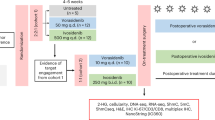
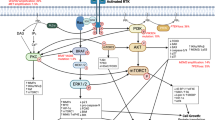
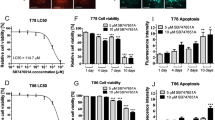
Abstract
Gliomas are the most common malignant primary brain tumours in adults and cannot usually be cured with standard cancer treatments. Gliomas show intratumoural and intertumoural heterogeneity at the histological and molecular levels, and they frequently contain mutations in the isocitrate dehydrogenase 1 (IDH1) or IDH2 gene. IDH-mutant adult-type diffuse gliomas are subdivided into grade 2, 3 or 4 IDH-mutant astrocytomas and grade 2 or 3 IDH-mutant, 1p19q-codeleted oligodendrogliomas. The product of the mutated IDH genes, d-2-hydroxyglutarate (D-2-HG), induces global DNA hypermethylation and interferes with immunity, leading to stimulation of tumour growth. Selective inhibitors of mutant IDH, such as ivosidenib and vorasidenib, have been shown to reduce D-2-HG levels and induce cellular differentiation in preclinical models and to induce MRI-detectable responses in early clinical trials. The phase III INDIGO trial has demonstrated superiority of vorasidenib, a brain-penetrant pan-mutant IDH inhibitor, over placebo in people with non-enhancing grade 2 IDH-mutant gliomas following surgery. In this Review, we describe the pathway of development of IDH inhibitors in IDH-mutant low-grade gliomas from preclinical models to clinical trials. We discuss the practice-changing implications of the INDIGO trial and consider new avenues of investigation in the field of IDH-mutant gliomas.
Key points
-
Gliomas are the most common malignant primary brain tumours in adults, and they frequently contain mutations in the isocitrate dehydrogenase 1 (IDH1) or IDH2 gene. Mutant IDH produces the oncometabolite d-2-hydroxyglutarate (D-2-HG), which induces DNA and histone hypermethylation and interferes with immunity, thereby stimulating tumour growth.
-
In preclinical models, selective inhibitors of mutant IDH were shown to decrease the production of d-2-HG, slow tumour growth and promote cellular differentiation.
-
In early clinical trials, ivosidenib, a mutant IDH1 inhibitor, and vorasidenib, a brain-penetrant pan-mutant IDH inhibitor, were found to reduce D-2-HG concentrations and induce high rates of MRI-detectable glioma stabilization or response.
-
The phase III INDIGO trial has shown superiority of vorasidenib over placebo in residual or recurrent non-enhancing grade 2 gliomas after surgery, thereby raising the prospect of delaying of chemoradiotherapy.
-
Future studies should evaluate vorasidenib in enhancing tumours of different grades of malignancy, investigate novel IDH inhibitors and combination of drugs, and develop advanced tools to monitor response and relapse.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
van den Bent, M. J., Smits, M., Kros, J. M. & Chang, S. M. Diffuse infiltrating oligodendroglioma and astrocytoma. J. Clin. Oncol. 35, 2394–2401 (2017).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Horbinski, C., Kofler, J., Kelly, L. M., Murdoch, G. H. & Nikiforova, M. N. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J. Neuropathol. Exp. Neurol. 68, 1319–1325 (2009).
Capper, D., Zentgraf, H., Balss, J., Hartmann, C. & von Deimling, A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 118, 599–601 (2009).
Pusch, S. et al. Glioma IDH1 mutation patterns off the beaten track. Neuropathol. Appl. Neurobiol. 37, 428–430 (2011).
Ward, P. S. et al. Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production. Oncogene 31, 2491–2498 (2012).
Gupta, R. et al. Expanding the spectrum of IDH1 mutations in gliomas. Mod. Pathol. 26, 619–625 (2013).
Ganz, J. et al. Rates and patterns of clonal oncogenic mutations in the normal human brain. Cancer Discov. 12, 172–185 (2022).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Rudà, R., Bello, L., Duffau, H. & Soffietti, R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 14, 55–64 (2012).
