Abstract
Secondary aquatic adaptations evolved independently more than 30 times from terrestrial vertebrate ancestors1,2. For decades, non-avian dinosaurs were believed to be an exception to this pattern. Only a few species have been hypothesized to be partly or predominantly aquatic3,4,5,6,7,8,9,10,11. However, these hypotheses remain controversial12,13, largely owing to the difficulty of identifying unambiguous anatomical adaptations for aquatic habits in extinct animals. Here we demonstrate that the relationship between bone density and aquatic ecologies across extant amniotes provides a reliable inference of aquatic habits in extinct species. We use this approach to evaluate the distribution of aquatic adaptations among non-avian dinosaurs. We find strong support for aquatic habits in spinosaurids, associated with a marked increase in bone density, which precedes the evolution of more conspicuous anatomical modifications, a pattern also observed in other aquatic reptiles and mammals14,15,16. Spinosaurids are revealed to be aquatic specialists with surprising ecological disparity, including subaqueous foraging behaviour in Spinosaurus and Baryonyx, and non-diving habits in Suchomimus. Adaptation to aquatic environments appeared in spinosaurids during the Early Cretaceous, following their divergence from other tetanuran theropods during the Early Jurassic17.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data described and used in this manuscript are freely available. The measurements and provenance information for fossil specimens can be found in the extended data figures and in the Supplementary Dataset. The phylogenetic datasets and the R coding are available as Supplementary Material. The CT scan datasets collected for this study are available in Morphosource (specific links for each taxon can be found in the Supplementary Dataset).
References
Kelley, N. P. & Pyenson, N. D. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science 348, aaa3716 (2015).
Gutarra, S. & Rahman, I. A. The locomotion of extinct secondarily aquatic tetrapods. Biol. Rev. 97, 67–98 (2022).
Owen, R. A description of a portion of the skeleton of the Cetiosaurus, a gigantic extinct saurian reptile occurring in the oolitic formations of different portions of England. Proc. Geol. Soc. Lond. 3, 457–462 (1841).
Cope, E. On the characters of the skull in the Hadrosauridae. Proc. Natl Acad. Nat. Sci. USA 35, 97–107 (1883).
Bidar, A., Demay, L. & Thomel, G. Compsognathus corallestris, une nouvelle espèce de dinosaurien théropode du Portlandien de Canjuers (Sud-Est de la France). Annales Muséum d’Histoire Naturelle de Nice 1, 9–40 (1972).
Norell, M. A., Makovicky, P. J. & Currie, P. J. The beaks of ostrich dinosaurs. Nature 412, 873–874 (2001).
Tereschenko, V. S. Adaptive features of protoceratopoids (Ornithischia: Neoceratopsia). Paleontol. J. 42, 273–286 (2008).
Lee, Y. N. et al. Resolving the long-standing enigmas of a giant ornithomimosaur Deinocheirus mirificus. Nature 515, 257–260 (2014).
Ibrahim, N. et al. Semiaquatic adaptations in a giant predatory dinosaur. Science 345, 1613–1616 (2014).
Cau, A. et al. Synchrotron scanning reveals amphibious ecomorphology in a new clade of bird-like dinosaurs. Nature 552, 395–399 (2017).
Ibrahim, N. et al. Tail-propelled aquatic locomotion in a theropod dinosaur. Nature 581, 67–70 (2020).
Henderson, D. M. A buoyancy, balance and stability challenge to the hypothesis of a semi-aquatic Spinosaurus Stromer, 1915 (Dinosauria: Theropoda). PeerJ 6, e5409 (2018).
Hone, D. W. E. & Holtz, T. R. Jr Evaluating the ecology of Spinosaurus: shoreline generalist or aquatic pursuit specialist? Palaeontol. Electronica 24, a03 (2021).
Thewissen, J. G., Cooper, L. N., Clementz, M. T., Bajpai, S. & Tiwari, B. N. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 450, 1190–1194 (2007).
Houssaye, A. Bone histology of aquatic reptiles: what does it tell us about secondary adaptation to an aquatic life? Biol. J. Linn. Soc. 108, 3–21 (2013).
Motani, R. et al. A basal ichthyosauriform with a short snout from the Lower Triassic of China. Nature 517, 485–488 (2015).
