Abstract
Plastics are key components of almost any technology today. Although their production consumes substantial feedstock resources, plastics are largely disposed of after their service life. In terms of a circular economy1,2,3,4,5,6,7,8, reuse of post-consumer sorted polymers (‘mechanical recycling’) is hampered by deterioration of materials performance9,10. Chemical recycling1,11 via depolymerization to monomer offers an alternative that retains high-performance properties. The linear hydrocarbon chains of polyethylene12 enable crystalline packing and provide excellent materials properties13. Their inert nature hinders chemical recycling, however, necessitating temperatures above 600 degrees Celsius and recovering ethylene with a yield of less than 10 per cent3,11,14. Here we show that renewable polycarbonates and polyesters with a low density of in-chain functional groups as break points in a polyethylene chain can be recycled chemically by solvolysis with a recovery rate of more than 96 per cent. At the same time, the break points do not disturb the crystalline polyethylene structure, and the desirable materials properties (like those of high-density polyethylene) are fully retained upon recycling. Processing can be performed by common injection moulding and the materials are well-suited for additive manufacturing, such as 3D printing. Selective removal from model polymer waste streams is possible. In our approach, the initial polymers result from polycondensation of long-chain building blocks, derived by state-of-the-art catalytic schemes from common plant oil feedstocks, or microalgae oils15. This allows closed-loop recycling of polyethylene-like materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on request.
References
Vollmer, I. et al. Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 59, 15402–15423 (2020).
The New Plastics Economy—rethinking the future of plastics. Ellen MacArthur Foundation https://www.ellenmacarthurfoundation.org/assets/downloads/EllenMacArthurFoundation_TheNewPlasticsEconomy_Pages.pdf (2016).
Hees, T., Zhong, F., Stürzel, M. & Mülhaupt, R. Tailoring hydrocarbon polymers and all-hydrocarbon composites for circular economy. Macromol. Rapid Commun. 40, 1800608 (2019).
De Hoe, G. X. et al. Sustainable polyester elastomers from lactones: synthesis, properties, and enzymatic hydrolyzability. J. Am. Chem. Soc. 140, 963–973 (2018).
Rahimi, A. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
Zhang, X., Fevre, M., Jones, G. O. & Waymouth, R. M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 118, 839–885 (2018).
Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Eagan, J. M. et al. Combining polyethylene and polypropylene: enhanced performance with PE/iPP multiblock polymers. Science 355, 814–816 (2017).
Ragaert, K., Delva, L. & van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017).
Lopez, G., Artetxe, M., Amutio, M., Bilbao, J. & Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 73, 346–368 (2017).
Stürzel, M., Mihan, S. & Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 116, 1398–1433 (2016).
Strobl, G. R. The Physics of Polymers (Springer, 2007).
Kaminsky, W. Production of polyolefins by metallocene catalysts and their recycling by pyrolysis. Macromol. Symp. 360, 10–22 (2016).
Stempfle, F., Ortmann, P. & Mecking, S. Long-chain aliphatic polymers to bridge the gap between semicrystalline polyolefins and traditional polycondensates. Chem. Rev. 116, 4597–4641 (2016).
Chikkali, S. & Mecking, S. Refining of plant oils to chemicals by olefin metathesis. Angew. Chem. Int. Ed. 51, 5802–5808 (2012).
Technology—manufacturing. Elevance Renewable Sciences https://elevance.com/technology/?highlight=press%20release (2013).
Biermann, U., Bornscheuer, U., Meier, M. A. R., Metzger, J. O. & Schäfer, H. J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 50, 3854–3871 (2011).
Ortmann, P., Heckler, I. & Mecking, S. Physical properties and hydrolytic degradability of polyethylene-like polyacetals and polycarbonates. Green Chem. 16, 1816–1827 (2014).
Mutlu, H., Hofsäß, R., Montenegro, R. E. & Meier, M. A. R. Self-metathesis of fatty acid methyl esters: full conversion by choosing the appropriate plant oil. RSC Adv. 3, 4927–4934 (2013).