Weller, M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 18, 170–186 (2021).
Miller, J. J. et al. Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol. 25, 4–25 (2023).
Baumert, B. G. et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 17, 1521–1532 (2016).
Buckner, J. C. et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N. Engl. J. Med. 374, 1344–1355 (2016).
Douw, L. et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 8, 810–818 (2009).
Johnson, B. E. et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343, 189–193 (2014).
DiNardo, C. D. et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 378, 2386–2398 (2018).
Abou-Alfa, G. K. et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 796–807 (2020).
Tap, W. D. et al. Phase I study of the mutant IDH1 inhibitor ivosidenib: safety and clinical activity in patients with advanced chondrosarcoma. J. Clin. Oncol. 38, 1693–1701 (2020).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2010).
Chowdhury, R. et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 12, 463–469 (2011).
Koivunen, P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17, 510–522 (2010).
Lai, A. et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J. Clin. Oncol. 29, 4482–4490 (2011).
Carrillo, J. A. et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. Am. J. Neuroradiol. 33, 1349–1355 (2012).
Stockhammer, F. et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure 21, 194–197 (2012).
Chen, R. et al. Hominoid-specific enzyme GLUD2 promotes growth of IDH1R132H glioma. Proc. Natl Acad. Sci. USA 111, 14217–14222 (2014).
Waitkus, M. S. et al. Adaptive evolution of the GDH2 allosteric domain promotes gliomagenesis by resolving IDH1(R132H)-induced metabolic liabilities. Cancer Res. 78, 36–50 (2018).
Watanabe, T., Nobusawa, S., Kleihues, P. & Ohgaki, H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am. J. Pathol. 174, 1149–1153 (2009).
Wolter, M. et al. Oligodendroglial tumors frequently demonstrate hypermethylation of the CDKN2A (MTS1, p16INK4a), p14ARF, and CDKN2B (MTS2, p15INK4b) tumor suppressor genes. J. Neuropathol. Exp. Neurol. 60, 1170–1180 (2001).
Alonso, M. E. et al. Aberrant promoter methylation of multiple genes in oligodendrogliomas and ependymomas. Cancer Genet. Cytogenet. 144, 134–142 (2003).
Kuo, L. T. et al. Genetic and epigenetic alterations in primary-progressive paired oligodendroglial tumors. PLoS ONE 8, e67139 (2013).
Unruh, D. et al. Methylation and transcription patterns are distinct in IDH-mutant gliomas compared to other IDH-mutant cancers. Sci. Rep. 9, 8946 (2019).
Unruh, D. et al. Methylation-dependent tissue factor suppression contributes to the reduced malignancy of IDH1 mutant gliomas. Clin. Cancer Res. 25, 747–759 (2019).
Flavahan, W. A. et al. Insulator dysfunction and oncogene activation in IDH-mutant gliomas. Nature 529, 110–114 (2016).
Rahme, G. J. et al. Modeling epigenetic lesions that cause gliomas. Cell 186, 3674–3685.e14 (2023).
Gunn, K. et al. (R)-2-Hydroxyglutarate inhibits KDM5 histone lysine demethylases to drive transformation in IDH-mutant cancers. Cancer Discov. 13, 1478–1497 (2023).
Han, S. et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br. J. Cancer 11, 1580–1589 (2020).
Linninger, A. et al. Modeling the diffusion of D-2-hydroxyglutarate from IDH1 mutant gliomas in the central nervous system. Neuro Oncol. 20, 1197–1206 (2018).
Berghoff, A. S. et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol. 19, 1460–1468 (2017).
Bunse, L. et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 24, 1192–1203 (2018).
Notarangelo, G. et al. Oncometabolite D-2HG alters T cell metabolism to impair CD8+ T cell function. Science 377, 1519–1529 (2022).
Zhang, X. et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro Oncol. 18, 1402–1412 (2016).
Friedrich, M. et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 25, 263–276 (2023).
Sasaki, M. et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488, 656–659 (2012).
Losman, J. A. et al. (R)-2-Hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 339, 1621–1625 (2013).
