Abstract
Of the many peculiarities that enable the giant panda (Ailuropoda melanoleuca), a member of the order Carnivora, to adapt to life as a dedicated bamboo feeder, its extra “thumb” is arguably the most celebrated yet enigmatic. In addition to the normal five digits in the hands of most mammals, the giant panda has a greatly enlarged wrist bone, the radial sesamoid, that acts as a sixth digit, an opposable “thumb” for manipulating bamboo. We report the earliest enlarged radial sesamoid, already a functional opposable “thumb,” in the ancestral panda Ailurarctos from the late Miocene site of Shuitangba in Yunnan Province, China. However, since the late Miocene, the “thumb” has not enlarged further because it must be balanced with the constraints of weight bearing while walking in a plantigrade posture. This morphological adaptation in panda evolution thus reflects a dual function of the radial sesamoid for both bamboo manipulation and weight distribution. The latter constraint could be the main reason why the panda’s false thumb never evolved into a full digit. This crude “thumb” suggests that the origin of the panda’s dedicated bamboo diet goes back to as early as 6–7 Ma.
Similar content being viewed by others
Introduction
The false thumb of the giant panda (“panda” throughout text below unless otherwise specified) fascinated early naturalists1,2,3. In recent decades, as popularized by Gould4,5, it has become a celebrated case of evolutionary adaptation to independently acquire an opposable thumb-like structure when the need arose. Gould’s essay also highlights an exclusive association of this unique anatomic structure with an equally unique diet of bamboo herbivory, although a false thumb has been shown also to have evolved independently in the red panda and its distant relatives6,7, in tremarctine bears (either convergently or as a shared plesiomorphic trait)8, as well as in a distant relative of the giant panda clade9. In fact, the giant panda is a striking example of a highly specialized member of the bear family (Ursidae) that has become a dedicated herbivore, a rare case of a large carnivore with a short, carnivorous digestive tract10 that became a low-level consumer with a greatly altered gut microbiota11.
Despite its celebrated status, the panda’s false thumb is a small, flat structure that barely protrudes out of the palmar surface, and this relatively obscure anatomy understandably baffled early anatomists (e.g., Wood-Jones3). Such a relatively small and flat radial sesamoid has also been documented in fossil pandas from the late Pleistocene (ca. 102–49 Ka) Shuanghe Cave12. If bamboo manipulation is the main function of this feature, why did pandas not evolve a markedly more elongated radial sesamoid, one that more closely resembles a true opposable thumb for the efficient gripping of bamboo, given that mammalian sesamoids seem to be readily elongated with relatively little developmental constraint13? Until now, this question has not been answerable due to a lack of fossil evidence beyond late Pleistocene within the Ailurarctos-Ailuropoda lineage.
We report here the earliest occurrence of an enlarged radial sesamoid, as well as an isolated M2, a broken canine, and a partial humerus, all assigned to Ailurarctos, from Shuitangba, a late Miocene site in the Zhaotong Basin, Yunnan Province (Fig. 1). The morphology of the preserved dentition closely matches that of the stem genus Ailurarctos (tribe Ailuropodini) from Lufeng and Yuanmou14,15, the most basal panda so far known. The false thumb in Ailurarctos shows an intermediate morphology (see below), and thus documents, for the first time, the likely timing and steps in the evolution of bamboo feeding in pandas.
Map of southern China showing late Miocene localities with panda specimens in Yunnan (red symbols), the giant panda’s modern distribution (orange)16 and historical range (yellow)16,17,18, present approximate bamboo forest distribution in China (green)19, and Pleistocene and later fossil distribution (solid and open black circles and triangles)16,18,19,20,21,22,23,24. We eliminated the humerus record from Zhoukoudian locality 1 following Jiangzuo et al.25 (see discussion in online Supplementary material). Topographical map generated by GeoMapApp (version 3.6.10) under CC BY license26.
Institutional Abbreviations. IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing; KIZ, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming; USNM, National Museum of Natural History, Smithsonian Institution, Washington, D.C.; YV, Yunnan Institute of Cultural Relics and Archaeology, Kunming, Yunnan Province; ZT, Zhaotong collection, Yunnan Institute of Cultural Relics and Archaeology, Kunming, Yunnan Province.
Systematic paleontology
Order Carnivora Bowdich, 1821.
Family Ursidae Fischer von Waldheim, 1817.
Subfamily Ailuropodinae Grevé, 1894.
Tribe Ailuropodini Grevé, 1894.
Genus Ailurarctos Qiu and Qi, 1989.
Ailurarctos cf. A. lufengensis Qiu and Qi, 1989.
