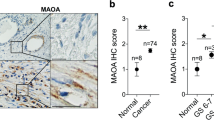
Abstract
Androgen Receptor (AR) activity in prostate stroma is required to maintain prostate homeostasis. This is mediated through androgen-dependent induction and secretion of morphogenic factors that drive epithelial cell differentiation. However, stromal AR expression is lost in aggressive prostate cancer. The mechanisms leading to stromal AR loss and morphogen production are unknown. We identified TGFβ1 and TNFα as tumor-secreted factors capable of suppressing AR mRNA and protein expression in prostate stromal fibroblasts. Pharmacological and RNAi approaches identified NF-κB as the major signaling pathway involved in suppressing AR expression by TNFα. In addition, p38α- and p38δ-MAPK were identified as suppressors of AR expression independent of TNFα. Two regions of the AR promoter were responsible for AR suppression through TNFα. FGF10 and Wnt16 were identified as androgen-induced morphogens, whose expression was lost upon TNFα treatment and enhanced upon p38-MAPK inhibition. Wnt16, through non-canonical Jnk signaling, was required for prostate basal epithelial cell survival. These findings indicate that stromal AR loss is mediated by secreted factors within the TME. We identified TNFα/TGFβ as two possible factors, with TNFα mediating its effects through NF-κB or p38-MAPK to suppress AR mRNA transcription. This leads to loss of androgen-regulated stromal morphogens necessary to maintain normal epithelial homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Cunha GR. Growth factors as mediators of androgen action during male urogenital development. Prostate Suppl. 1996;6:22–5.
Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–34.
Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99:13498–503.
Yu S, Yeh CR, Niu Y, Chang HC, Tsai YC, Moses HL, et al. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate. 2012;72:437–49.
Yu S, Zhang C, Lin CC, Niu Y, Lai KP, Chang HC, et al. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate. 2011;71:517–24.
Hahn AW, Siddiqui BA, Leo J, Dondossola E, Basham KJ, Miranti CK, et al. Cancer cell-extrinsic roles for the androgen receptor in prostate cancer. Endocrinology. 2023;164:bqad078.
Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–86.
Jiang L, Ivich F, Tahsin S, Tran M, Frank SB, Miranti CK, et al. Human stroma and epithelium co-culture in a microfluidic model of a human prostate gland. Biomicrofluidics. 2019;13:064116.
Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, et al. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63:131–40.
Toivanen R, Shen MM. Prostate organogenesis: tissue induction, hormonal regulation and cell type specification. Development. 2017;144:1382–98.
Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction. 2001;121:187–95.
Pu Y, Huang L, Birch L, Prins GS. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology. 2007;148:1697–706.
Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–8.
Planz B, Wang Q, Kirley SD, Lin CW, McDougal WS. Androgen responsiveness of stromal cells of the human prostate: regulation of cell proliferation and keratinocyte growth factor by androgen. J Urol. 1998;160:1850–5.
Nakano K, Fukabori Y, Itoh N, Lu W, Kan M, McKeehan WL, et al. Androgen-stimulated human prostate epithelial growth mediated by stromal-derived fibroblast growth factor-10. Endocr J. 1999;46:405–13.
Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev Biol. 2005;278:396–414.
Fasciana C, van der Made AC, Faber PW, Trapman J. Androgen regulation of the rat keratinocyte growth factor (KGF/FGF7) promoter. Biochem Biophys Res Commun. 1996;220:858–63.
Nemeth JA, Zelner DJ, Lang S, Lee C. Keratinocyte growth factor in the rat ventral prostate: androgen-independent expression. J Endocrinol. 1998;156:115–25.
Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–701.
Ropiquet F, Giri D, Kwabi-Addo B, Schmidt K, Ittmann M. FGF-10 is expressed at low levels in the human prostate. Prostate. 2000;44:334–8.
