Abstract
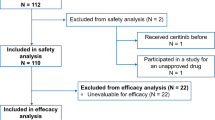
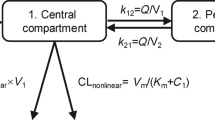
This study aimed to analyze potential ethnic disparities in the dose–exposure–response relationships of trilaciclib, a first-in-class intravenous cyclin-dependent kinase 4/6 inhibitor for treating chemotherapy-induced myelosuppression in patients with extensive-stage small cell lung cancer (ES-SCLC). This investigation focused on characterizing these relationships in both Chinese and non-Chinese patients to further refine the dosing regimen for trilaciclib in Chinese patients with ES-SCLC. Population pharmacokinetic (PopPK) and exposure–response (E–R) analyses were conducted using pooled data from four randomized phase 2/3 trials involving Chinese and non-Chinese patients with ES-SCLC. PopPK analysis revealed that trilaciclib clearance in Chinese patients was approximately 17% higher than that in non-Chinese patients with ES-SCLC. Sex and body surface area influenced trilaciclib pharmacokinetics in both populations but did not exert a significant clinical impact. E–R analysis demonstrated that trilaciclib exposure increased with a dosage escalation from 200 to 280 mg/m2, without notable changes in myeloprotective or antitumor efficacy. However, the incidence of infusion site reactions, headaches, and phlebitis/thrombophlebitis rose with increasing trilaciclib exposure in both Chinese and non-Chinese patients with ES-SCLC. These findings suggest no substantial ethnic disparities in the dose–exposure–response relationship between Chinese and non-Chinese patients. They support the adoption of a 240-mg/m2 intravenous 3-day or 5-day dosing regimen for trilaciclib in Chinese patients with ES-SCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dhillon S. Trilaciclib: first approval. Drugs. 2021;81:867–74.
Zugazagoitia J, Paz-Ares L. Extensive-stage small-cell lung cancer: first-line and second-line treatment options. J Clin Oncol. 2022;40:671–80.
He S, Roberts PJ, Sorrentino JA, Bisi JE, Storrie-White H, Tiessen RG, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9:eaal3986.
Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC. Preclinical characterization of G1T28: a novel CDK4/6 inhibitor for reduction of chemotherapy-induced myelosuppression. Mol Cancer Ther. 2016;15:783–93.
Lai AY, Sorrentino JA, Dragnev KH, Weiss JM, Owonikoko TK, Rytlewski JA, et al. CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J Immunother Cancer. 2020;8:e000847.
George MA, Qureshi S, Omene C, Toppmeyer DL, Ganesan S. Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front Oncol. 2021;11:693104.
Li C, Rich B, Bullock JM, Barriere O, Marier JF, Beelen A. Population pharmacokinetics and exposure-response of trilaciclib in extensive-stage small cell lung cancer and triple-negative breast cancer. Br J Clin Pharmacol. 2023;89:1067–79.
Li C, Horton JK, Sale M, Curd L, Goti V, Tao W, et al. Pharmacokinetic drug-drug interaction studies between trilaciclib and midazolam, metformin, rifampin, itraconazole, and topotecan in healthy volunteers and patients with extensive-stage small-cell lung cancer. Clin Drug Invest. 2022;42:679–92.
Hart LL, Ferrarotto R, Andric ZG, Beck JT, Subramanian J, Radosavljevic DZ, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: results from a randomized, double-blind, placebo-controlled phase II study. Adv Ther. 2021;38:350–65.
Daniel D, Kuchava V, Bondarenko I, Ivashchuk O, Reddy S, Jaal J, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: a multicentre, randomised, double-blind, placebo-controlled Phase II trial. Int J Cancer. 2020;148:2557–70.
Weiss JM, Csoszi T, Maglakelidze M, Hoyer RJ, Beck JT, Domine Gomez M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30:1613–21.
Qiu J, Sheng D, Lin F, Jiang P, Shi N. The efficacy and safety of trilaciclib in preventing chemotherapy-induced myelosuppression: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2023;14:1157251.
Abraham I, Goyal A, Deniz B, Moran D, Chioda M, MacDonald KM, et al. Budget impact analysis of trilaciclib for decreasing the incidence of chemotherapy-induced myelosuppression in patients with extensive-stage small cell lung cancer in the United States. J Manag Care Spec Pharm. 2022;28:435–48.
