Abstract
Background
We previously reported that hydrogen (H2) gas combined with therapeutic hypothermia (TH) improved short-term neurological outcomes in asphyxiated piglets. However, the effect on seizure burden was unclear. Using amplitude-integrated electroencephalography (aEEG), we compared TH + H2 with TH alone in piglets 24 h after hypoxic–ischemic (HI) insult.
Methods
After a 40-min insult and resuscitation, 36 piglets ≤24 h old were divided into three groups: normothermia (NT, n = 14), TH alone (33.5 ± 0.5 °C, 24 h, n = 13), and TH + H2 (2.1–2.7% H2 gas, 24 h, n = 9). aEEG was recorded for 24 h post-insult and its background pattern, status epilepticus (SE; recurrent seizures lasting >5 min), and seizure occurrence (Sz; occurring at least once but not fitting the definition of SE) were evaluated. Background findings with a continuous low voltage and burst suppression were considered abnormal.
Results
The percentage of piglets with an abnormal aEEG background (aEEG-BG), abnormal aEEG-BG+Sz and SE was lower with TH + H2 than with TH at 24 h after HI insult. The duration of SE was shorter with TH + H2 and significantly shorter than with NT.
Conclusions
H2 gas combined with TH ameliorated seizure burden 24 h after HI insult.
Impact
-
In this asphyxiated piglet model, there was a high percentage of animals with an abnormal amplitude-integrated electroencephalography background (aEEG-BG) after hypoxic-ischemic (HI) insult, which may correspond to moderate and severe hypoxic-ischemic encephalopathy (HIE).
-
Therapeutic hypothermia (TH) was associated with a low percentage of piglets with EEG abnormalities up to 6 h after HI insult but this percentage increased greatly after 12 h, and TH was not effective in attenuating seizure development.
-
H2 gas combined with TH was associated with a low percentage of piglets with an abnormal aEEG-BG and with a shorter duration of status epilepticus at 24 h after HI insult.
Similar content being viewed by others
Introduction
Perinatal hypoxic–ischemic encephalopathy (HIE) occurs in 1–3% of term or near-term births as a result of hypoxic and/or ischemic insults during labor and delivery.1,2,3 Outcome improvements have been found in neonates with HIE with therapeutic hypothermia (TH) in three randomized controlled studies.1,2,4 However, hypothermia was insufficient to avert death or moderate-to-severe neurodevelopmental disabilities in more than 45% of patients. To further improve outcomes, we need to develop a new therapeutic strategy involving TH.
HIE continues to be a common cause of seizures in neonates.5,6 Prior to the introduction of TH, electrographic seizures were reported in more than 50% of neonates with HIE, status epilepticus (SE) was a frequent occurrence, and the overall seizure burden was high.7 Seizure burden is consistently associated with both brain injury and adverse neurodevelopmental outcomes,8,9,10 although it is unclear if seizures independently contribute to brain damage after HIE or merely reflect the underlying evolution of the injury resulting from the hypoxic–ischemia.
Previously, Ohsawa et al.11 reported the neuroprotective properties of molecular hydrogen while Domoki et al.12 determined that hydrogen (H2) gas ventilation ameliorated the neurologic deficits associated with perinatal asphyxia using a piglet model. As an adjunct to TH, we reported the neuroprotective potential of combined TH and H2 gas ventilation (TH + H2) via an assessment of short-term neurological outcomes and histological findings in 5-day neonatal HIE piglets.13 As one of the possible explanations for this brain protective mechanism, we have already reported improvements in cerebral hemodynamics and oxygenation.14 However, it remains unclear how H2 gas combined with TH influences electrocortical activity and seizure burden.
We hypothesized that H2 gas combined with TH would reduce seizure burden more than TH alone in asphyxiated piglets. We thus examined the percentage of piglets with an abnormal amplitude-integrated electroencephalography (aEEG) background pattern or SE and the duration of SE in the first 24 h after HI insult in three treatment groups: a normothermia (NT) group, TH group, and TH + H2 gas group.