Lamb LE, Knudsen BS, Miranti CK. E-cadherin-mediated survival of androgen-receptor-expressing secretory prostate epithelial cells derived from a stratified in vitro differentiation model. J Cell Sci. 2010;123:266–76.
Heer R, Collins AT, Robson CN, Shenton BK, Leung HY. KGF suppresses α2β1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium. J Cell Sci. 2006;119:1416–24.
Frank SB, Miranti CK. Disruption of prostate epithelial differentiation pathways and prostate cancer development. Front Oncol. 2013;3:273.
Frank SB, Berger PL, Ljungman M, Miranti CK. Human prostate luminal cell differentiation requires NOTCH3 induction by p38-MAPK and MYC. J Cell Sci. 2017;130:1952–64.
Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61:423–7.
Wikstrom P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69:799–809.
Leach DA, Need EF, Toivanen R, Trotta AP, Palethorpe HM, Tamblyn DJ, et al. Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome. Oncotarget. 2015;6:16135–50.
Palethorpe HM, Leach DA, Need EF, Drew PA, Smith E. Myofibroblast androgen receptor expression determines cell survival in co-cultures of myofibroblasts and prostate cancer cells in vitro. Oncotarget. 2018;9:19100–14.
Tang Q, Cheng B, Dai R, Wang R. The role of qndrogen receptor in cross talk between stromal cells and prostate cancer epithelial cells. Front Cell Dev Biol. 2021;9:729498.
Liao CP, Chen LY, Luethy A, Kim Y, Kani K, MacLeod AR, et al. Androgen receptor in cancer-associated fibroblasts influences stemness in cancer cells. Endocr Relat Cancer. 2017;24:157–70.
Caligiuri G, Tuveson DA. Activated fibroblasts in cancer: perspectives and challenges. Cancer Cell. 2023;41:434–49.
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–23.
Ishii K, Mizokami A, Tsunoda T, Iguchi K, Kato M, Hori Y, et al. Heterogenous induction of carcinoma-associated fibroblast-like differentiation in normal human prostatic fibroblasts by co-culturing with prostate cancer cells. J Cell Biochem. 2011;112:3604–11.
Verona EV, Elkahloun AG, Yang J, Bandyopadhyay A, Yeh IT, Sun LZ. Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 2007;67:5737–46.
Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. 2010;15:166–79.
Kruslin B, Ulamec M, Tomas D. Prostate cancer stroma: an important factor in cancer growth and progression. Bosn J Basic Med Sci. 2015;15:1–8.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
Chen S, Supakar PC, Vellanoweth RL, Song CS, Chatterjee B, Roy AK. Functional role of a conformationally flexible homopurine/homopyrimidine domain of the androgen receptor gene promoter interacting with Sp1 and a pyrimidine single strand DNA-binding protein. Mol Endocrinol. 1997;11:3–15.
Faber PW, van Rooij HC, Schipper HJ, Brinkmann AO, Trapman J. Two different, overlapping pathways of transcription initiation are active on the TATA-less human androgen receptor promoter. The role of Sp1. J Biol Chem. 1993;268:9296–301.
Hay CW, Hunter I, MacKenzie A, McEwan IJ. An Sp1 modulated regulatory region unique to higher primates regulates human androgen receptor promoter activity in prostate cancer cells. PLoS ONE. 2015;10:e0139990.
Hunter I, Hay CW, Esswein B, Watt K, McEwan IJ. Tissue control of androgen action: The ups and downs of androgen receptor expression. Mol Cell Endocrinol. 2018;465:27–35.
Nadiminty N, Tummala R, Lou W, Zhu Y, Zhang J, Chen X, et al. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287:1527–37.
Grad JM, Dai JL, Wu S, Burnstein KL. Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol. 1999;13:1896–911.
Mizokami A, Yeh SY, Chang C. Identification of 3’,5’-cyclic adenosine monophosphate response element and other cis-acting elements in the human androgen receptor gene promoter. Mol Endocrinol. 1994;8:77–88.