Weiss J, Goldschmidt J, Andric Z, Dragnev KH, Gwaltney C, Skaltsa K, et al. Effects of trilaciclib on chemotherapy-induced myelosuppression and patient-reported outcomes in patients with extensive-stage small cell lung cancer: pooled results from three Phase II randomized, double-blind, placebo-controlled studies. Clin Lung Cancer. 2021;22:449–60.
Hussein M, Maglakelidze M, Richards DA, Sabatini M, Gersten TA, Lerro K, et al. Myeloprotective effects of trilaciclib among patients with small cell lung cancer at increased risk of chemotherapy-induced myelosuppression: pooled results from three phase 2, randomized, double-blind, placebo-controlled studies. Cancer Manag Res. 2021;13:6207–18.
Ferrarotto R, Anderson I, Medgyasszay B, Garcia-Campelo MR, Edenfield W, Feinstein TM, et al. Trilaciclib prior to chemotherapy reduces the usage of supportive care interventions for chemotherapy-induced myelosuppression in patients with small cell lung cancer: Pooled analysis of three randomized phase 2 trials. Cancer Med. 2021;10:5748–56.
Domine Gomez M, Csoszi T, Jaal J, Kudaba I, Nikolov K, Radosavljevic D, et al. Exploratory composite endpoint demonstrates benefit of trilaciclib across multiple clinically meaningful components of myeloprotection in patients with small cell lung cancer. Int J Cancer. 2021;149:1463–72.
Abraham I, Onyekwere U, Deniz B, Moran D, Chioda M, MacDonald K, et al. Trilaciclib and the economic value of multilineage myeloprotection from chemotherapy-induced myelosuppression among patients with extensive-stage small cell lung cancer treated with first-line chemotherapy. J Med Econ. 2021;24:71–83.
Cheng Y, Wu L, Huang DZ, Wang QM, Fan Y, Liu ZF, et al. EP08.02-078 myeloprotection with trilaciclib in Chinese patients with extensive-stage small cell lung cancer receiving standard chemotherapy (TRACES). J Thorac Oncol. 2022;17:S437.
Liu Y, Wu L, Huang D, Wang Q, Fan Y, Liu Z, et al. 1998P antitumor activity and safety profile of trilaciclib in Chinese patients with extensive-stage small cell lung cancer (ES-SCLC) receiving chemotherapy (TRACES): updated results from TRACES. Ann Oncol 2023;34:S1066.
Population Pharmacokinetics: Guidance for Industry. 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics.
Guideline on reporting the results of population pharmacokinetic analyses. 2007. https://www.ema.europa.eu/en/reporting-results-population-pharmacokinetic-analyses-scientific-guideline.
Ruiz-Garcia A, Baverel P, Bottino D, Dolton M, Feng Y, Gonzalez-Garcia I, et al. A comprehensive regulatory and industry review of modeling and simulation practices in oncology clinical drug development. J Pharmacokinet Pharmacodyn 2023;50:147–72.
Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11:371–80.
Irby DJ, Ibrahim ME, Dauki AM, Badawi MA, Illamola SM, Chen M, et al. Approaches to handling missing or “problematic” pharmacology data: pharmacokinetics. CPT Pharmacomet Syst Pharmacol. 2021;10:291–308.
Xu XS, Yuan M, Karlsson MO, Dunne A, Nandy P, Vermeulen A. Shrinkage in nonlinear mixed-effects population models: quantification, influencing factors, and impact. AAPS J. 2012;14:927–36.
Ramalingam SS, Kummar S, Sarantopoulos J, Shibata S, LoRusso P, Yerk M, et al. Phase I study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. J Clin Oncol 2010;28:4507–12.
Paglialunga S, Offman E, Ichhpurani N, Marbury TC, Morimoto BH. Update and trends on pharmacokinetic studies in patients with impaired renal function: practical insight into application of the FDA and EMA guidelines. Expert Rev Clin Pharmacol 2017;10:273–83.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Ahamadi M, Largajolli A, Diderichsen PM, de Greef R, Kerbusch T, Witjes H, et al. Operating characteristics of stepwise covariate selection in pharmacometric modeling. J Pharmacokinet Pharmacodyn. 2019;46:273–85.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther 2007;82:17–20.
Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharmacol Res. 2007;24:2187–97.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;2:e38.
Mao JH, Han L, Liu XQ, Jiao Z. Significant predictors for olanzapine pharmacokinetics: a systematic review of population pharmacokinetic studies. Expert Rev Clin Pharmacol. 2023;16:575–88.
Qin Y, Zhang LL, Ye YR, Chen YT, Jiao Z. Parametric population pharmacokinetics of linezolid: a systematic review. Br J Clin Pharmacol. 2022;88:4043–66.
Li ZR, Wang CY, Zhu X, Jiao Z. Population pharmacokinetics of levetiracetam: a systematic review. Clin Pharmacokinet. 2021;60:305–18.
Chen YT, Wang CY, Yin YW, Li ZR, Lin WW, Zhu M, et al. Population pharmacokinetics of oxcarbazepine: a systematic review. Expert Rev Clin Pharmacol. 2021;14:853–64.
Exposure-response relationships — study design, data analysis, and regulatory applications. 2003. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/exposure-response-relationships-study-design-data-analysis-and-regulatory-applications.
Guideline for exposure-response analysis of drugs. 2020. https://www.pmda.go.jp/files/000235605.pdf.
Li C, Hart L, Owonikoko TK, Aljumaily R, Rocha Lima CM, Conkling PR, et al. Trilaciclib dose selection: an integrated pharmacokinetic and pharmacodynamic analysis of preclinical data and Phase Ib/IIa studies in patients with extensive-stage small cell lung cancer. Cancer Chemother Pharmacol. 2021;87:689–700.
Iwata H, Umeyama Y, Liu Y, Zhang Z, Schnell P, Mori Y, et al. Evaluation of the association of polymorphisms with palbociclib-induced neutropenia: pharmacogenetic analysis of PALOMA-2/-3. Oncologist. 2021;26:e1143–e55.
Sathe C, Accordino MK, DeStephano D, Shah M, Wright JD, Hershman DL. Social determinants of health and CDK4/6 inhibitor use and outcomes among patients with metastatic breast cancer. Breast Cancer Res Treat. 2023;200:85–92.
Whitaker KD, Wang X, Ascha M, Showalter TN, Lewin HG, Calip GS, et al. Racial inequities in second-line treatment and overall survival among patients with metastatic breast cancer. Breast Cancer Res Treat. 2022;196:163–73.
Schreier A, Munoz-Arcos L, Alvarez A, Sparano JA, Anampa JD. Racial disparities in neutrophil counts among patients with metastatic breast cancer during treatment with CDK4/6 inhibitors. Breast Cancer Res Treat. 2022;194:337–51.
Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D’Amico TA, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:1441–64.
Dingemans AC, Fruh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32:839–53.
Acknowledgements
We would like to thank all the subjects in these trials. Special thanks go to Dr. Jun-jie Ding from University of Oxford and Dr. Liang Li from Gracell Biotechnologies Group for their valuable comments. We thank Editage (www.editage.cn) for English language editing. This study was sponsored by Simcere Zaiming Pharmaceutical Co. Ltd.
Author information
Authors and Affiliations
Contributions
HRD: Methodology, Software, Formal analysis, Validation, Visualization, Writing–Original Draft, and Writing–Review and Editing; YY: Methodology, Investigation, Data curation, Formal analysis, Validation, Supervision, Writing–Original Draft, and Writing–Review and Editing; CYW: Methodology, Software, Validation, Visualization, Investigation, Writing–Original Draft, and Writing–Review and Editing; YTC: Methodology, Software, Investigation, and Writing–Review and Editing; YFC: Methodology, Software, Investigation; PJL: Investigation, Data curation, Supervision, and Writing–Review and Editing; JC: Investigation, Supervision, and Writing–Review and Editing; CY: Supervision, and Writing–Review and Editing; ZJ: Conceptualization, Supervision, Methodology, Writing–Original Draft, and Writing–Review and Editing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
YY, PJL, JC, and CY are employees of Simcere Zaiming Pharmaceutical Co. Ltd. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Hr., Yang, Y., Wang, Cy. et al. Trilaciclib dosage in Chinese patients with extensive-stage small cell lung cancer: a pooled pharmacometrics analysis. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01297-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01297-6