Methods
Animal procedures
Thirty-six newborn piglets (Camborough; Daiwa Chikusan, Kagawa, Japan) within 24 h of birth and weighing 1.5–2.1 kg were obtained for the study and divided into the three treatment groups: HI insult treated with NT (NT group, n = 14), HI insult treated with TH (TH group, n = 13), and HI insult treated with TH and H2 gas ventilation (TH + H2 group, n = 9).
Anesthesia, ventilation, and monitoring of physiological variables
The piglets were initially anesthetized with 1–2% isoflurane in air using a facemask. Each piglet was then intubated and mechanically ventilated using an infant ventilator. The umbilical vein and artery were cannulated using a neonatal umbilical catheter for drip infusion and blood pressure monitoring/blood sampling, respectively. After cannulation, pancuronium bromide was used at an initial dose of 0.1 mg/kg, followed by infusion at 0.1 mg/kg/h to induce paralysis. Fentanyl citrate was then administered at an initial dose of 10 μg/kg, followed by infusion at 5 μg/kg/h for anesthesia. A maintenance solution of electrolytes plus 2.7% glucose (KN3B; Otsuka Pharmaceutical Co., Tokyo, Japan) was continuously infused at a rate of 4 mL/kg/h via the umbilical vein. Arterial blood samples were taken throughout the experiment at critical time points and when clinically indicated. Each piglet was then placed under a radiant warmer to maintain a mean ± standard deviation (SD) rectal temperature of 39.0 ± 0.5 °C. The inspired gas was prepared by mixing oxygen and nitrogen (N2) gases to obtain the oxygen concentrations required for the experiment. Ventilation was adjusted to maintain arterial oxygen tension (PaO2) and arterial carbon dioxide tension within their normal ranges.
Amplitude-integrated electroencephalography
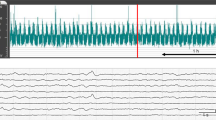
Neural activity was measured by aEEG (Nicolet One; Cardinal Health, Inc., Dublin, OH). All electrical devices and the copper mesh shield were grounded. The signal was displayed on a semi-logarithmic scale at a low speed (6 cm/h). Measurements were conducted every second. Gold-plated electrode needles were placed at the P3 and P4 positions, which corresponded to the left and right parietal regions of the head. A maximum amplitude <5 µV was defined as low-amplitude EEG (LAEEG) (Fig. 1a). As shown in Fig. 1b, a minimum amplitude >5 µV and a maximum amplitude >10 µV were defined as continuous normal voltage, whereas a minimum amplitude of 3–5 µV and a maximum amplitude >10 µV were defined as discontinuous normal voltage. Traces with continuous normal voltage or discontinuous normal voltage backgrounds were classified as normal aEEG background (aEEG-BG). A maximum amplitude of 5–10 µV was defined as continuous low voltage. A minimum amplitude <5 µV and a maximum amplitude >25 µV were defined as burst suppression. Recurrent seizures lasting more than 5 min were defined as SE, in the same way as in humans.15 Traces with LAEEG, continuous low voltage, burst suppression, and SE backgrounds were classified as abnormal aEEG-BG. We defined “Sz” as any seizure that occurred at least once but did not fit the definition of SE from the previous evaluation period to the current evaluation period (e.g., if single seizure(s) occurred from 3 to 6 h after HI insult, the evaluation at 6 h was considered to be Sz). Using the above evaluation method, the percentage of piglets with an abnormal aEEG-BG, aEEG-BG+Sz, and SE at 24 h after HI insult and the duration of SE (Fig. 1c) were examined in each group. All EEG findings were assessed after the experiment by a single rater (Y.S.) who did not have information about the intervention. In addition, all seizures were confirmed by raw EEG.
Representative example of aEEG at the initial phase of HI insult and LAEEG (a), aEEG background patterns of piglets (b), and representative example of aEEG with SE within 24 h after HI insult (c). a A maximum amplitude <5 µV was defined as LAEEG. The green zone indicates the HI insult, the red line indicates the start of resuscitation (Res), and the blue zone indicates LAEEG. b Normal aEEG background (aEEG-BG): continuous normal voltage and discontinuous normal voltage. Abnormal aEEG-BG: continuous low voltage, burst suppression, and status epilepticus (SE). c Convulsions lasting more than 5 min were defined as SE (orange zone).