Yang L, Xie S, Jamaluddin MS, Altuwaijri S, Ni J, Kim E, et al. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2005;280:33558–65.
Shiota M, Yokomizo A, Tada Y, Inokuchi J, Kashiwagi E, Masubuchi D, et al. Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene. 2010;29:237–50.
Kang HY, Huang HY, Hsieh CY, Li CF, Shyr CR, Tsai MY, et al. Activin A enhances prostate cancer cell migration through activation of androgen receptor and is overexpressed in metastatic prostate cancer. J Bone Miner Res. 2009;24:1180–93.
Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, et al. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006;25:3436–44.
Qiao L, Tasian GE, Zhang H, Cao M, Ferretti M, Cunha GR, et al. Androgen receptor is overexpressed in boys with severe hypospadias, and ZEB1 regulates androgen receptor expression in human foreskin cells. Pediatr Res. 2012;71:393–8.
Wu D, Sunkel B, Chen Z, Liu X, Ye Z, Li Q, et al. Three-tiered role of the pioneer factor GATA2 in promoting androgen-dependent gene expression in prostate cancer. Nucleic Acids Res. 2014;42:3607–22.
He B, Lanz RB, Fiskus W, Geng C, Yi P, Hartig SM, et al. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc Natl Acad Sci USA. 2014;111:18261–6.
Alimirah F, Panchanathan R, Chen J, Zhang X, Ho SM, Choubey D. Expression of androgen receptor is negatively regulated by p53. Neoplasia. 2007;9:1152–9.
Davis JN, Wojno KJ, Daignault S, Hofer MD, Kuefer R, Rubin MA, et al. Elevated E2F1 inhibits transcription of the androgen receptor in metastatic hormone-resistant prostate cancer. Cancer Res. 2006;66:11897–906.
Valdez CD, Davis JN, Odeh HM, Layfield TL, Cousineau CS, Berton TR, et al. Repression of androgen receptor transcription through the E2F1/DNMT1 axis. PLoS ONE. 2011;6:e25187.
Dai JL, Burnstein KL. Two androgen response elements in the androgen receptor coding region are required for cell-specific up-regulation of receptor messenger RNA. Mol Endocrinol. 1996;10:1582–94.
Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457–71.
Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, et al. NF-κB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–99.
Zaidi G, Supakar PC. Identification of a nuclear protein interacting with a novel site on rat androgen receptor promoter after transcription factor NFkB is displaced from adjacent site. Mol Biol Rep. 2003;30:121–5.
Song CS, Jung MH, Supakar PC, Chen S, Vellanoweth RL, Chatterjee B, et al. Regulation of androgen action by receptor gene inhibition. Ann N Y Acad Sci. 1995;761:97–108.
Delfino FJ, Boustead JN, Fix C, Walker WH. NF-κB and TNFα stimulate androgen receptor expression in Sertoli cells. Mol Cell Endocrinol. 2003;201:1–12.
Ko S, Shi L, Kim S, Song CS, Chatterjee B. Interplay of NF-κB and B-myb in the negative regulation of androgen receptor expression by tumor necrosis factor alpha. Mol Endocrinol. 2008;22:273–86.
Hay CW, Watt K, Hunter I, Lavery DN, MacKenzie A, McEwan IJ. Negative regulation of the androgen receptor gene through a primate-specific androgen response element present in the 5’ UTR. Horm Cancer. 2014;5:299–311.
Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci USA. 2006;103:15969–74.
Yeap BB, Krueger RG, Leedman PJ. Differential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and breast cancer cells. Endocrinology. 1999;140:3282–91.
Lee DK, Chang C. Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88:4043–54.
Lamprecht S, Sigal-Batikoff I, Shany S, Abu-Freha N, Ling E, Delinasios GJ, et al. Teaming up for trouble: Cancer cells, transforming growth factor-β1 signaling and the epigenetic corruption of stromal naïve fibroblasts. Cancers. 2018;10:61.
Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107.
Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, et al. Immune activation of NF-κB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–34.
Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 2001;15:1900–12.
Thiefes A, Wolter S, Mushinski JF, Hoffmann E, Dittrich-Breiholz O, Graue N, et al. Simultaneous blockade of NFkappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor [corrected] target genes. J Biol Chem. 2005;280:27728–41.
Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–90.
Zimmerman EI, Gibson AA, Hu S, Vasilyeva A, Orwick SJ, Du G, et al. Multikinase inhibitors induce cutaneous toxicity through OAT6-mediated uptake and MAP3K7-driven cell death. Cancer Res. 2016;76:117–26.
Monteiro C, Miarka L, Perea-García M, Priego N, García-Gómez P, Álvaro-Espinosa L, et al. Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nat Med. 2022;28:752–65.
Zhang X, Zheng S, Hu C, Li G, Lin H, Xia R, et al. Cancer-associated fibroblast-induced lncRNA UPK1A-AS1 confers platinum resistance in pancreatic cancer via efficient double-strand break repair. Oncogene. 2022;41:2372–89.
Wang H, Xu Q, Xiao F, Jiang Y, Wu Z. Involvement of the p38 mitogen-activated protein kinase α, β, and γ isoforms in myogenic differentiation. Mol Biol Cell. 2008;19:1519–28.
Richards Z, McCray T, Marsili J, Zenner ML, Manlucu JT, Garcia J, et al. Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience. 2019;12:304–17.
Berger PL, Frank SB, Schulz VV, Nollet EA, Edick MJ, Holly B, et al. Transient induction of ING4 by Myc drives prostate epithelial cell differentiation and its disruption drives prostate tumorigenesis. Cancer Res. 2014;74:3357–68.
Watson J, Francavilla C. Regulation of FGF10 signaling in development and disease. Front Genet. 2018;9:500.
Madueke IC, Hu WY, Huang L, Prins GS. WNT2 is necessary for normal prostate gland cyto-differentiation and modulates prostate growth in an FGF10 dependent manner. Am J Clin Exp Urol. 2018;6:154–63.
Lai KP, Yamashita S, Vitkus S, Shyr CR, Yeh S, Chang C. Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor. Mol Endocrinol. 2012;26:52–66.
Tanner MJ, Welliver RC Jr, Chen M, Shtutman M, Godoy A, Smith G, et al. Effects of androgen receptor and androgen on gene expression in prostate stromal fibroblasts and paracrine signaling to prostate cancer cells. PLoS ONE. 2011;6:e16027.
Bostwick DG. Prostatic intraepithelial neoplasia (PIN): current concepts. J Cell Biochem Suppl. 1992;16h:10–9.
Acknowledgements
We thank Carol Kepler in the Tissue Acquisition and Cellular Molecular Analysis Shared Resource (TACMASR) for helping to secure primary prostate stromal cells, Dr. Sander Frank for his cloning expertise, and Dr. Ghassan Mouneimne for critical editing. ST, NS, BC, LJ, YZ, and CKM were supported by funding from NIH/NCI MPI R01 CA254200 and TACMASR by P30 CA023075.
Author information
Authors and Affiliations
Contributions
ST was responsible for designing, carrying out, and interpreting the experiments, as well as the primary writer of the manuscript. NSS and BC designed and carried out experiments and helped edit the manuscript. LJ and YZ assisted with interpretation of data and editing the manuscript. BRL contributed patient tissues and helped with manuscript editing. CM directed the project, assisted in data interpretation, did manuscript writing and editing, and is the contributing author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tahsin, S., Sane, N.S., Cernyar, B. et al. AR loss in prostate cancer stroma mediated by NF-κB and p38-MAPK signaling disrupts stromal morphogen production. Oncogene (2024). https://doi.org/10.1038/s41388-024-03064-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03064-7