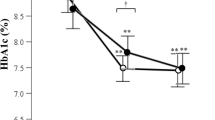
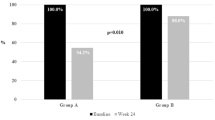
Abstract
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) injected periodically have been shown to not increase and, for some members of this class, decrease the risk of cardiovascular events. The cardiovascular safety of delivering a continuous subcutaneous infusion of the GLP-1RA exenatide (ITCA 650) is unknown. Here, we randomly assigned patients with type 2 diabetes with, or at risk for, atherosclerotic cardiovascular disease (ASCVD) to receive ITCA 650 or placebo to assess cardiovascular safety in a pre-approval trial (NCT01455896). The primary outcome was a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for unstable angina. On the basis of 2008 guidance from the US Food and Drug Administration, a non-inferiority margin of 1.8 for the upper bound of the 95% confidence interval (CI) of the hazard ratio (HR) was used. We randomized 4,156 patients (2,075 assigned to receive ITCA 650 and 2,081 assigned to receive placebo) who were followed for a median of 16 months. The primary outcome occurred in 4.6% (95/2,075) of patients in the ITCA 650 group and 3.8% (79/2,081) of patients in the placebo group, meeting the pre-specified non-inferiority criterion (HR = 1.21, 95% CI, 0.90–1.63, Pnon-inferiority = 0.004). Serious adverse events were similar between the two groups. Adverse events were more frequent in the ITCA 650 group (72%, 1,491/2,074) than in the placebo group (63.9%, 1,325/2,070), mainly due to an increase in gastrointestinal events and disorders while on ITCA 650. In patients with type 2 diabetes with, or at risk for, ASCVD, ITCA 650 was non-inferior to placebo. A larger and longer-duration cardiovascular outcomes trial is needed to define more precisely the cardiovascular effects of ITCA 650 in this population.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data from these analyses cannot be made publicly available owing to contractual obligations with the sponsor. We encourage parties interested in collaboration and data sharing to submit an application for non-commercial use to the corresponding author (C.T.R.). Applications should outline specifically what data they are interested in receiving and how the data will be used. Any data that can be shared will need approval from the sponsor and require a material transfer agreement. All data shared will be de-identified.
Code availability
Detailed descriptions of analyses and methods are provided in the Trial Protocol and the Statistical Analysis Plan, which have been provided as separate documents. All statistical computations were performed with SAS software, version 9.4 (SAS Institute).
References
Emerging Risk Factors, C. et al. Association of cardiometabolic multimorbidity with mortality. JAMA 314, 52–60 (2015).
Menke, A., Casagrande, S., Geiss, L. & Cowie, C. C. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 314, 1021–1029 (2015).
US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf (2008).
Kristensen, S. L. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7, 776–785 (2019).
Zelniker, T. A. et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393, 31–39 (2019).
Zelniker, T. A. et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 139, 2022–2031 (2019).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
Henry, R. R., Logan, D., Alessi, T. & Baron, M. A. A randomized, open-label, multicenter, 4-week study to evaluate the tolerability and pharmacokinetics of ITCA 650 in patients with type 2 diabetes. Clin. Ther. 35, 634–645 (2013).
Henry, R. R. et al. Randomized trial of continuous subcutaneous delivery of exenatide by ITCA 650 versus twice-daily exenatide injections in metformin-treated type 2 diabetes. Diabetes Care 36, 2559–2565 (2013).
Henry, R. R. et al. Continuous subcutaneous delivery of exenatide via ITCA 650 leads to sustained glycemic control and weight loss for 48 weeks in metformin-treated subjects with type 2 diabetes. J. Diabetes Complications 28, 393–398 (2014).
Henry, R. et al. Treatment satisfaction with ITCA 650, a novel drug-device delivering continuous exenatide, versus twice-daily injections of exenatide in type 2 diabetics using metformin. Diabetes Obes. Metab. 20, 638–645 (2018).
Henry, R. R. et al. Clinical impact of ITCA 650, a novel drug-device GLP-1 receptor agonist, in uncontrolled type 2 diabetes and very high baseline HbA(1c): the FREEDOM-1 HBL (high baseline) study. Diabetes Care 41, 613–619 (2018).
Rosenstock, J. et al. Efficacy and safety of ITCA 650, a novel drug-device GLP-1 receptor agonist, in type 2 diabetes uncontrolled with oral antidiabetes drugs: the FREEDOM-1 trial. Diabetes Care 41, 333–340 (2018).
Rosenstock, J. et al. Superior efficacy of ITCA 650 vs sitagliptin in uncontrolled type 2 diabetes on metformin: the FREEDOM 2 randomized, double-blind, 1-year study. Diabetes 65, 183–1 (2016).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Riley, R. D., Lambert, P. C. & Abo-Zaid, G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 340, c221 (2010).
Lachin, J. M. & Foulkes, M. A. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics 42, 507–519 (1986).