Referred specimens. From Shuitangba, Zhaotong Basin, Yunnan: ZT-2015-0124, an isolated left M2 (Fig. 2A-C); ZT-2015-0056, left radial sesamoid (Figs. 3, 4, S1); ZT-2007-02-097, partial lower canine (Fig. S2); ZT-2007-62-251, distal half of left humerus (Fig. S3). See online Supplementary material for depositional context and associated fauna.
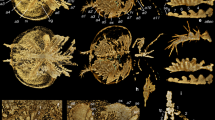
Ailurarctos cf. A. lufengensis from Zhaotong (A–C) compared to A. lufengensis from the type locality of Lufeng (D–F) and A. yuanmouensis from type locality of Yuanmou (G). ZT-2015–0124, (A) lingual, (B) labial, and (C) occlusal (stereophoto) of left M2; (D) right M2 (reversed), IVPP V6892.4; (E) left M2, IVPP V6892.5, and (F) left M2, IVPP V6892.6; and (G) left M2, YV 2509.2, A. yuanmouensis. Images for A. lufengensis and A. yuanmouensis are courtesy of Qigao Jiangzuo.
Giant panda’s false thumb. Dorsal (A) and ventral (C) views of the modern giant panda left hand, as compared with an isolated left radial sesamoid of Ailurarctos cf. A. lufengensis (B and D, ZT-2015–0056) at a similar angle and relative size. Mounted skeleton of the giant panda on display at KIZ exhibition hall, probably a zoo specimen.
Comparison of M2, taxonomic assignment, and diet. Three M2s of Ailurarctos lufengensis (Fig. 2D-F) and one of A. yuanmouensis (Fig. 2G) are available and offer a sense of the variation within and between species. The three M2s from Lufeng display a range of morphology: presence or absence of a lingual cingulum and reduction of buccal cingulum, and presence or absence of a clearly delineated metaconule (RMe3 in Jiangzuo et al.27). Of these, the Shuitangba M2 (ZT-2015-0124) has a distinct lingual cingulum but lacks an elevated metaconule. The Lufeng sample, however, consistently has a more distinct ridge on the lingual side of the preparacrista (RPa1.2 in Jiangzuo et al.27) not seen in ZT-2015-0124. An M2 of A. yuanmouensis has substantially less crenulation in its trigon, whereas its talon has achieved the level of complexity seen in those from Lufeng and Shuitangba. The overall proportion of the Shuitangba M2 is slightly more elongated than in the Lufeng sample and the elevated talon anterocentrally is not seen in the Lufeng M2s. Since M2s in Ailuropoda are more quadrate with greater width to length ratios (Table 1), the narrower ZT-2015-0124 appears slightly more primitive. Given the above dental comparisons, the Shuitangba M2 reliably belongs to Ailurarctos, in terms of both the length/width proportions and the detailed cusp morphology, and we tentatively assign it to Ailurarctos cf. A. lufengensis pending additional materials becoming available.
Among carnivorans, ursids have the most complex molars due to their unique morphology related to hypocarnivory. Within ursids, dental patterns in ailuropodines are some of the most elaborate, with numerous, highly distinct crown cuspules27, advantageous for crushing tough bamboo, i.e., durophagous mastication20. These features are associated with a robust mandible28 and lateral movements of the temporomandibular joint29. It is evident that the dental pattern of Ailurarctos has reached the level of complexity of modern Ailuropoda, as recognized by Qiu and Qi14. In fact, the degree of enamel crenulation on most M2s of Ailurarctos is even greater than in Ailuropoda. If it is accepted that the robust cuspation in Ailuropoda is linked to a bamboo diet, dental specializations in Ailurarctos strongly suggest both an ancestral relationship to Ailuropoda as well as a diet including bamboo20. See additional description and comparison of other specimens in online Supplementary material.