Rohle, D. et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340, 626–630 (2013).
Saha, S. K. et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature 513, 110–114 (2014).
Jin, Y. et al. Mutant IDH1 dysregulates the differentiation of mesenchymal stem cells in association with gene-specific histone modifications to cartilage- and bone-related genes. PLoS ONE 10, e0131998 (2015).
Suijker, J., Baelde, H. J., Roelofs, H., Cleton-Jansen, A. M. & Bovee, J. V. The oncometabolite D-2-hydroxyglutarate induced by mutant IDH1 or -2 blocks osteoblast differentiation in vitro and in vivo. Oncotarget 6, 14832–14842 (2015).
Lu, C. et al. Induction of sarcomas by mutant IDH2. Genes Dev. 27, 1986–1998 (2013).
Hirata, M. et al. Mutant IDH is sufficient to initiate enchondromatosis in mice. Proc. Natl Acad. Sci. USA 112, 2829–2834 (2015).
Hao, Z. et al. Idh1 mutations contribute to the development of T-cell malignancies in genetically engineered mice. Proc. Natl Acad. Sci. USA 113, 1387–1392 (2016).
Gu, Y. et al. IDH1 mutation contributes to myeloid dysplasia in mice by disturbing heme biosynthesis and erythropoiesis. Blood 137, 945–958 (2021).
Sasaki, M. et al. D-2-Hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev. 26, 2038–2049 (2012).
Bardella, C. et al. Expression of Idh1(R132H) in the murine subventricular zone stem cell niche recapitulates features of early gliomagenesis. Cancer Cell 30, 578–594 (2016).
Pirozzi, C. J. et al. Mutant IDH1 disrupts the mouse subventricular zone and alters brain tumor progression. Mol. Cancer Res. 15, 507–520 (2017).
Horbinski, C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 125, 621–636 (2013).
Zong, H., Parada, L. F. & Baker, S. J. Cell of origin for malignant gliomas and its implication in therapeutic development. Cold Spring Harb. Perspect. Biol. 7, a020610 (2015).
Turcan, S. et al. Mutant-IDH1-dependent chromatin state reprogramming, reversibility, and persistence. Nat. Genet. 50, 62–72 (2018).
Venteicher, A. S. et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, 6332 (2017).
Kannan, K. et al. Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 3, 1194–1203 (2012).
Liu, X. Y. et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 124, 615–625 (2012).
Abedalthagafi, M. et al. The alternative lengthening of telomere phenotype is significantly associated with loss of ATRX expression in high-grade pediatric and adult astrocytomas: a multi-institutional study of 214 astrocytomas. Mod. Pathol. 26, 1425–1432 (2013).
Ferreira, M. S. V. et al. Alternative lengthening of telomeres is the major telomere maintenance mechanism in astrocytoma with isocitrate dehydrogenase 1 mutation. J. Neurooncol. 147, 1–14 (2020).
Lovejoy, C. A. et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 8, e1002772 (2012).
Clynes, D. et al. ATRX dysfunction induces replication defects in primary mouse cells. PLoS ONE 9, e92915 (2014).
Danussi, C. et al. Atrx inactivation drives disease-defining phenotypes in glioma cells of origin through global epigenomic remodeling. Nat. Commun. 9, 1057 (2018).
Mukherjee, J. et al. Mutant IDH1 cooperates with ATRX loss to drive the alternative lengthening of telomere phenotype in glioma. Cancer Res. 78, 2966–2977 (2018).
Borodovsky, A. et al. A model of a patient-derived IDH1 mutant anaplastic astrocytoma with alternative lengthening of telomeres. J. Neurooncol. 121, 479–487 (2015).
Udugama, M. et al. Mutations inhibiting KDM4B drive ALT activation in ATRX-mutated glioblastomas. Nat. Commun. 12, 2584 (2021).
Grandin, N. et al. The level of activity of the alternative lengthening of telomeres correlates with patient age in IDH-mutant ATRX-loss-of-expression anaplastic astrocytomas. Acta Neuropathol. Commun. 7, 175 (2019).
