation (DMRF). V.K. was supported through the Cancer Research Training Program (CRTP) of the Beatrice Hunter Cancer Research Institute (BHCRI). P.K. was funded by the Killam Predoctoral Scholarship. J.G.P. is supported by the Site de Recherche intégrée sur le Cancer (SIRIC) Cancer Research and Personalized Medicine (CARPEM), Institut National du Cancer (INCa), Cancer ITMO (Multi-Organisation Thematic Institute) of the French Alliance for Life Sciences and Health (AVIESAN) and Fondation pour la Recherche Médicale (FRM). G.K. is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; European Research Council Advanced Investigator Grant “ICD-Cancer”, FRM; a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council (ICD-Cancer), European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC CARPEM. This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. J.C.B. is supported by the CIHR, the CCS, BioCanRx and the Terry Fox Research Institute (TFRI).
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by S.G., J.G.P., J.C.B. and G.K. Writing and editing was done by S.G., J.G.P., V.K., P.K., M.L.-G., J.C.B. and G.K. Project administration was handled by S.G., J.G.P., J.C.B. and G.K.
Corresponding authors
Ethics declarations
Competing interests
J.G.P. is named as an inventor on patents for cancer vaccination involving an oncolytic rhabdovirus. These patents have been licensed to Turnstone Biologics. G.K. holds research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys and Vascage. G.K. consults for Reithera. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. G.K. is on the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. G.K. is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. J.C.B. is a co-founder of Turnstone Biologics and Esphera Synbio. J.C.B. holds multiple patents involving the development of OV-based therapeutics. J.C.B. is on the advisory boards of Transgene, Cytonus, Abalos and hCBioscience.
Peer review
Peer review information
Nature Protocols thanks Howard Kaufman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gujar, S., Pol, J.G., Kumar, V. et al. Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00985-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-00985-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.