Description and comparison of the radial sesamoid. The radial sesamoid, ZT-2015-0056 (Figs. 3, 4), resembles in all essential details those of Ailuropoda previously described10,12,30. Compared to those of Indarctos arctoides, a possible stem ailuropodine in the late Miocene of Spain, the Ailurarctos radial sesamoid is considerably larger, relatively wider, and more hooked (see relative size of radial sesamoid to metacarpal I of I. arctoides in Fig. 8 of Abella et al.9), although we lack knowledge of Ailurarctos metacarpals and Abella et al. did not publish lengths of metacarpal I. The proximal articulating facets are also much larger with a more concave facet for the scapholunar in Ailurarctos, while the distal end of the I. arctoides radial sesamoid still preserves a possible cartilaginous tip (see Fig. 3 of Abella et al.9), which is also present in some extant Ailurus7 but presumably absent in Ailurarctos (lacking a distinct rim seen in Indarctos). The radial sesamoid of Ailurarctos is slightly larger than those of modern pandas, by 8% if compared to the maximum length of the largest radial sesamoid of living panda measured by Li and others31, but relative to body size (using M2 length as a proxy), it is significantly larger than its modern counterparts (Table 2). It is gently convex on the external (approximately ventral) surface and concave on the internal surface (orientation assuming a plantigrade posture). At the proximal end, a large, elongate, concave facet (16 mm in maximum longitudinal dimension) articulates with the medial process of the scapholunar, whereas a much smaller, oval-shaped, flat facet (7 × 9 mm) articulates with the medial face of the first metacarpal. The distal end thickens slightly and bends toward the palm, as if to oppose to the fingers.
Besides its comparatively large size, the radial sesamoid in Ailurarctos differs from that in modern Ailuropoda in other ways. A prominent tubercle arising from the inner edge of the articular facet for the scapholunar, presumably for the attachment of the opponens pollicis muscle10,32, is present in ZT-2015-0056 but is not seen in living Ailuropoda. Of more importance, a distinct hook in the distal end10,32, bending sharply inward toward the palm (Fig. 4C), and a correspondingly flattened external surface due to the thinning of the distal plate (Fig. 5C, D), both evident in extant Ailuropoda, are not present in Ailurarctos (see Dual Functions below for their functional significance). This flattened external, distal end corresponds to the accessory pad for the false thumb of the panda (Fig. 5E).

modified from Davis8. Muscles (dark red bundles) between the radial sesamoid and first metacarpal are abductor pollicis brevis and opponens pollicis, following Endo et al.30. Note the small distal hook and flat ventral surface of the radial sesamoid in extant Ailuropoda, which are derived features that function for better grasping (small hook) as well as walking (flattened palm surface) in contrast to the primitive conditions seen in Ailurarctos (Fig. 3).
Comparison of the radial sesamoid in the basal ursoid, Ailuropoda, and Homo and the positioning of the radial sesamoid. Illustrations are of left hands. (A) A basal ursoid from the early Oligocene of North Dakota (USNM 637,259) showing the primitive condition of an unenlarged radial sesamoid; (B) grasping hand in extant Ailuropoda; (C) grasping hand of modern human; (D) walking hand of extant Ailuropoda in a plantigrade posture; (E) external ventral surface of the hand of Ailuropoda showing a fleshy, plantar pad that corresponds to the radial sesamoid (red dash lines),
Dual functions of false thumb
Best developed in humans and their close relatives, precision grip by a true opposable thumb (capable of closure of the pollex to opposing fingers) requires not only flexibility of the joints but also complex interactions of flexor and extensor muscles. Endo et al.33 demonstrated that grasping in pandas is fundamentally different from that in humans. Instead of a human thumb that is capable of independent movements against other fingers, the panda’s radial sesamoid forms a functional complex in rigid articulation with the first metacarpal and scapholunar, which collectively rotate with other metacarpals. Once fully flexed, the radial sesamoid functional complex couples with the pisiform on the lateral side of the hand to function as a double stop against the pincer-like actions of the bending phalanges (but see Fig. 6, which shows only the radial sesamoid is used in the pincer action and the pisiform is not). Small muscles (such as abductor pollicis brevis and opponens pollicis) between the radial sesamoid and first metacarpal serve as a cushion for the bamboo stems grasped between the radial sesamoid and phalanges (Fig. 5). Such a passive system of gripping, far less effective than that of humans, nonetheless offers the panda the tightness of grip it needs for bamboo feeding. Furthermore, from an evolutionary point of view, such a simple passive mechanism of grasping can be functionally useful even with a slight initial enlargement of the radial sesamoid. Natural selection would be effective from the early stages of enlargement, i.e., even a small, protruding lump at the wrist can be a modest help in preventing bamboo from slipping off bent fingers.
Giant panda gripping and chewing a thick, dried bamboo stem at Chengdu Research Base of Giant Panda Breeding on April 21, 2016. Inset: a semitransparent radial sesamoid bone is placed at its approximate position inside the fleshy pad (actual orientation of this bone may differ slightly from our placement). This photo also shows that the pisiform plays no role in bamboo grasping, contra Endo et al.33 Reproduction of photo by permission from Sharon Fisher.