Modrek, A. S. et al. Low-grade astrocytoma mutations in IDH1, P53, and ATRX cooperate to block differentiation of human neural stem cells via repression of SOX2. Cell Rep. 21, 1267–1280 (2017).
Bérubé, N. G. et al. The chromatin-remodeling protein ATRX is critical for neuronal survival during corticogenesis. J. Clin. Invest. 115, 258–267 (2005).
Conte, D. et al. Loss of Atrx sensitizes cells to DNA damaging agents through p53-mediated death pathways. PLoS ONE 7, e52167 (2012).
Ritchie, K., Watson, L. A., Davidson, B., Jiang, Y. & Bérubé, N. G. ATRX is required for maintenance of the neuroprogenitor cell pool in the embryonic mouse brain. Biol. Open 3, 1158–1163 (2014).
Akıncılar, S. C. et al. Long-range chromatin interactions drive mutant TERT promoter activation. Cancer Discov. 6, 1276–1291 (2016).
Reifenberger, J. et al. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am. J. Pathol. 145, 1175–1190 (1994).
Griffin, C. A. et al. Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J. Neuropathol. Exp. Neurol. 65, 988–994 (2006).
Jenkins, R. B. et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 66, 9852–9861 (2006).
Bettegowda, C. et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333, 1453–1455 (2011).
Sahm, F. et al. CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol. 123, 853–860 (2012).
Yip, S. et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J. Pathol. 226, 7–16 (2012).
Baumgarten, P. et al. Loss of FUBP1 expression in gliomas predicts FUBP1 mutation and is associated with oligodendroglial differentiation, IDH1 mutation and 1p/19q loss of heterozygosity. Neuropathol. Appl. Neurobiol. 40, 205–216 (2014).
Chan, A. K. et al. Loss of CIC and FUBP1 expressions are potential markers of shorter time to recurrence in oligodendroglial tumors. Mod. Pathol. 27, 332–342 (2014).
Wijnenga, M. M. J. et al. Prognostic relevance of mutations and copy number alterations assessed with targeted next generation sequencing in IDH mutant grade II glioma. J. Neurooncol. 139, 349–357 (2018).
Piaskowski, S. et al. Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br. J. Cancer 104, 968–970 (2011).
Luchman, H. A. et al. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol. 14, 184–191 (2012).
Luchman, H. A., Chesnelong, C., Cairncross, J. G. & Weiss, S. Spontaneous loss of heterozygosity leading to homozygous R132H in a patient-derived IDH1 mutant cell line. Neuro Oncol. 15, 979–980 (2013).
Pietrak, B. et al. A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of αHG. Biochemistry 50, 4804–4812 (2011).
Ward, P. S. et al. The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization. J. Biol. Chem. 288, 3804–3815 (2013).
Ichimura, K. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro. Oncol. 11, 341–347 (2009).
Lass, U. et al. Clonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1- mutation as common tumor initiating event. PLoS ONE 7, e41298 (2012).
Walker, E. J. et al. Monoallelic expression determines oncogenic progression and outcome in benign and malignant brain tumors. Cancer Res. 72, 636–644 (2012).
Jin, G. et al. Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Cancer Res. 73, 496–501 (2013).
Mazor, T. et al. Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proc. Natl Acad. Sci. USA 114, 10743–10748 (2017).
Wakimoto, H. et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin. Cancer Res. 20, 2898–2909 (2014).
Amankulor, N. M. et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 31, 774–786 (2017).
Philip, B. et al. Mutant IDH1 promotes glioma formation in vivo. Cell Rep. 23, 1553–1564 (2018).
Núñez, F. J. et al. IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci. Transl. Med. 11, 1427 (2019).
Drumm, M. et al. Postoperative risk of IDH-mutant glioma-associated seizures and their potential management with IDH-mutant inhibitors. J. Clin. Invest. 133, 168035 (2023).
Konteatis, Z. et al. Vorasidenib (AG-881): a first-in-class, brain-penetrant dual inhibitor of mutant IDH1 and 2 for treatment of glioma. ACS Med. Chem. Lett. 11, 101–107 (2020).