Rauhut, O. W. & Pol, D. Probable basal allosauroid from the early Middle Jurassic Cañadón Asfalto Formation of Argentina highlights phylogenetic uncertainty in tetanuran theropod dinosaurs. Sci. Rep. 9, 1–9 (2019).
You, H. L. et al. A nearly modern amphibious bird from the Early Cretaceous of northwestern China. Science 312, 1640–1643 (2006).
Wilson, L. E. & Chin, K. Comparative osteohistology of Hesperornis with reference to pygoscelid penguins: the effects of climate and behaviour on avian bone microstructure. R. Soc. Open Sci. 1, 140245 (2014).
Gatesy, S. M. & Dial, K. P. Locomotor modules and the evolution of avian flight. Evolution 50, 331–340 (1996).
Amiot, R. et al. Oxygen isotope evidence for semi-aquatic habits among spinosaurid theropods. Geology 38, 139–142 (2010).
Hassler, A. et al. Calcium isotopes offer clues on resource partitioning among Cretaceous predatory dinosaurs. Proc. R. Soc. B 285, 20180197 (2018).
Larramendi, A., Paul, G. S. & Hsu, S. Y. A review and reappraisal of the specific gravities of present and past multicellular organisms, with an emphasis on tetrapods. Anat. Rec. 304, 1833–1888 (2021).
Charig, A. J. & Milner, A. C. Baryonyx, a remarkable new theropod dinosaur. Nature 324, 359–361 (1986).
Schoener, T. W. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429 (2011).
Houssaye, A. “Pachyostosis” in aquatic amniotes: a review. Integr. Zool. 4, 325–340 (2009).
Houssaye, A., Sander, M. P. & Klein, N. Adaptive patterns in aquatic amniote bone microanatomy—more complex than previously thought. Integr. Comp. Biol. 56, 1349–1369 (2016).
Quemeneur, S., De Buffrenil, V. & Laurin, M. Microanatomy of the amniote femur and inference of lifestyle in limbed vertebrates. Biol. J. Linn. Soc. 109, 644–655 (2013).
Canoville, A., de Buffrénil, V. & Laurin, M. Microanatomical diversity of amniote ribs: an exploratory quantitative study. Biol. J. Linn. Soc. 118, 706–733 (2016).
Amson, E., de Muizon, C., Laurin, M., Argot, C. & de Buffrénil, V. Gradual adaptation of bone structure to aquatic lifestyle in extinct sloths from Peru. Proc. R. Soc. B 281, 20140192 (2014).
Grafen, A. The phylogenetic regression. Philos. Trans. R. Soc. B 326, 119–157 (1989).
Liem, K. F. Adaptive significance of intra-and interspecific differences in the feeding repertoires of cichlid fishes. Am. Zool. 20, 295–314 (1980).
Turner, A. H., Pol, D., Clarke, J. A., Erickson, G. M. & Norell, M. A. A basal dromaeosaurid and size evolution preceding avian flight. Science 317, 1378–1381 (2007).
Voeten, D. F. et al. Wing bone geometry reveals active flight in Archaeopteryx. Nat. Commun. 9, 1319 (2018).
Houssaye, A., Martin, F., Boisserie, J. R. & Lihoreau, F. Paleoecological inferences from long bone microanatomical specializations in Hippopotamoidea (Mammalia, Artiodactyla). J. Mamm. Evol. 28, 847–870 (2021).
Amson, E. & Bibi, F. Differing effects of size and lifestyle on bone structure in mammals. BMC Biol. 19, 87 (2021).
Malafaia, E. et al. A new spinosaurid theropod (Dinosauria: Megalosauroidea) from the upper Barremian of Vallibona, Spain: Implications for spinosaurid diversity in the Early Cretaceous of the Iberian Peninsula. Cret. Res. 106, 104221 (2020).
Sereno, P. C. et al. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302 (1998).
Aureliano, T. et al. Semi-aquatic adaptations in a spinosaur from the Lower Cretaceous of Brazil. Cret. Res. 90, 283–295 (2018).
Barker, C. T. et al. New spinosaurids from the Wessex Formation (Early Cretaceous, UK) and the European origins of Spinosauridae. Sci. Rep. 11, 19340 (2021).
Taquet, P. Géologie et Paléontologie du Gisement de Gadoufaoua (Aptien du Niger) (Éditions du Centre national de la Recherche Scientifique, 1976).