Radial sesamoids in living giant pandas have a rather abrupt, inward hook near the distal end (Figs. 4C, 5B, 7) as illustrated by Wood-Jones30 and Endo et al.32, and described by Davis10 and Wang et al.12. The function of this hook can be intuitively understood as a passive pincer in a single element grasping system, in contrast to that in humans with a two-segmented pollex in which the distal segment can be bent to facilitate grasping (Fig. 5). The lack of a distal hook in Ailurarctos indicates a two-step evolution, with an initial simple elongation in the false thumb followed by the subsequent appearance of a more refined distal hook (perhaps by late Pleistocene12), concomitant with a slight shortening of the tip.

adopted from Wang et al.10, which was referred to A. melanoleuca. A major hiatus exists in the Pliocene, likely reflecting poor Pliocene records in South China. Chronology of Lufeng and Yuanmou faunas is based on Dong and Qi33.
Phylogram of the giant panda showing the relevant steps discussed in the text and evolution of the false thumb. Pleistocene panda species ranges and chronospecies scheme follow Jin et al.20. The largest species, Ailuropoda baconi, was thought to be restricted to middle and late Pleistocene, with the modern species, A. melanoleuca, as Holocene20, although Sheng et al.34 recognized the initial divergence of the living lineage in the late Pleistocene. The false thumb from late Pleistocene Shuanghe Cave was
The radial sesamoid in Ailurarctos exceeds that of its modern descendants, both in absolute and relative size (radial sesamoid index = 1.89 for Ailurarctos from Shuitangba; 0.92 for Shuanghe Cave fossil; 0.84–1.28 for living Ailuropoda melanoleuca) (Tables 1, 2). If a longer radial sesamoid alone was being selected, it would be expected that modern pandas would have increased the length of the radial sesamoid in the intervening six million years. Yet, modern pandas have a shorter radial sesamoid relative to their increased body size (as compared to their fossil ancestors), adding only a slight hook at the distal end. This raises the question of why the false thumb of pandas did not elongate further, as a longer digit would surely enhance capabilities for grasping thicker bundles of bamboo.
We propose that the lack of further elongation is the result of a functional compromise between the need for grasping larger bundles of bamboo and the weight-bearing function of the false thumb (Fig. 5). All ursids are fully plantigrade in their standing postures, i.e., the palm of the hand touches the ground while walking. A highly elongate radial sesamoid designed for bamboo manipulation would inevitably result in a conflict with walking long distances, thus compromising the radial sesamoid’s dual functions—its inner surface for grasping (Fig. 5B) and its outer surface for weight bearing (Fig. 5D). Due to its position in plantigrade posture, any further enlargement of the radial sesamoid would result in greater ventral protrusion and interference with walking. We view the flattened distal surface of the Ailuropoda radial sesamoid as a means to spread the load within the external accessory pad to cushion stride impact, an additional feature indicative of the dual functional demands of the radial sesamoid in both food procurement and locomotion. Living giant pandas thus balance these conflicting demands with a sharp bend inward on the distal end to form a hook and, at the same time, reducing external protrusion by flattening the external walking surface of the false thumb (Fig. 7).
As illustrated in the evolution of the false thumb (Fig. 5), more efficient consumption of bamboo cannot override requirements for weight-bearing while walking because the panda inherited a plantigrade posture. Potential alternatives to overcome such a constraint include a digitigrade posture (lifting the palm off the ground, freeing the wrist area from weight bearing), as in cursorial carnivorans (canids, felids, hyaenids), but this may not have been feasible for ursids given their evolutionary history of plantigrady in addition to arboreality. All digitigrade families evolved from a small, agile ancestor and efficient digitigrady evolved over millions of years, in contrast to ursids who were already large-bodied by the late Miocene and fully plantigrade36. Furthermore, pandas are partly arboreal, which is also facilitated by being plantigrade. Of the living ursids, only the giant pandas have a large fleshy pad (Fig. 6) to cushion the radial sesamoid10, signaling the importance of the weight-bearing function for this bone. While the panda solution may not be the most elegant, its functionality is evidenced by a long history of at least 6–7 million years (Fig. 1).
Grasp volume and grip strength
The abundance of bamboo in the giant panda’s habitat makes daily foraging distance a very small component of the feeding strategy. Instead, eating fast and in large quantities appear to be of greater importance37. Perhaps the most demanding function of the false thumb is to maintain a tight grip on bamboo stems while the panda uses its teeth to tear and shred stems into bite size portions for consumption. The high strength of bamboo, especially the woody stems during the winter months, requires considerable grip strength by the hands to twist and jerk, countering the powerful biting and tearing by the jaws (see, for example, a panda cam at the San Diego Zoo: https://www.facebook.com/watch/live/?v=562351354170625&ref=watch_permalink). Therefore, it seems likely that a tight grip is more critical to panda’s feeding ability than the volume of their grasp.