Radoul, M. et al. Early noninvasive metabolic biomarkers of mutant IDH inhibition in glioma. Metabolites 11, 109 (2021).
Kadiyala, P. et al. Inhibition of 2-hydroxyglutarate elicits metabolic reprogramming and mutant IDH1 glioma immunity in mice. J. Clin. Invest. 131, 139542 (2021).
Chuntova, P. et al. Inhibition of D-2HG leads to upregulation of a proinflammatory gene signature in a novel HLA-A2/HLA-DR1 transgenic mouse model of IDH1R132H-expressing glioma. J. Immunother. Cancer 10, e004644 (2022).
Carney, S. V. et al. Zinc finger MYND-type containing 8 (ZMYND8) is epigenetically regulated in mutant isocitrate dehydrogenase 1 (IDH1) glioma to promote radioresistance. Clin. Cancer Res. 29, 1763–1782 (2023).
Chen, H. et al. Mutant IDH1 and seizures in patients with glioma. Neurology 88, 1805–1813 (2017).
Mellinghoff, I. K. et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J. Clin. Oncol. 38, 3398–3406 (2020).
Louis, D. N. et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 (2007).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010).
van den Bent, M. J. et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 12, 583–593 (2011).
Mellinghoff, I. K. et al. Vorasidenib, a dual inhibitor of mutant IDH1/2, in recurrent or progressive glioma; results of a first-in-human phase I trial. Clin. Cancer Res. 27, 4491–4499 (2021).
Houillier, C. et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75, 1560–1566 (2010).
Wahl, M. et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 19, 242–251 (2017).
Rudà, R. et al. Efficacy of initial temozolomide for high-risk low grade gliomas in a phase II AINO (Italian Association for Neuro-Oncology) study: a post-hoc analysis within molecular subgroups of WHO 2016. J. Neurooncol. 145, 115–123 (2019).
Mellinghoff, I. K. et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: a randomized, perioperative phase 1 trial. Nat. Med. 29, 615–622 (2023).
Harding, J. J. et al. Isoform switching as a mechanism of acquired resistance to mutant isocitrate dehydrogenase inhibition. Cancer Discov. 8, 1540–1547 (2018).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820 (2016).
Mellinghoff, I. K. et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N. Engl. J. Med. 389, 589–601 (2023).
Wen, P. Y. et al. LTBK-06. Impact of vorasidenib treatment on mutant IDH1 or IDH2 diffuse glioma tumor growth rate: results from the randomized, double-blind, phase 3 INDIGO study. Neuro Oncol. 25, v310–v311 (2023).
Preusser, M., Geurts, M., Hainfellner, J. A. & van den Bent, M. J. What is an isocitrate dehydrogenase-mutated central nervous system World Health Organization grade 2 glioma, or who should receive vorasidenib? Neuro Oncol. 25, 1915–1917 (2023).
Reifenberger, G. et al. in Central Nervous System Tumors — Fifth Edition World Health Organization Classification of Tumors, 28–38 (IARC, 2021).
Buckingham, S. C. et al. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 17, 1269–1274 (2011).
Yuen, T. I. et al. Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology 79, 883–889 (2012).
Rudà, R., Bruno, F. & Pellerino, A. Epilepsy in gliomas: recent insights into risk factors and molecular pathways. Curr. Opin. Neurol. 36, 557–563 (2023).
Vo, A. H., Ambady, P. & Spencer, D. The IDH1 inhibitor ivosidenib improved seizures in a patient with drug-resistant epilepsy from IDH1 mutant oligodendroglioma. Epilepsy Behav. Rep. 18, 100526 (2022).
Avila, E. K. et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 19, 12–21 (2017).
Pusch, S. et al. Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of IDH1 mutant astrocytoma in vivo. Acta Neuropathol. 133, 629–644 (2017).