Rayfield, E. J., Milner, A. C., Xuan, V. B. & Young, P. G. Functional morphology of spinosaur ‘crocodile-mimic’ dinosaurs. J. Vertebr. Paleontol. 27, 892–901 (2007).
Benson, R. B., Butler, R. J., Carrano, M. T. & O’Connor, P. M. Air‐filled postcranial bones in theropod dinosaurs: physiological implications and the ‘reptile’–bird transition. Biol. Rev. 87, 168–193 (2012).
Reid, R. E. H. Zonal “growth rings” in dinosaurs. Mod. Geol. 15, 19–48 (1990).
Chinsamy, A. & Raath, M. A. Preparation of fossil bone for histological examination. Palaeont. Afr. 29, 39–44 (1992).
Griffin, C. T. et al. Assessing ontogenetic maturity in extinct saurian reptiles. Biol. Rev. 96, 470–525 (2021).
Carrano, M. T., Benson, R. B. & Sampson, S. D. The phylogeny of Tetanurae (Dinosauria: Theropoda). J. Syst. Palaeontol. 10, 211–300 (2012).
Ibrahim, N. et al. Geology and paleontology of the Upper Cretaceous Kem Kem Group of eastern Morocco. ZooKeys 928, 1–216 (2020).
Smyth, R. S., Ibrahim, N. & Martill, D. M. Sigilmassasaurus is Spinosaurus: a reappraisal of African spinosaurines. Cret. Res. 114, 104520 (2020).
Goloboff, P. A., Farris, J. S. & Nixon, K. C. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008).
Erickson, G. M. Assessing dinosaur growth patterns: a microscopic revolution. Trends Ecol. Evol. 20, 677–684 (2005).
Hayashi, S. et al. Bone inner structure suggests increasing aquatic adaptations in Desmostylia (Mammalia, Afrotheria). PLoS ONE 8, e59146 (2013).
Straehl, F. R., Scheyer, T. M., Forasiepi, A. M., MacPhee, R. D. E. & Sánchez-Villagra, M. R. Evolutionary patterns of bone histology and bone compactness in xenarthran mammal long bones. PLoS ONE 8, e69275 (2013).
Houssaye, A., Tafforeau, P., de Muizon, C. & Gingerich, P. D. Transition of Eocene whales from land to sea: evidence from bone microstructure. PLoS ONE 10, e0118409 (2015).
Girondot, M. & Laurin, M. Bone profiler: a tool to quantify, model, and statistically compare bone-section compactness profiles. J. Vertebr. Paleontol. 23, 458–461 (2003).
De Ricqlès, A. J., Padian, K., Horner, J. R., Lamm, E. T. & Myhrvold, N. Osteohistology of Confuciusornis sanctus (Theropoda: Aves). Journ. Vertebr. Paleontol. 23, 373–386 (2003).
Maddison, W. P. Mesquite: a modular system for evolutionary analysis. Evolution 62, 1103–1118 (2008).
Upham, N. S., Esselstyn, J. A. & Jetz, W. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019).
Simoes, T. R. et al. The origin of squamates revealed by a Middle Triassic lizard from the Italian Alps. Nature 557, 706–709 (2018).
Nesbitt, S. J. et al. The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature 544, 484–487 (2017).
Langer, M. C. et al. Untangling the dinosaur family tree. Nature 551, E1–E3 (2017).
Brusatte, S. L., Lloyd, G. T., Wang, S. C. & Norell, M. A. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr. Biol. 24, 2386–2392 (2014).
Prum, R. O. et al. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573 (2015).
Bapst, D. W. paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods Ecol. Evol. 3, 803–807 (2012).
Schmitz, L. & Motani, R. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332, 705–708 (2011).
Motani, R. & Schmitz, L. Phylogenetic versus functional signals in the evolution of form–function relationships in terrestrial vision. Evolution 65, 2245–2257 (2011).