The strength of the giant panda grip is dependent on the flexor muscles of the fingers, with the radial sesamoid acting as a passive stop against flexion of the fingers. Pandas are good climbers, especially for evading danger37, which necessitates powerful digital flexor muscles for the claws to penetrate into tree bark. Such musculature also serves well for gripping bamboo during feeding. Because of the functional constraints imposed on the length of the radial sesamoid noted above, pandas never evolved sufficiently long false thumbs to seize large bundles of bamboo, a task that, while desirable, is not critical for survival. It is instead the ability to grip tightly on bamboo stems to oppose strong twisting forces by the jaws that is essential and on which selection has acted. While the giant panda’s false thumb is not the most elegant or dexterous, the persistence of this distinctive morphology for the last six million years suggests that it has fulfilled an essential function for survival of the lineage.
Bulk feeding as a tradeoff in low quality but year-round availability of bamboo
Besides having a false thumb, much else about the giant panda is also unusual and/or enigmatic. Pandas traded the high-protein, omnivorous diet of their ursid ancestors for bamboo, a woody grass of high fiber and low nutrition, but with year-round availability in South China and Southeast Asia. To make this tradeoff work, pandas eat prodigious quantities of bamboo, up to 45 kg/day (depending on the season), and spend ~ 15 h/day eating37. The panda’s short digestive tract, inherited from its carnivoran ancestors, is also poorly suited for extracting nutrients, absorbing less than 20% of digestible dry matter38. Furthermore, pandas lack the high-crowned teeth that most ungulate mammals possess for grinding tough plant fibers into a fine mush and consequently make minimal use of microbials to break down cellulose to extract the structural carbohydrates11. As a result, the panda’s gastrointestinal tract allows a rapid passage of digesta in less than 12 h38, too fast for fermentation when compared to fore- and hindgut fermenting mammalian herbivores, and necessitating an equally prodigious quantity of defecation, up to 100 times/day39.
This extreme bulk feeding strategy thus prioritizes dietary quantity over nutritional quality and nutrient extraction rate. This is made possible by the wide availability of bamboo, practically eliminating energy expenditure for foraging while maximizing the net rate of energy intake40. In modern panda habitats, bamboo (such as Fargesia and Sinarundinaria) are in plentiful, year-round supply, typically more than what pandas can consume (except during periodical bamboo flowering and die outs). With 99% of their food being bamboo and without major competitors for this abundant food resource, nor the need to avoid predators, pandas can thus reduce daily foraging range to within tens of meters of their resting dens, permitting a highly efficient foraging strategy of spending large portions of daily activities feeding and resting within small areas37.
Pandas usually feed while sitting, hooking bamboo stems toward the mouth using curved paws and while biting the leaves/stems, use grasping hands to jerk the stems up and down to help sever them37. The hands are also capable of a twisting action to tear off strips in the mouth, which requires a tight grip. It is difficult to imagine that the panda’s crude false thumb can be useful for conventional omnivorous purposes such as gathering of seeds, nuts, berries, or even low grasses, suggesting that the sole dietary purpose of an enlarged radial sesamoid is bamboo feeding, although a tree-climbing function for the false thumb has been suggested for ailurids6,7. If the dietary function is paramount, the panda’s false thumb must be a crucial adaptation for efficient bamboo procurement within this lineage. Once pandas committed to this bulk feeding strategy, the false thumb was an advantageous solution to the challenge of bamboo manipulation.
Despite the seeming inefficiency of its digestive system, the giant panda’s bulk feeding strategy permitted it to successfully expand to much of South China and Southeast Asia and become a prominent member of the Giant Panda-Stegodon fauna in the Pleistocene41 (Fig. 1). Deep inside the Chinese bamboo forests, giant pandas adopt a solitary, reclusive life of quiet herbivory, retreating from the more dominant position in the food chain of their distant relatives. In adopting a low-quality, year-round bamboo diet, pandas are also unable to store sufficient fat to hibernate, a crucial strategy for ursids to expand into high latitudes and to migrate across Beringia into North America42,43. The panda’s historic range is thus consistent with both the availability of bamboo and a warm climate without the need for hibernation (Fig. 1; note that a previous purported panda record from Zhoukoudian locality 144, a jarring presence in a cold climate, has now been shown to be that of a cave bear25).