Hypoxic–ischemic insult protocol
Hypoxia was induced at least 120 min after induction of anesthesia by decreasing the fractional concentration of inspired oxygen (FiO2) to 0.04. The hypoxic insult was continued for 30 min. The FiO2 was decreased (in 0.01 decrements) to a minimum of 0.02 or increased (in 0.01 increments) during the insult to maintain the LAEEG at <5 μV, heart rate (HR) at >130 beats/min, and mean arterial pressure (MAP) at >60% of baseline. When the criteria for LAEEG, HR, or MAP were satisfied during the first 20 min of the insult, the FiO2 was returned to 0.04. For the final 10 min of the 30-min insult, hypotension was induced by decreasing the FiO2 until the MAP decreased to below 60% of baseline. The criteria for resuscitation after the first 20 min of the insult were as follows: if the cerebral blood volume (CBV) value dropped below 33% during the insult, the insult was stopped and resuscitation was started (change in CBV during insult = [value of CBV at end of insult − value of CBV before insult]/[maximum value of CBV during insult − value of CBV before insult] × 100 [%]), even if the MAP was not maintained below 60% of baseline for 10 min.
Hypoxia was terminated by resuscitation with 100% oxygen. NaHCO3 was used to correct a base deficit (base excess below −5.0 mEq/L) to maintain a pH of 7.3–7.5. After 10 min of 100% FiO2, the ventilator rate and FiO2 were gradually reduced to maintain an SpO2 of 95–98%. We measured blood gas, glucose, lactate, and hemoglobin levels using a blood gas analyzer (ABL90 FLEX PLUS; Radiometer Co., Ltd., Copenhagen, Denmark).
Post-insult treatment
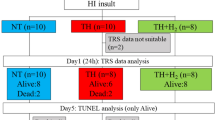
After the HI insult, whole-body hypothermia was induced after resuscitation in the TH and TH + H2 groups by using a cooling blanket (Medicool; MAC8 Inc., Tokyo, Japan) (Fig. 2). The piglets were cooled to 33.5 ± 0.5 °C for 24 h and then rewarmed at 1 °C/h using a blanket. Rectal temperature was used as the body temperature. The temperature of the incubator was maintained at 28–32 °C. For H2 inhalation, two types of cylinders were used, one containing a gas mixture comprising 3.8% H2 and 96.2% N2, and the other containing 100% O2, as in a previous study. The H2 concentration depended on the oxygen requirement of each piglet and was usually between 2.1 and 2.7 (FiO2 range, 0.21–0.4) during the therapy. H2 gas was delivered through the ventilator for 24 h. The concentration of H2 gas was measured by a portable gas monitor (TP-70D; Riken Keiki Co., Ltd., Tokyo, Japan). After 24 h of treatment, the H2–N2 gas mixture was replaced with compressed air again. For piglets given TH, their temperature was automatically controlled to maintain the target temperature (rectal temperature, 33–34 °C) during TH and rewarmed at 1 °C/h by a cooling blanket. The anesthesia was stopped at the beginning of the rewarming period. For NT piglets, the rectal temperature was monitored continuously to maintain a normal range (38–39 °C) under the radiant warmer under anesthesia–ventilation for 24 h after insult.
Piglets were assigned to the NT (n = 14), TH (n = 13), or TH + H2 (n = 9) groups. Within 24 h after insult, all piglets in the NT and TH + H2 groups had survived, but one piglet had died in the TH group. Thus, aEEG analysis was performed in 14 piglets in the NT group, 12 piglets in the TH group, and 9 piglets in the TH + H2 group.
