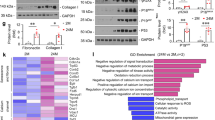
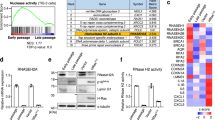
Abstract
Senescence, an intricate and inevitable biological process, characterized by the gradual loss of homeostasis and declining organ functions. The pathological features of cellular senescence, including cell cycle arrest, metabolic disruptions, and the emergence of senescence-associated secretory phenotypes (SASP), collectively contribute to the intricate and multifaceted nature of senescence. Beyond its classical interaction with p53, murine double minute gene 2 (MDM2), traditionally known as an E3 ubiquitin ligase involved in protein degradation, plays a pivotal role in cellular processes governing senescence. Histone deacetylase (HDAC), a class of histone deacetylases mainly expressed in the nucleus, has emerged as a critical contributor to renal tissues senescence. In this study we investigated the interplay between MDM2 and HDAC1 in renal senescence. We established a natural aging model in mice over a 2-year period that was verified by SA-β-GAL staining and increased expression of senescence-associated markers such as p21, p16, and TNF-α in the kidneys. Furthermore, we showed that the expression of MDM2 was markedly increased, while HDAC1 expression underwent downregulation during renal senescence. This phenomenon was confirmed in H2O2-stimulated HK2 cells in vitro. Knockout of renal tubular MDM2 alleviated renal senescence in aged mice and in H2O2-stimulated HK2 cells. Moreover, we demonstrated that MDM2 promoted renal senescence by orchestrating the ubiquitination and subsequent degradation of HDAC1. These mechanisms synergistically accelerate the aging process in renal tissues, highlighting the intricate interplay between MDM2 and HDAC1, underpinning the age-related organ function decline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Childs BG, Durik M, Baker DJ, Van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–35.
Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–96.
Sturmlechner I, Durik M, Sieben CJ, Baker DJ, Van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol. 2017;13:77–89.
Noodén LD, Guiamét JJ, John I. Senescence mechanisms. Physiol Plant. 1997;101:746–53.
Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238–46.
Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10:228–30.
Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–76.
Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1:993–1000.
Wiley CD, Schaum N, Alimirah F, Lopez-Dominguez JA, Orjalo AV, Scott G, et al. Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Sci Rep. 2018;8:2410.
Mulay SR, Thomasova D, Ryu M, Anders H-J. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012;81:1199–211.
Wu D, Prives C. Relevance of the p53–MDM2 axis to aging. Cell Death Differ. 2018;25:169–79.
Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Trends Cell Biol. 2021;31:298–310.
Piette J, Neel H, Maréchal V. Mdm2: keeping p53 under control. Oncogene. 1997;15:1001–10.
Bhattacharya S, Chaum E, Johnson DA, Johnson LR. Age-related susceptibility to apoptosis in human retinal pigment epithelial cells is triggered by disruption of p53–Mdm2 association. Invest Ophthalmol Vis Sci. 2012;53:8350–66.
Ye C, Tang H, Zhao Z, Lei CT, You CQ, Zhang J, et al. MDM2 mediates fibroblast activation and renal tubulointerstitial fibrosis via a p53-independent pathway. Am J Physiol Ren Physiol. 2017;312:F760–F8.
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49.
Willis-Martinez D, Richards HW, Timchenko NA, Medrano EE. Role of HDAC1 in senescence, aging, and cancer. Exp Gerontol. 2010;45:279–85.
Liu M, Zhang Y, Zhan P, Sun W, Dong C, Liu X, et al. Histone deacetylase 9 exacerbates podocyte injury in hyperhomocysteinemia through epigenetic repression of Klotho. Pharmacol Res. 2023;198:107009.
Guo J, Wang Z, Wu J, Liu M, Li M, Sun Y, et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3. 2-GATA5 signaling. Circ Res. 2019;124:1448–61.
Rangel PXM, Cross E, Liu C, Pedigo CE, Tian X, Gutiérrez-Calabrés E, et al. Cell cycle and senescence regulation by podocyte histone deacetylase 1 and 2. J Am Soc Nephrol. 2023;34:433–50.
Inoue K, Gan G, Ciarleglio M, Zhang Y, Tian X, Pedigo CE, et al. Podocyte histone deacetylase activity regulates murine and human glomerular diseases. J Clin Invest. 2019;129:1295–313.
Wang G-L, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 cooperates with C/EBPα in the inhibition of liver proliferation in old mice. J Biol Chem. 2008;283:26169–78.
Pasyukova EG, Vaiserman AM. HDAC inhibitors: a new promising drug class in anti-aging research. Mech Ageing Dev. 2017;166:6–15.
Kwon D-H, Eom GH, Ko JH, Shin S, Joung H, Choe N, et al. MDM2 E3 ligase-mediated ubiquitination and degradation of HDAC1 in vascular calcification. Nat Commun. 2016;7:10492.
Itahana K, Dimri G, Campisi J. Regulation of cellular senescence by p53. Eur J Biochem. 2001;268:2784–91.
Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. Role of p53 in the regulation of cellular senescence. Biomolecules. 2020;10:420.
Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–15.
Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–8.
Ganguli G, Wasylyk B. p53-independent functions of MDM2. Mol Cancer Res. 2003;1:1027–35.
Baboota RK, Rawshani A, Bonnet L, Li X, Yang H, Mardinoglu A, et al. BMP4 and Gremlin 1 regulate hepatic cell senescence during clinical progression of NAFLD/NASH. Nat Metab. 2022;4:1007–21.
Liu B, Yi J, Yang X, Liu L, Lou X, Zhang Z, et al. MDM2-mediated degradation of WRN promotes cellular senescence in a p53-independent manner. Oncogene. 2019;38:2501–15.
Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 2017;8:1–15.
Wang X, Liu J, Zhen J, Zhang C, Wan Q, Liu G, et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014;86:712–25.
Shi W, Wei X, Wang Z, Han H, Fu Y, Liu J, et al. HDAC 9 exacerbates endothelial injury in cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2016;20:1139–49.
Zhang Y, Yang Y, Yang F, Liu X, Zhan P, Wu J, et al. HDAC9-mediated epithelial cell cycle arrest in G2/M contributes to kidney fibrosis in male mice. Nat Commun. 2023;14:3007.
Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, et al. MDM2–HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21:6236–45.
Minamoto T, Buschmann T, Habelhah H, Matusevich E, Tahara H, Boerresen-Dale AL, et al. Distinct pattern of p53 phosphorylation in human tumors. Oncogene. 2001;20:3341–7.
Vaziri H, Dessain SK, Eaton EN, Imai SI, Frye RA, Pandita TK, et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59.
Wang D, Kon N, Lasso G, Jiang L, Leng W, Zhu WG, et al. Acetylation-regulated interaction between p53 and SET reveals a widespread regulatory mode. Nature. 2016;538:118–22.
Luo J, Nikolaev AY, Imai SI, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–48.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (82370728, 81974096, 82202911, 81800610 and 82170773), the National Key Research and Development Program of China (2021YFC2500200), and the Key Research and Development Program of Hubei Province (2023BCB034).
Author information
Authors and Affiliations
Contributions
CZ and HLX designed the experiments. HLX performed the experiments and wrote the manuscript. QY reviewed and edited the manuscript. JYZ, ZYX, and HZZ collected the data. JH, ANS and JX analyzed the data. All the authors made editorial suggestions and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, Hl., Yuan, Q., Zeng, Jy. et al. MDM2 accelerated renal senescence via ubiquitination and degradation of HDAC1. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01294-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01294-9