The panda’s transition from a broad, omnivorous diet to a highly specialized bamboo diet necessitated multiple changes in anatomy and physiology, as well as their genetics underpinning45,46,47. However, even after at least six million years of a bamboo diet, these transformations are still limited, mostly focused on food handling while the digestive system remains that of a carnivore48. The fact that there was no further elongation of the false thumb in the panda lineage after the late Miocene, suggests that an adequate grip for bamboo had been obtained, i.e., good enough for grasping a single stem or small bundle, and that further enlargement was inhibited by countervailing selection for weight-bearing and walking (Fig. 8). We caution, however, that the fossil record is too incomplete to allow a full understanding of this process and future discoveries will likely reveal unforeseen details.
An artist reconstruction of Ailurarctos from Shuitangba. The grasping function of its false thumb (shown in the right individual) has reached to the level of modern pandas, whereas the radial sesamoid may have protruded slightly more than its modern counterpart during walking (seen in the left individual). Art by Mauricio Antón.
Steven J. Gould’s4 insightful remarks still stand: “the panda’s true thumb is committed to another role, too specialized for a different function to become an opposable, manipulating digit. So the panda must use parts on hand and settle for an enlarged wrist bone and a somewhat clumsy, but quite workable, solution”. However, he would probably have been delighted to learn that the historic contingency of the panda’s false thumb requires that while being a better finger was favored by selection, it also had to bear the burden of considerable body weight.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Lankester, E. R. On the affinities of Aeluropus melanoleucus, A. Milne-Edwards. Trans. Linn. Soc. Lond. Ser. 2 Zool. 8, 163–165 (1901).
Pocock, R. I. Some external characters of the giant panda (Ailuropoda melanoleuca). Proc. Zool. Soc. Lond. 1928, 975–981 (1928).
Wood-Jones, F. The ‘thumb’ of the giant panda. Nature 143, 246. https://doi.org/10.1038/143246b0 (1939).
Gould, S. J. The panda’s peculiar thumb. Nat. Hist. 87(9), 20–30 (1978).
Gould, S.J. The Panda’s Thumb, More Reflections in Natural History paperback edn. (W. W. Norton & Company, New York, 1982).
Salesa, M. J., Antón, M., Peigné, S. & Morales, J. Evidence of a false thumb in a fossil carnivore clarifies the evolution of pandas. Proc. Nat. Acad. Sci. 103, 379–382 (2006).
Antón, M., Salesa, M. J., Pastor, J. F., Peigné, S. & Morales, J. Implications of the functional anatomy of the hand and forearm of Ailurus fulgens (Carnivora, Ailuridae) for the evolution of the ‘false-thumb’ in pandas. J. Anat. 209, 757–764 (2006).
Salesa, M. J., Siliceo, G., Antón, M., Montoya, P. & Morales, J. Anatomy of the “false thumb” of Tremarctos ornatus (Carnivora, Ursidae, Tremarctinae): phylogenetic and functional implications. Est. Geol. 62, 389–394 (2006).
Abella, J. et al. Tracing the origin of the panda’s thumb. Sci. Nat. 102, 1–13. https://doi.org/10.1007/s00114-015-1286-3 (2015).
Davis, D. D. The giant panda, a morphological study of evolutionary mechanisms. Fieldiana Zool. Mem. 3, 1–339 (1964).
Xue, Z. et al. The bamboo-eating giant panda harbors a carnivore-like gut microbiota, with excessive seasonal variations. MBio 6, e00022-e115. https://doi.org/10.1128/mBio.00022-15 (2015).
Wang, D. et al. Significance of the preservation of ‘pseudo-thumb’ in fossil skeletons of giant panda (Ailuropoda melanoleuca) in Shuanghe Cave, Guizhou Province, southern China. Hist. Biol. https://doi.org/10.1080/08912963.2021.2006195 (2021).
Amador, L. I. Sesamoids and morphological variation: a hypothesis on the origin of rod-like skeletal elements in aerial mammals. J. Mamm. Evol. https://doi.org/10.1007/s10914-021-09571-8 (2021).
Qiu, Z.-X. & Qi, G.-Q. Ailuropod found from the late Miocene deposits in Lufeng, Yunnan. Vert. PalAsiat. 27, 153–169 (1989).
Zong, G.-F. in Yuanmou Hominoid Fauna (ed Z.-Q. He) pp 69–89 (Yunnan Science and Technology Press, 1997).
Han, H. et al. Diet evolution and habitat contraction of giant pandas via stable isotope analysis. Curr. Biol. 29, 664–669. https://doi.org/10.1016/j.cub.2018.12.051 (2019).