Data analysis
GraphPad Prism 9.3.1 (GraphPad Software, La Jolla, CA) was used for all statistical analyses. All values are expressed as the mean ± SD for physiological and blood gas data after insult in the TH and TH + H2 groups. Physiological data, blood gas data, and measurement of HR, MAP, and the percentage of piglets with an abnormal aEEG-BG, aEEG-BG+Sz, and SE were compared among the three groups at each time point using two-way repeated-measures analysis of variance (ANOVA) followed by Tukey’s post hoc analysis. The duration of SE was compared among the three groups at 24 h after the HI insult using two-way repeated-measures ANOVA followed by Dunnett’s post hoc analysis. A p value < 0.05 was considered significant.
Results
The mean ± SD body weights were 1768 ± 128 g in the NT group, 1794 ± 174 g in the TH group, and 1829 ± 204 g in the TH + H2 group. At 24 h after insult, all piglets were alive in the NT group (n = 14; 6 males and 8 females), all were alive in the TH group except one (12 of 13; 11 males and 2 females), and all were alive in the TH + H2 group (n = 9; 6 males and 3 females; Fig. 2).
The biochemical parameters of pH, PaO2, PaCO2, base excess, lactate, glucose, and hemoglobin at baseline showed no significant differences among the three groups (Table 1). At 3–12 h after insult, pH was significantly lower in the TH + H2 group than in the NT group and, at 24 h after, was lowest in the TH + H2 group. PaCO2 was essentially maintained at a constant value for 24 h after insult, although PaO2 was significantly higher in the TH + H2 group than in the other groups at some time points. The base excess was significantly acidotic in the TH group at the end of the insult (0 h) and at 1 h after insult compared with the TH + H2 group and was significantly higher in the NT group at 3, 6, and 24 h after insult compared with the other groups. There were no significant differences among the three groups in terms of HR, MBP, or rectal temperature at baseline (Table 2). All three groups showed significant reductions in HR and MAP at 0 h that gradually returned to baseline. In the TH + H2 group, HR continued to be lower than in the other groups from 3 h after insult, although there was no tendency for MAP to be consistently lower than in the other groups.
The mean duration of LAEEG (SD: min) after insult was not significantly different among the groups [NT, 18 (8) min; TH, 19 (13) min; TH + H2, 13 (8) min].
For the percentage of piglets with an abnormal aEEG-BG, the NT group showed an increase with time after insult, while the TH group showed a sharp increase from 12 h after insult. The TH + H2 group did not show much of an increase, even at 24 h after insult (Fig. 3a). For the percentage of piglets with an abnormal aEEG-BG+Sz, the NT and TH groups exhibited results similar to those of the aEEG-BG group. Although the percentage of piglets with an abnormal aEEG-BG+Sz at 3 h in the TH + H2 group was slightly higher than that with an abnormal aEEG-BG, it was decreased at 6 h and remained below 40% at 12 and 24 h, similar to aEEG-BG. The percentage of piglets with an abnormal aEEG-BG and, aEEG-BG+Sz did not increase much in the TH + H2 group, even 24 h after insult (Fig. 3b).
Similar results were found for the percentage of piglets with SE, with an increase with time after insult in the NT group, a sharp increase from 12 h after insult in the TH group, and no notable increase in the TH + H2 group, even 24 h after insult (Fig. 4). The mean duration of SE (standard error of the mean [SEM]) was non-significantly shorter in the TH + H2 group [341 (178) min] than in the TH group [613 (151) min] and was significantly shorter than in the NT group [877 (144) min] (Fig. 5).
Discussion
In this asphyxiated piglet model, there was a high percentage of animals with an abnormal aEEG-BG after HI insult, which may correspond to moderate and severe HIE. In addition, we found that with TH there was a low percentage of piglets with EEG abnormalities up to 6 h after insult, but this percentage was high after 12 h and TH was not effective in attenuating seizure development. On the other hand, H2 gas combined with TH was associated with a lower percentage of piglets with an abnormal aEEG-BG or aEEG-BG+Sz and with a shorter duration of SE at 24 h after insult. We speculate that this improvement in aEEG findings with TH + H2 did not reflect an alleviation of brain injury due to seizures, but rather an alleviation of brain injury by some neuroprotective mechanism.
