Abstract
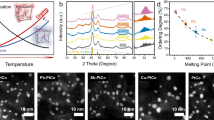
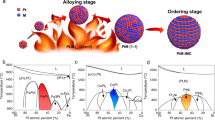
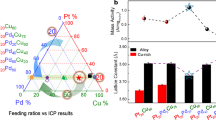
Structurally ordered L10-PtM (M = Fe, Co, Ni and so on) intermetallic nanocrystals, benefiting from the chemically ordered structure and higher stability, are one of the best electrocatalysts used for fuel cells. However, their practical development is greatly plagued by the challenge that the high-temperature (>600 °C) annealing treatment necessary for realizing the ordered structure usually leads to severe particle sintering, morphology change and low ordering degree, which makes it very difficult for the gram-scale preparation of desirable PtM intermetallic nanocrystals with high Pt content for practical fuel cell applications. Here we report a new concept involving the low-melting-point-metal (M′ = Sn, Ga, In)-induced bond strength weakening strategy to reduce Ea and promote the ordering process of PtM (M = Ni, Co, Fe, Cu and Zn) alloy catalysts for a higher ordering degree. We demonstrate that the introduction of M′ can reduce the ordering temperature to extremely low temperatures (≤450 °C) and thus enable the preparation of high-Pt-content (≥40 wt%) L10-Pt-M-M′ intermetallic nanocrystals as well as ten-gram-scale production. X-ray spectroscopy studies, in situ electron microscopy and theoretical calculations reveal the fundamental mechanism of the Sn-facilitated ordering process at low temperatures, which involves weakened bond strength and consequently reduced Ea via Sn doping, the formation and fast diffusion of low-coordinated surface free atoms, and subsequent L10 nucleation. The developed L10-Ga-PtNi/C catalysts display outstanding performance in H2–air fuel cells under both light- and heavy-duty vehicle conditions. Under the latter condition, the 40% L10-Pt50Ni35Ga15/C catalyst delivers a high current density of 1.67 A cm−2 at 0.7 V and retains 80% of the current density after extended 90,000 cycles, which exceeds the United States Department of Energy performance metrics and represents among the best cathodic electrocatalysts for practical proton-exchange membrane fuel cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Fan, J. T. et al. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 6, 475–486 (2021).
Cullen, D. A. et al. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 6, 462–474 (2021).
Luo, M. C. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Yan, Y. C. et al. Intermetallic nanocrystals: syntheses and catalytic applications. Adv. Mater. 29, 1605997 (2017).
Liang, J. S. et al. Atomic arrangement engineering of metallic nanocrystals for energy-conversion electrocatalysis. Joule 3, 956–991 (2019).
Zhou, M., Li, C. & Fang, J. Y. Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications. Chem. Rev. 121, 736–795 (2021).
Wang, D. L. et al. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12, 81–87 (2013).
Bu, L. Z. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016).
Li, Q. et al. New approach to fully ordered fct-FePt nanoparticles for much enhanced electrocatalysis in acid. Nano Lett. 15, 2468–2473 (2015).
Du, X., He, Y., Wang, X. & Wang, J. Fine-grained and fully ordered intermetallic PtFe catalysts with largely enhanced catalytic activity and durability. Energy Environ. Sci. 9, 2623–2632 (2016).
Liang, J. et al. Tungsten-doped L10-PtCo ultrasmall nanoparticles as a high-performance fuel cell cathode. Angew. Chem. Int. Ed. 58, 15471–15477 (2019).
Cheng, Q. et al. High-loaded sub-6 nm Pt1Co1 intermetallic compounds with high-efficient performance expression in PEMFCs. Energy Environ. Sci. 15, 278–286 (2021).
Wang, T. et al. Sub‐6 nm fully ordered L10-Pt-Ni-Co nanoparticles enhance oxygen reduction via Co doping induced ferromagnetism enhancement and optimized surface strain. Adv. Energy Mater. 9, 1803771 (2019).
Porter, D. A. & Easterling, K. E. Phase Transformations in Metals and Alloys (Revised Reprint) (CRC Press, 2009).
Zhou, J. H. et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 570, 500–503 (2019).
Alloyeau, D. et al. Size and shape effects on the order-disorder phase transition in CoPt nanoparticles. Nat. Mater. 8, 940–946 (2009).
Yang, C. L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Chen, M. et al. High-platinum-content catalysts on atomically dispersed and nitrogen coordinated single manganese site carbons for heavy-duty fuel cells. J. Electrochem. Soc. 169, 034510 (2022).
Zeng, Y. et al. Regulating catalytic properties and thermal stability of Pt and PtCo intermetallic fuel-cell catalysts via strong coupling effects between single-metal site-rich carbon and Pt. J. Am. Chem. Soc. 145, 17643–17655 (2023).
Sassin, M., Garsany, Y., Atkinson, R. III, Hjelm, R. & Swider-Lyons, K. Understanding the interplay between cathode catalyst layer porosity and thickness on transport limitations en route to high-performance PEMFCs. Int. J. Hydrogen Energy 44, 16944–16955 (2019).