Wick, A. et al. Phase I assessment of safety and therapeutic activity of BAY1436032 in patients with IDH1-mutant solid tumors. Clin. Cancer Res. 27, 2723–2733 (2021).
de Botton, S. et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed or refractory IDH1-mutated AML. Blood Adv. 7, 3117–3127 (2023).
de la Fuente, M. I. et al. Olutasidenib (FT-2102) in patients with relapsed or refractory IDH1-mutant glioma: a multicenter, open-label, phase Ib/II trial. Neuro Oncol. 25, 146–156 (2023).
Machida, Y. et al. A potent blood-brain barrier-permeable mutant IDH1 inhibitor suppresses the growth of glioblastoma with IDH1 mutation in a patient-derived orthotopic xenograft model. Mol. Cancer Ther. 19, 375–383 (2020).
Natsume, A. et al. The first-in-human phase I study of a brain-penetrant mutant IDH1 inhibitor DS-1001 in patients with recurrent or progressive IDH1-mutant gliomas. Neuro Oncol. 25, 326–336 (2023).
Platten, M., Bunse, L. & Wick, W. Emerging targets for anticancer vaccination: IDH. ESMO Open 6, 100214 (2021).
Gallus, M. et al. Immunotherapy approaches in isocitrate-dehydrogenase-mutant low-grade glioma. Cancers 15, 3726 (2023).
Platten, M. et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 592, 463–468 (2021).
Bunse, L. et al. AMPLIFY-NEOVAC: a randomized, 3-arm multicenter phase I trial to assess safety, tolerability and immunogenicity of IDH1-vac combined with an immune checkpoint inhibitor targeting programmed death-ligand 1 in isocitrate dehydrogenase 1 mutant gliomas. Neurol. Res. Pract. 4, 20 (2022).
Turcan, S. et al. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT inhibitor decitabine. Oncotarget 4, 1729–1736 (2013).
Yamashita, A. S. et al. Demethylation and epigenetic modification with 5-azacytidine reduces IDH1 mutant glioma growth in combination with temozolomide. Neuro Oncol. 21, 189–200 (2019).
Montesinos, P. et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N. Engl. J. Med. 386, 1519–1531 (2022).
Sulkowski, P. L. et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med. 9, 2463 (2017).
Mandonnet, E. et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann. Neurol. 53, 524–528 (2003).
Pallud, J. et al. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: a plea for systematic measurement of growth rates. Neurosurgery 71, 729–739 (2012).
Huang, R. Y. et al. Volumetric analysis of IDH-mutant lower-grade glioma: a natural history study of tumor growth rates before and after treatment. Neuro Oncol. 22, 1822–1830 (2020).
Ellingson, B. M. et al. Hypothetical generalized framework for a new imaging endpoint of therapeutic activity in early phase clinical trials in brain tumors. Neuro Oncol. 24, 1219–1229 (2022).
Ellingson, B. M. et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: evidence from a phase I trial of ivosidenib. Neuro Oncol. 24, 770–778 (2022).
Bhatia, A. et al. Tumor volume growth rates and doubling times during active surveillance of IDH-mutant low-grade glioma. Clin. Cancer Res. 30, 106–115 (2024).
Cho, N. S. et al. Early volumetric, perfusion, and diffusion MRI changes after mutant isocitrate dehydrogenase (IDH) inhibitor treatment in IDH1-mutant gliomas. Neurooncol. Adv. 4, vdac124 (2022).
Andronesi, O. C. et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med. 4, 116ra4 (2012).
Pope, W. B. et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J. Neurooncol. 107, 197–205 (2012).
Branzoli, F. et al. Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro Oncol. 20, 907–916 (2018).
Suh, C. H., Kim, H. S., Jung, S. C., Choi, C. G. & Kim, S. J. 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol. 20, 1573–1583 (2018).
de la Fuente, M. I. et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 18, 283–290 (2016).
Choi, C. et al. Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J. Clin. Oncol. 34, 4030–4039 (2016).
Di Stefano, A. L. et al. In vivo 2-hydroxyglutarate monitoring with edited MR spectroscopy for the follow-up of IDH-mutant diffuse gliomas: the IDASPE prospective study. Neurology 100, e94–e106 (2023).
Emir, U. E. et al. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer Res. 76, 43–49 (2016).