Loucks, C. J. et al. Giant pandas in a changing landscape. Science 294, 1465–1465. https://doi.org/10.1126/science.1064710 (2001).
Jablonski, N. G. et al. Remains of Holocene giant pandas from Jiangdong Mountain (Yunnan, China) and their relevance to the evolution of quaternary environments in south-western China. Hist. Biol. 24, 527–536. https://doi.org/10.1080/08912963.2011.640400 (2012).
Chen, Y.-P., Ellison, A. M. & Lu, Y.-L. Establish a special conservation zone for the captive giant panda. Ecosyst. Health Sustain. 4, 29–33. https://doi.org/10.1080/20964129.2018.1455990 (2018).
Jin, C.-Z. et al. The first skull of the earliest giant panda. Proc. Nat. Acad. Sci. 104, 10932–10937 (2007).
Wan, Q.-H., Wu, H. & Fang, S.-G. A new subspecies of giant panda (Ailuropoda melanoleuca) from Shaanxi, China. J. Mammal. 86, 397–402. https://doi.org/10.1644/brb-226.1 (2005).
Wang, T.-K. On the taxonomic status of species, geological distribution and evolutionary history of Ailuropoda. Acta Zool. Sin. 20, 191–201 (1974).
Tougard, C., Chaimanee, Y., Suteethorn, V., Triamwichanon, S. & Jaeger, J.-J. Extension of the geographic distribution of the giant panda (Ailuropoda) and search for the reasons for its pregressive disappearance in Southeast Asia during the latest Middle Pleistocene. Comptes Rendus de l’Académie des Sciences Paris, Series II A 323, 973–979 (1996).
Li, T., Lai, X.-L., Wang, W. & Zhou, X.-G. Taxonomy and evolution of giant panda. Geol. Sci. Technol. Inform. 23, 40–46 (2004).
Jiangzuo, Q.-G. et al. Presence of the middle pleistocene cave bears in China confirmed: evidence from Zhoukoudian area. Quat. Sci. Rev. 199, 1–17. https://doi.org/10.1016/j.quascirev.2018.09.012 (2018).
Ryan, W. B. F. et al. Global multi-resolution topography synthesis. Geochem. Geophys. Geosyst. https://doi.org/10.1029/2008gc002332 (2009).
Jiangzuo, Q.-G., Liu, J.-Y. & Chen, J. Morphological homology, evolution, and proposed nomenclature for bear dentition. Acta Palaeontol. Polonica 64, 693–710 (2019).
Zhang, S., Pan, R., Li, M., Oxnard, C. & Wei, F. Mandible of the giant panda (Ailuropoda melanoleuca) compared with other Chinese carnivores: functional adaptation. Biol. J. Linnean Soc. 92, 449–456. https://doi.org/10.1111/j.1095-8312.2007.00876.x (2007).
Vallittu, P. K. et al. Temporomandibular joint and giant panda’s (Ailuropoda melanoleuca) adaptation to bamboo diet. Sci. Rep. 11, 14252. https://doi.org/10.1038/s41598-021-93808-2 (2021).
Wood-Jones, F. The forearm and manus of the giant panda, Ailuropoda melanoleuca, M.-Edw. with an account of the mechanism of its grasp. Proc. Zool. Soc. Lond. B109, 113–129. https://doi.org/10.1111/j.1469-7998.1939.tb00026.x (1939).
Li, Y.-W., et al. Morphology of the Giant Panda, Systematic Anatomy and Organ-Histology (in Chinese). (Science Press, 1986).
Endo, H. et al. Functional anatomy of the radial sesamoid bone in the giant panda (Ailuropoda melanoleuca). J. Anat. 189, 587–592 (1996).
Endo, H. et al. Role of the giant panda’s ‘pseudo-thumb’. Nature 397, 309–310. https://doi.org/10.1038/16830 (1999).
Sheng, G.-L. et al. Ancient DNA from giant panda (Ailuropoda melanoleuca) of south-western China reveals genetic diversity loss during the Holocene. Genes https://doi.org/10.3390/genes9040198 (2018).
Dong, W. & Qi, G.-Q. in Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology (eds Xiaoming W., Lawrence, J.F., & Mikael F.) pp 293–313 (Columbia University Press, 2013).
Abella, J. et al. Kretzoiarctos gen. nov., the oldest member of the giant panda clade. PLoS ONE 7, e48985. https://doi.org/10.1371/journal.pone.0048985 (2012).
Schaller, G. B., Hu, J.-C., Pan, W.-S. & Zhu, J. The Giant Pandas of Wolong. (University of Chicago Press, 1985).