Tang, M., Zhang, S. & Chen, S. Pt utilization in proton exchange membrane fuel cells: structure impacting factors and mechanistic insights. Chem. Soc. Rev. 51, 1529–1546 (2022).
Li, J. et al. Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 140, 2926–2932 (2018).
Liu, X. et al. Introducing electron buffers into intermetallic Pt alloys against surface polarization for high-performing fuel cells. J. Am. Chem. Soc. 146, 2033–2042 (2024).
Kim, H. Y. et al. Self-supported mesostructured Pt-based bimetallic nanospheres containing an intermetallic phase as ultrastable oxygen reduction electrocatalysts. Small 12, 5347–5353 (2016).
Zhao, X. et al. High-performance nitrogen-doped intermetallic PtNi catalyst for the oxygen reduction reaction. ACS Catal. 10, 10637–10645 (2020).
Kim, H. Y. et al. Intermetallic PtCu nanoframes as efficient oxygen reduction electrocatalysts. Nano Lett. 20, 7413–7421 (2020).
Gong, M. X. et al. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction. Appl. Catal. B 282, 119617 (2021).
Zhao, X. R. et al. Rhombohedral ordered intermetallic nanocatalyst boosts the oxygen reduction reaction. ACS Catal. 11, 184–192 (2021).
Qi, Z. Y. et al. Sub-4 nm PtZn intermetallic nanoparticles for enhanced mass and specific activities in catalytic electrooxidation reaction. J. Am. Chem. Soc. 139, 4762–4768 (2017).
Liang, J. S. et al. Biaxial strains mediated oxygen reduction electrocatalysis on Fenton reaction resistant L10-PtZn fuel cell cathode. Adv. Energy Mater. 10, 2000179 (2020).
Han, A. J. et al. Isolating contiguous Pt atoms and forming Pt-Zn intermetallic nanoparticles to regulate selectivity in 4-nitrophenylacetylene hydrogenation. Nat. Commun. 10, 3787 (2019).
Li, J. et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule 3, 124–135 (2019).
Zhang, S. et al. Tuning nanoparticle structure and surface strain for catalysis optimization. J. Am. Chem. Soc. 136, 7734–7739 (2014).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2, 454–460 (2010).
Luo, M. C. & Guo, S. J. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
Li, M. et al. Lavender-like Ga-doped Pt3Co nanowires for highly stable and active electrocatalysis. ACS Catal. 10, 3018–3026 (2020).
Chong, L. et al. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Zhao, Z. P. et al. Tailoring a three-phase microenvironment for high-performance oxygen reduction reaction in proton exchange membrane fuel cells. Matter 3, 1774–1790 (2020).
Kissinger, H. E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl Bur. Stand. 57, 217–221 (1956).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396–1396 (1997).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Heyden, A., Bell, A. T. & Keil, F. J. Efficient methods for finding transition states in chemical reactions: comparison of improved dimer method and partitioned rational function optimization method. J. Chem. Phys. 123, 224101 (2005).
Acknowledgements
This work is supported by the National Key Research and Development Program of China (no. 2021YFA1501001, to S.G. and Q.L.), National Nature Science Foundation of China (grants 22122202 and 21972051, to Q.L.; grant 52025133, to S.G.; grant 22209061, to Y.W.), Start-up Fund for Senior Talents in Jiangsu University (21JDG060, to Y.W.), NSF-PREM program (grant DMR-1828019, to G.L.) and New Cornerstone Science Foundation through the XPLORER PRIZE (S.G.). In situ STEM experiments were carried out on Hitachi HF-5000; we appreciate the help from H. Matsumoto from Hitachi. We thank the Analytical and Testing Center of Huazhong University of Science and Technology (HUST) for carrying out the TEM, DSC, X-ray fluorescence and XRD measurements. The X-ray adsorption spectroscopy experiments were performed at BL11B beamline, Shanghai.
Author information
Authors and Affiliations
Contributions
Q.L. and S.G. conceived the idea and designed the experiments. J. Liang, X.L., S.L., F.L. and Y.S. carried out the sample synthesis, characterization and measurements. H.L. and G.W. performed the in situ XRD measurements. J.X., Z.D. and J.H. performed the pair distribution function tests. Y.W., J. Liu and G.L. provided the theoretical calculations. J. Liang, X.L. and S.L. performed the fuel cell measurements. J. Liang, Q.L., Y.H. and S.G. wrote and revised the paper. All authors contributed to the overall scientific discussion and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
Q.L. and J. Liang hold a Chinese patent through Huazhong University of Science and Technology (Chinese patent no. ZL202110438703.2) on the technology related to the synthesis of Pt-based intermetallic catalysts by low-melting-point-metal (Ga, Sn, In) doping. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–61, Tables 1–6 and Notes 1–6.
Supplementary Video 1
Phase transition process of Pt50Ni35Sn15 NC recorded by in-situ STEM at 480 °C
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, J., Wan, Y., Lv, H. et al. Metal bond strength regulation enables large-scale synthesis of intermetallic nanocrystals for practical fuel cells. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01901-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-01901-4