Perinatal brain damage after HI evolves over time and involves multiple mechanisms, such as excitotoxicity, oxidative stress, dysregulated inflammation, and seizures. It is challenging to untangle the impact of seizures from brain injury due to HI, and the direction of the causality of seizures for brain injury is still not clear.16 However, many animal and clinical studies have reported that seizure burden is correlated with brain injury in neonates with HIE.10,17 Previous studies have reported an SE incidence in HIE neonates undergoing TH of 10-25% and demonstrated that neonates with HIE undergoing hypothermia who exhibit electrographic seizures and high seizure burden are associated with more severe brain injury on magnetic resonance imaging (MRI).17 Hence, seizure burden in the HIE neonate is a critical marker for evaluating not only the severity of brain injuries, but also the efficacy of TH and other neuroprotective therapies such as H2 gas.
In the present study, the percentage of piglets with an abnormal aEEG-BG, aEEG-BG+Sz, and SE at 6 h after HI insult was lower in both the TH and TH + H2 groups than in the NT group, although the TH group showed a much higher percentage of piglets with such abnormal findings at 12 h after insult. In contrast, the TH + H2 group maintained a low percentage of piglets with an abnormal aEEG-BG, aEEG-BG+Sz, and SE at 24 h after insult. These results may indicate the limitation of the treatment efficacy of TH and the enhanced neuroprotective effect of H2 gas combined with TH. In terms of the efficacy of H2 gas for ameliorating seizure burden, no clinical trial results have been reported, only the results of animal research. Nemeth et al. developed an HIE piglet model and determined in this model that H2 gas inhalation for 4 h could enhance EEG recovery and significantly protect neurons at 24 h after insult.18
Additionally, it has been reported that antiepileptic drugs used to treat neonatal seizures are often ineffective after an initial loading dose.10,19 Furthermore, animal studies suggest that anticonvulsants have the potential to cause unwanted adverse effects in the developing brain.20,21 H2 gas may have the potential to be a new agent for preventing or treating seizure burden.
The mechanisms underlying the neuroprotective properties of molecular H2 are unclear. However, it may have anti-inflammatory and antioxidant effects.22 It has been reported in animal studies that seizures in HIE are due to vascular endothelial damage in the blood–brain barrier (BBB). Reactive oxygen species (ROS) directly destroy lipids, proteins, and nucleic acids, damaging vascular endothelial cells and the basement membrane.23 In a rat model of global cerebral ischemia, inhalation of H2 gas alleviated brain edema and BBB disruption, reduced neuronal apoptosis, and improved neurological function.24 In our previous study focusing on changes in cerebral circulating oxygen metabolism, the TH + H2 gas group showed a significant increase in CBV at 24 h after insult compared to the TH group, and the amount of the CBV increase was significantly correlated with the decrease in the number of TUNEL-positive cells. Another clinical study using magnetic resonance spectroscopy suggested that seizures were associated with a mismatch in oxygen supply and demand, such that an increased seizure burden was linked to elevated lactate and a reduced NAA/choline ratio, a marker of neuronal injury.25 These results suggest that the recovery of vascular endothelial function resulted in the maintenance of cerebral blood flow to meet the oxygen demand for neuronal activity, thereby reducing neuronal necrosis and leading to a decrease in seizures.
Some animal models are problematic in that large numbers of animals either die or survive with considerable variability in the degree of injury. It is important that the model effectively produces a high proportion of animals that not only survive the HI insult, but also sustain a consistent degree of neuropathological damage. We had previously reported that, with our insult protocol, 88.9% of the piglets have histopathological brain injuries and 11.1% have no histopathological damage.26 In our model, the pattern of histopathological injuries at day 5 after insult is similar to the typical pattern seen in term neonates with HIE, which show cortical lesions in the watershed areas of the hemispheres (parasagittal region, depths of the sulci), the hippocampus, and the cerebellum.26,27 Thus, because our insult protocol can produce a high percentage of piglets with histopathological brain damage on day 5, we can presume that the NT group in this study also has a similarly high percentage of piglets showing brain damage. Electrocortical activity measured by aEEG is useful for evaluating the severity of brain damage. Previous animal studies had already reported that the duration of LAEEG during and after an HI insult could reflect the severity of histopathological brain injuries in the piglet26,28,29 and also indicated the strength of the insult.30 In this study, the fact that the duration of LAEEG after insult was not significantly different among the three groups could indicate that the strength of the insult was the same in all piglets.