Dierenfeld, E. S., Hintz, H. F., Robertson, J. B., Van Soest, P. J. & Oftedal, O. T. Utilization of bamboo by the giant panda. J. Nutrit. 112, 636–641. https://doi.org/10.1093/jn/112.4.636 (1982).
Hume, I. D. Optimal digestive strategies in mammalian herbivores. Physiol. Zool. 62, 1145–1163 (1989).
Townsend, C. R. & Hughes, R. H. in Physiological Ecology: An Evolutionary Approach to Resource Use (eds C. R. Townsend & P. Callow) 86–108 (Sunderland, 1981).
Rink, W. J., Wei, W., Bekken, D. & Jones, H. L. Geochronology of Ailuropoda-Stegodon fauna and Gigantopithecus in Guangxi Province, southern China. Quat. Res. 69, 377–387. https://doi.org/10.1016/j.yqres.2008.02.008 (2008).
Wang, X., Rybczynski, N., Harington, C. R., White, S. C. & Tedford, R. H. A basal ursine bear (Protarctos abstrusus) from the Pliocene High Arctic reveals Eurasian affinities and a diet rich in fermentable sugars. Sci. Rep. 7, 17722. https://doi.org/10.1038/s41598-017-17657-8 (2017).
Garshelis, D. L. et al. in Bears of the World: Ecology, Conservation and Management (eds Mario, M., & Vincenzo, P.) 53–62 (Cambridge University Press, 2020).
Pei, W.-C. On the Carnivora from Locality 1 of Choukoutien. Palaeontol. Sin. Ser. C 8, 1–216 (1934).
Hu, Y. et al. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Nat. Acad. Sci. 114, 1081–1086. https://doi.org/10.1073/pnas.1613870114 (2017).
Wei, F. et al. Giant pandas are not an evolutionary cul-de-sac: evidence from multidisciplinary research. Mol. Biol. A Evol. 32, 4–12. https://doi.org/10.1093/molbev/msu278 (2014).
Zhao, H., Yang, J.-R., Xu, H. & Zhang, J. Pseudogenization of the umami taste receptor gene Tas1r1 in the giant panda coincided with its dietary switch to bamboo. Mol. Biol. Evol. 27, 2669–2673. https://doi.org/10.1093/molbev/msq153 (2010).
Nie, Y. et al. Giant pandas are macronutritional carnivores. Curr. Biol. 29, 1677–1682. https://doi.org/10.1016/j.cub.2019.03.067 (2019).
Pei, W.-Z. Carnivora, proboscidea and rodentia from the liucheng Gigantopithecus cave and other caves in Guangxi. Mem. Instit. Verteb. Paleontol. Paleoanthropol. Chin. Acad. Sci. 18, 1–115 (1987).
Colbert, E. H. & Hooijer, D. A. Pleistocene mammals from the limestone fissures of Szechwan, China. Bull. Am. Mus. Nat. Hist. 102, 1–134 (1953).
Acknowledgements
We are indebted to Qigao Jiangzuo, who kindly provided us high resolution scanned images of Ailurarctos lufengensis, photos of A. yuanmouensis, and his unpublished measurements of M2 length from Shuanghe Cave. We thank Yanping Song for photographic image processing. See online Supplementary material for additional acknowledgments. We thank Sharon Fisher for her permission to use the panda photograph in Fig. 6. We greatly appreciate comments and suggestions by Juan Abella and an anonymous reviewer, which improved this manuscript. Field excavations between 2007 and 2010 were supported by the United States National Science Foundation (BCS 1035897 to DFS and NGJ, BCS 0321893 to F.C. Howell and T. White, BCS 1227964 to DFS, BCS 1227927 to NGJ, BCS 1227838 to JK), and the Yunnan Natural Science Foundation and Government of Zhaotong (2010CC010 to XPJ). Field excavation in 2015 was supported by special excavation funds from the IVPP, the National Natural Science Foundation of China (41430102 to TD), and the governments of Zhaotong and Zhaoyang.
Author information
Authors and Affiliations
Contributions
D.F.S., N.G.J., J.K., T.D., and X.J. co-led field works at Shuitangba and coordinated work on biostratigraphy and geochronology. X.W. conceived the project, analyzed the data, and produced the figures. X.W. wrote the manuscript text and all authors contributed to reviewing and revising the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Su, D.F., Jablonski, N.G. et al. Earliest giant panda false thumb suggests conflicting demands for locomotion and feeding. Sci Rep 12, 10538 (2022). https://doi.org/10.1038/s41598-022-13402-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13402-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.