Andronesi, O. C. et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat. Commun. 9, 1474 (2018).
Roelcke, U. et al. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol. 18, 744–751 (2016).
Suchorska, B. et al. Identification of time-to-peak on dynamic 18F-FET-PET as a prognostic marker specifically in IDH1/2 mutant diffuse astrocytoma. Neuro Oncol. 20, 279–288 (2018).
Albert, N. L., Furtner, J., van den Bent, M. J. & Preusser, M. The potential of amino acid PET imaging for prediction and monitoring of vorasidenib response in IDH-mutant gliomas. Neuro Oncol. 26, 403–406 (2024).
Albert, N. L. et al. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): a report of the RANO group. Lancet Oncol. 25, 29–41 (2024).
Wollring, M. M. et al. Clinical applications and prospects of PET imaging in patients with IDH-mutant gliomas. J. Neurooncol. 162, 481–488 (2023).
Choi, Y. S. et al. Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro Oncol. 23, 304–313 (2021).
Chakrabarty, S., LaMontagne, P., Shimony, J., Marcus, D. S. & Sotiras, A. MRI-based classification of IDH mutation and 1p/19q codeletion status of gliomas using a 2.5D hybrid multi-task convolutional neural network. Neurooncol. Adv. 5, vdad023 (2023).
Lombardi, G. et al. Diagnostic value of plasma and urinary 2-hydroxyglutarate to identify patients with isocitrate dehydrogenase-mutated glioma. Oncologist 20, 562–567 (2015).
Kalinina, J. et al. Selective detection of the D-enantiomer of 2-hydroxyglutarate in the CSF of glioma patients with mutated isocitrate dehydrogenase. Clin. Cancer Res. 22, 6256–6265 (2016).
Ballester, L. Y. et al. Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol. Commun. 6, 85 (2018).
Fujita, Y. et al. IDH1 p.R132H ctDNA and D-2-hydroxyglutarate as CSF biomarkers in patients with IDH-mutant gliomas. J. Neurooncol. 159, 261–270 (2022).
Riviere-Cazaux, C. et al. Cerebrospinal fluid 2-hydroxyglutarate as a monitoring biomarker for IDH-mutant gliomas. Neurooncol. Adv. 5, vdad061 (2023).
Martínez-Ricarte, F. et al. Molecular diagnosis of diffuse gliomas through sequencing of cell-free circulating tumor DNA from cerebrospinal fluid. Clin. Cancer Res. 24, 2812–2819 (2018).
Zhang, S. et al. Noninvasive detection of brain gliomas using plasma cell-free DNA 5-hydroxymethylcytosine sequencing. Int. J. Cancer 152, 1707–1718 (2023).
Author information
Authors and Affiliations
Contributions
R.R. designed the manuscript, wrote the clinical parts, and reviewed and edited the manuscript before submission. C.H. wrote the sections on oncogenesis in IDH-mutant gliomas and IDH inhibitors in preclinical models. M.v.d.B. wrote the conclusions. M.P. and R.S. reviewed the entire manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.R. has received honoraria for lectures from UCB, Servier, Genenta, Novocure and Curevac. M.v.d.B. reports consulting for Boehringer Ingelheim, F. Hoffman-La Roche, Fore Biotherapeutics, Genenta, Incyte Corporation, AnHeart therapeutics, Mundipharma, SymBio Pharma and Servier Affaires Medicales. M.P. has received honoraria for lectures, consultation or advisory board participation from Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Janssen, Servier, Miltenyi, Böhringer Ingelheim and Telix, Medscape. R.S. has received honoraria for lectures, consultation or advisory board participation from Celldex Therapeutics, Mundipharma, Puma Technology, Astra Zeneca, Servier and Seagen. C.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks T. Jiang, who co-reviewed with K. Zhang; A. Molinaro; K. Lamszus; and J. Miller for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rudà, R., Horbinski, C., van den Bent, M. et al. IDH inhibition in gliomas: from preclinical models to clinical trials. Nat Rev Neurol (2024). https://doi.org/10.1038/s41582-024-00967-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41582-024-00967-7