Acknowledgements
We acknowledge P. Barrett and S. Chapman for access to the holotype of Baryonyx at the Natural History Museum, London, J. Scannella for access to thin sections of Tyrannosaurus housed at the Museum of the Rockies, and M. Fox and J. Gauthier for access to Poposaurus at the Yale Peabody Museum. The Moroccan Ministry of Energy, Mines, and the Environment is thanked for providing fieldwork permits to N. I. Members of the 2015–2019 expedition seasons are thanked for their assistance in the field. We thank J. Choiniere, P. Falkingham, S. Nesbitt and the other, anonymous, reviewer for constructive comments that improved the manuscript. Funding was received from the European Union’s Horizon 2020 research and innovation program 2014–2018, starting grant (R. B. J. B., 677774); a National Geographic Society grant (N.I., CP-143R-170); a National Geographic Emerging Explorer Grant (N.I.); the Jurassic Foundation (M.F.); the Paleontological Society grant (M.F.), as well as the Explorers Club (grant awarded to M.F.). The Lokschuppen (Rosenheim, Germany) and J. Pfauntsch provided additional financial support for fieldwork led by N.I. in Morocco.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.F. Data collection and curation: all authors. Data quantification: M.F. Methodology: M.F., G.N. and R.B.J.B. Formal analysis: M.F., G.N. and R.B.J.B. Resources: all authors. Writing, original draft preparation: M.F. Writing, review and editing: all authors. Visualization: M.F. and G.N.; Supervision: M.F., G.N., R.B.J.B. and N.I. Funding acquisition: M.F., R.B.J.B. and N.I.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jonah Choiniere, Peter Falkingham, Sterling Nesbitt and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
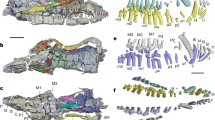
Extended Data Fig. 1 Comparative array of archosaurian femoral diaphysis included in the dataset.
Numerical values represent the bone density quantified for each taxon. Asterisks indicate femoral diaphysis that were retro-deformed before quantification of bone density due to taphonomic deformation and/or fragmentation present in the fossil.
Extended Data Fig. 2 Comparative array of non-avian and avian femoral diaphysis included in the dataset.
Numerical values represent the bone density quantified for each taxon.
Extended Data Fig. 3 Comparative array of avian and lepidosaur femoral diaphysis included in the dataset.
Numerical values represent the bone density quantified for each taxon.
Extended Data Fig. 4 Comparative array of amniote femoral diaphysis included in the dataset.
Numerical values represent the bone density quantified for each taxon.
Extended Data Fig. 5 Comparative array of mammalian femoral diaphysis included in the dataset.
Numerical values represent the bone density quantified for each taxon.
Extended Data Fig. 6 Comparative array of archosaurian dorsal rib cross sections included in the dataset.
Numerical values represent the bone density quantified for each taxon.
Extended Data Fig. 7 Comparative array of amniote dorsal rib cross sections included in the dataset.
Numerical values represent the bone density quantified for each taxon.
Extended Data Fig. 8
Bone density and femur diameter phylogenetic distribution plotted on the informal consensus tree used for discriminant analyses representing the phylogenetic relationships of the taxa included in our study.
Extended Data Fig. 9
Bone density and dorsal rib diameter phylogenetic distribution plotted on the informal consensus tree used for discriminant analyses representing the phylogenetic relationships of the taxa included in our study.
Extended Data Fig. 10 Qualitative comparison of bone compactness in selected skeletal elements between osteosclerotic spinosaurids and other non-avian dinosaurs.
Baryonyx and Spinosaurus possess dense, compact bone throughout the postcranial skeleton, namely in the neural spines, ribs, scapula, ilium, pubis, ischium, femur, and fibula. Increased bone density is found in postcranial elements of Spinosaurus as well; a reduced medullary cavity is present in the ribs, dorsal and caudal neural spines, manual phalanges, femur, tibia, and fibula. Abbreviations: bd=bone density.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–3 and Tables 1–10
Supplementary Dataset
This folder contains the list of taxa analysed in this study; R coding; phylogenetic dataset from Malafaia et al. (2020) and phylogenetic dataset from Rauhut & Pol (2019)
Rights and permissions
About this article
Cite this article
Fabbri, M., Navalón, G., Benson, R.B.J. et al. Subaqueous foraging among carnivorous dinosaurs. Nature 603, 852–857 (2022). https://doi.org/10.1038/s41586-022-04528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04528-0
This article is cited by
-
A new spinosaurid dinosaur species from the Early Cretaceous of Cinctorres (Spain)
Scientific Reports (2023)
-
Unravelling the postural diversity of mammals: Contribution of humeral cross-sections to palaeobiological inferences
Journal of Mammalian Evolution (2023)
-
A non-avian dinosaur with a streamlined body exhibits potential adaptations for swimming
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.