This study has some limitations. First, we were unable to clarify the relationship among vascular endothelial cell injury, cerebral circulation, and seizures. We are planning to clarify this relationship in future research. Second, we did not compare the results for H2 gas alone. This is because TH is already the standard of care for HIE in clinical practice and we considered its combination with TH to be the most feasible protocol. Many reports have suggested the antioxidant and anti-inflammatory effects of H2 gas. Our study does not focus on these mechanisms but only discusses their effects on seizure burden using aEEG, which is a critical marker for evaluating the severity of brain injuries. Finally, we were unable to identify the mechanism by which molecular hydrogen contributed to this seizure-reducing effect. We had previously reported that, in our piglet model, H2 gas ventilation did not significantly reduce albumin leakage; however, it had a tendency to reduce this leakage in response to moderate-to-severe HI insults, as determined by the ratio of albumin-stained to -unstained areas, even though histological images showed signs of improvement.31 We speculate that the mechanism underlying these signs may involve the protection of the BBB via the antioxidant, anti-inflammatory, and anti-apoptotic effects of H2 gas. In this study, this functional improvement in the BBB may have led to a reduction in seizures in this study. We hope to clarify these mechanisms in future studies.
This study showed that the combination of H2 gas and TH was associated with a lower rate of an abnormal aEEG-BG, aEEG-BG+Sz, and SE compared with NT up to 6 h after insult but that, after 12 h, the rate increased with TH but remained lower with H2. Combination therapy with H2 gas has the potential to improve the prognosis of patients with brain disorders that cannot be treated with TH alone, and aEEG is a useful noninvasive and simple bedside brain function monitor to determine the efficacy of H2 gas therapy.
Data availability
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, CD003311 (2013).
Azzopardi, D. et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK-analysis of national data. PLoS ONE 7, e38504 (2012).
Glass, H. C. et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology 82, 1239–1244 (2014).
Boylan, G. B., Kharoshankaya, L. & Wusthoff, C. J. Seizures and hypothermia: importance of electroencephalographic monitoring and considerations for treatment. Semin. Fetal Neonatal Med. 20, 103–108 (2015).
Zhou, K. Q. et al. Treating seizures after hypoxic-ischemic encephalopathy-current controversies and future directions. Int. J. Mol. Sci. 22, 7121 (2021).
Lin, Y. K., Hwang-Bo, S., Seo, Y. M. & Youn, Y. A. Clinical seizures and unfavorable brain MRI patterns in neonates with hypoxic ischemic encephalopathy. Medicine 100, e25118 (2021).
Basti, C. et al. Seizure burden and neurodevelopmental outcome in newborns with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: a single center observational study. Seizure 83, 154–159 (2020).
Glass, H. C. et al. Contemporary profile of seizures in neonates: a prospective cohort study. J. Pediatr. 174, 98–103.e101 (2016).
Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688–694 (2007).
Domoki, F. et al. Hydrogen is neuroprotective and preserves cerebrovascular reactivity in asphyxiated newborn pigs. Pediatr. Res. 68, 387–392 (2010).
Htun, Y. et al. Hydrogen ventilation combined with mild hypothermia improves short-term neurological outcomes in a 5-day neonatal hypoxia-ischaemia piglet model. Sci. Rep. 9, 4088 (2019).
Nakamura, S. et al. Impact of hydrogen gas inhalation during therapeutic hypothermia on cerebral hemodynamics and oxygenation in the asphyxiated piglet. Sci. Rep. 13, 1615 (2023).
Brophy, G. M. et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit. Care 17, 3–23 (2012).
Molloy, E. J. et al. Neuroprotective therapies in the NICU in term infants: present and future. Pediatr. Res. 93, 1819–1827 (2023).