Rothmann, M. et al. Design and analysis of non-inferiority mortality trials in oncology. Stat. Med. 22, 239–264 (2003).
O’Brien, P. C. & Fleming, T. R. A multiple testing procedure for clinical trials. Biometrics 35, 549–556 (1979).
Acknowledgements
Intarcia Therapeutics was the sponsor of this trial and was responsible for data collection.
Author information
Authors and Affiliations
Contributions
All authors had full access to the data. C.T.R. wrote the first draft of the manuscript. All co-authors (C.T.R., M.B., K.I., M.L.O., F.T.T. and M.S.S.) participated in subsequent revisions of the manuscript. C.T.R. and M.S.S. had final responsibility for the decision to submit the manuscript for publication, and they assume responsibility for the accuracy and completeness of the data and analyses.
Corresponding author
Ethics declarations
Competing interests
C.T.R. reports grant support from Boehringer Ingelheim, Daiichi Sankyo, MedImmune and the National Institutes of Health and has received consulting fees from Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Janssen, MedImmune, Pfizer, Portola and Anthos. M.B. and F.T.T. are employees of Intarcia Therapeutics, and M.B. owns stock in Intarcia Therapeutics. M.L.O. reports grants from Eisai, AstraZeneca, GlaxoSmithKline, Merck, Amgen, Janssen and The Medicines Company and has received consulting fees from Novartis, Janssen, Amgen and AstraZeneca. M.S.S. reports research grant support from Abbott Laboratories, Amgen, AstraZeneca, Bayer, Critical Diagnostics, Daiichi Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia Therapeutics, IONIS, Janssen Research and Development, The Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Quark Pharmaceuticals, Roche Diagnostics and Takeda and has received consulting fees from Alnylam, Amgen, AstraZeneca, Bristol Myers Squibb, CVS Caremark, Dyrnamix, Esperion, IFM Pharmaceuticals, Intarcia Therapeutics, Ionis, Janssen Research and Development, The Medicines Company, MedImmune, Merck, Moderna, MyoKardia, Novartis and Novo Nordisk. C.T.R., M.L.O., K.I. and M.S.S. are members of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, BRAHMS, Daiichi Sankyo, Eisai, GlaxoSmithKline, Intarcia Therapeutics, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company and Zora Biosciences.
Additional information
Peer review information Nature Medicine thanks Luke Ouma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Jennifer Sargent and Michael Basson were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ITCA 650.
ITCA 650 delivers a continuous subcutaneous infusion of exenatide by an osmotic mini-pump, which releases the drug at a predetermined rate based on the principle of osmosis.
Extended Data Fig. 2 Subgroup Analysis for Cardiovascular Death, Non-Fatal Myocardial Infarction, Non-Fatal Stroke, or Hospitalization for Unstable Angina.
Shown are hazard ratios, 95% confidence intervals, and two-sided p-values derived from a stratified Cox proportional-hazards model for the primary efficacy outcome of cardiovascular death, myocardial infarction, stroke, or hospitalization for unstable angina. There was no adjustment for multiple testing.
Extended Data Fig. 3 Additional Trials Included in Meta-Analysis.
Primary outcome: CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina. Key secondary outcome: CV death, non-fatal myocardial infarction, or non-fatal stroke.
Extended Data Fig. 4 IPD Meta-Analysis: Cardiovascular Death, Non-Fatal Myocardial Infarction, Non-Fatal Stroke, or Hospitalization for Unstable Angina.
Shown is the cumulative incidence of the primary efficacy outcome of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina.
Extended Data Fig. 5 IPD Meta-Analysis: Cardiovascular Death, Non-Fatal Myocardial Infarction, or Non-Fatal Stroke.
Shown is the cumulative incidence of the key secondary outcome of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
Extended Data Fig. 6 Initiation of New Antihyperglycemic Medication.
Shown is cumulative incidence of initiation of new antihyperglycemic medication.
Supplementary information
Supplementary Information
Supplementary Notes 1 (Site Investigator List), 2 (Inclusion and Exclusion Criteria) and 3 (Clinical Event Definition)
Supplementary Data
Statistical Analysis Plan
Supplementary Data
Trial Protocol
Rights and permissions
About this article
Cite this article
Ruff, C.T., Baron, M., Im, K. et al. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat Med 28, 89–95 (2022). https://doi.org/10.1038/s41591-021-01584-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01584-3
This article is cited by
-
Efficacy of antihyperglycemic therapies on cardiovascular and heart failure outcomes: an updated meta-analysis and meta-regression analysis of 35 randomized cardiovascular outcome trials
Cardiovascular Diabetology (2023)
-
Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action
Nature Reviews Cardiology (2023)
-
Incretins and cardiovascular disease: to the heart of type 2 diabetes?
Diabetologia (2023)
-
Cardiovascular outcomes trials: a paradigm shift in the current management of type 2 diabetes
Cardiovascular Diabetology (2022)
-
Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)
Diabetologia (2022)