Kharoshankaya, L. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic–ischemic encephalopathy. Dev. Med. Child Neurol. 58, 1242–1248 (2016).
Nemeth, J. et al. Molecular hydrogen affords neuroprotection in a translational piglet model of hypoxic-ischemic encephalopathy. J. Physiol. Pharmacol. 67, 677–689 (2016).
El-Dib, M. & Soul, J. S. The use of phenobarbital and other anti-seizure drugs in newborns. Semin. Fetal Neonatal Med. 22, 321–327 (2017).
Bittigau, P. et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl Acad. Sci. USA 99, 15089–15094 (2002).
Forcelli, P. A., Janssen, M. J., Vicini, S. & Gale, K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann. Neurol. 72, 363–372 (2012).
Htun, Y., Nakamura, S. & Kusaka, T. Hydrogen and therapeutic gases for neonatal hypoxic-ischemic encephalopathy: potential neuroprotective adjuncts in translational research. Pediatr. Res. 89, 753–759 (2020).
Fellman, V. & Raivio, K. O. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr. Res. 41, 599–606 (1997).
Nagatani, K. et al. Effect of hydrogen gas on the survival rate of mice following global cerebral ischemia. Shock 37, 645–652 (2012).
Miller, S. P. et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology 58, 542–548 (2002).
Nakamura, S. et al. Cerebral blood volume combined with amplitude-integrated EEG can be a suitable guide to control hypoxic/ischemic insult in a piglet model. Brain Dev. 35, 614–625 (2013).
Nakamura, M. et al. Cerebral blood volume measurement using near-infrared time-resolved spectroscopy and histopathological evaluation after hypoxic-ischemic insult in newborn piglets. Int. J. Dev. Neurosci. 42, 1–9 (2015).
Thoresen, M. et al. A piglet survival model of posthypoxic encephalopathy. Pediatr. Res. 40, 738–748 (1996).
Haaland, K., Loberg, E. M., Steen, P. A. & Thoresen, M. Posthypoxic hypothermia in newborn piglets. Pediatr. Res. 41, 505–512 (1997).
Bjorkman, S. T. et al. Hypoxic/ischemic models in newborn piglet: comparison of constant FiO2 versus variable FiO2 delivery. Brain Res. 1100, 110–117 (2006).
Htun, Y. et al. Conflicting findings on the effectiveness of hydrogen therapy for ameliorating vascular leakage in a 5-day post hypoxic-ischemic survival piglet model. Sci. Rep. 13, 10486 (2023).
Acknowledgements
We thank medical students at the Faculty of Medicine, Kagawa University, Kagawa, Japan for their assistance in this study.
Funding
This study was financially supported by JSPS KAKENHI grants (19K08253 (S.N.), 19K08349 (K.K.), 22K15923 (Y.N.), and 22K07822 (T.K.), 22K15899 (T.W.), 22K15922 (A.M.), and 23K07332 (S.N.)), and Kagawa University Faculty of Medicine School of Medicine Alumni Association Sanjukai Research Aid R1-1 (S.N.).
Author information
Authors and Affiliations
Contributions
T.T., S.N. and T.K. were involved in the initial study design and wrote the main text. K.K., Y.N., T.K. and S.N. obtained the necessary financial support for this project and provided study materials. T.T. and Y.S. were primarily responsible for evaluating the aEEG findings. Y.N., T. Mitsuie, E.I., K.I., M.A., K.K. and N.F. carried out the animal experiments and recorded blood gas and physiological data. K.M., S.K., K.O., M.U. and T. Miki. contributed to data analysis and performed the statistical analysis. All members drafted the article and critically revised it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and informed consent
The study protocol was approved by the Kagawa University Animal Care and Use Committee (15070–1) and was conducted in accordance with Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines and all other applicable guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsuchiya, T., Nakamura, S., Sugiyama, Y. et al. Hydrogen gas can ameliorate seizure burden during therapeutic hypothermia in asphyxiated newborn piglets. Pediatr Res 95, 1536–1542 (2024). https://doi.org/10.1038/s41390-024-03041-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-024-03041-6