Jeremic, D. in Ullmann’s Encyclopedia of Industrial Chemistry (ed. Elvers, B.) 1–42 (Wiley-VCH, 2014).
Sanchez, I. C. & Eby, R. K. Thermodynamics and crystallization of random copolymers. Macromolecules 8, 638–641 (1975).
Schirmeister, C. G., Hees, T., Licht, E. H. & Mülhaupt, R. 3D printing of high density polyethylene by fused filament fabrication. Addit. Manuf. 28, 152–159 (2019).
Witt, T., Häußler, M., Kulpa, S. & Mecking, S. Chain multiplication of fatty acids to precise telechelic polyethylene. Angew. Chem. Int. Ed. 56, 7589–7594 (2017).
Capello, C., Fischer, U. & Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 9, 927–934 (2007).
Huf, S., Krügener, S., Hirth, T., Rupp, S. & Zibek, S. Biotechnological synthesis of long-chain dicarboxylic acids as building blocks for polymers. Eur. J. Lipid Sci. Technol. 113, 548–561 (2011).
Hess, S. K., Lepetit, B., Kroth, P. G. & Mecking, S. Production of chemicals from microalgae lipids—status and perspectives. Eur. J. Lipid Sci. Technol. 120, 1700152 (2018).
Fowkes, F. M. Attractive forces at interfaces. Ind. Eng. Chem. 56, 40–52 (1964).
Stempfle, F., Quinzler, D., Heckler, I. & Mecking, S. Long-chain linear C19 and C23 monomers and polycondensates from unsaturated fatty acid esters. Macromolecules 44, 4159–4166 (2011).
Otera, J. & Nishikido, J. Esterification. Methods, Reactions, and Applications 2nd edn (Wiley-VCH, 2010).
Sun, J., Aly, K. I. & Kuckling, D. A novel one-pot process for the preparation of linear and hyperbranched polycarbonates of various diols and triols using dimethyl carbonate. RSC Adv. 7, 12550–12560 (2017).
AlAbbad, M., Giri, B. R., Szori, M., Viskolcz, B. & Farooq, A. A high temperature kinetic study for the thermal unimolecular decomposition of diethyl carbonate. Chem. Phys. Lett. 684, 390–396 (2017).
Acknowledgements
We thank M. Schnitte and L. Bolk for GPC and DSC measurements. Support of our studies of degradable polyethylenes by the ERC (Advanced Grant DEEPCAT, number 832480) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
S.M. conceived the concept. M.E., M.H. and S.M. jointly devised the experimental programme. M.H. and D.R. performed experiments with polycarbonates and 3D printing. M.E. performed experiments with polyesters. M.H., M.E. and S.M. wrote the manuscript. M.H. and M.E. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
A patent has been filed by the University of Konstanz on findings reported here.
Additional information
Peer review information Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
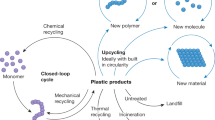
Extended Data Fig. 1 The presented closed-loop recycling concept.
Long-chain aliphatic building blocks (‘monomer’) obtained from biorefining of plant or microalgae oils (‘bio-based’, top inset) are polymerized (1) to give polycarbonates and polyesters with polyethylene-like properties. These materials can be processed (2) by common injection moulding and they are also well suited for additive manufacturing (such as 3D printing). After the useful service life of the polyethylene-like polymers—here the materials application is tensile testing (3)—chemical recycling (4) via a solvolysis process to the underlying monomers is enabled by the functional groups present in the polymer backbone. The recovered monomers can be repolymerized to materials with properties on a par with the initial polymers with an overall recycling rate of >96%.
Extended Data Fig. 2 Decarboxylation of diethyl carbonate endgroups.
To achieve high molecular weights for long-chain aliphatic polycarbonates, diethyl carbonate was employed as a monomer. Unlike the commonly used dimethyl carbonate19,31, this provides an additional pathway for molecular-weight build-up. In the final stages of the polycondensation reaction, condensation of two carbonate endgroups with liberation of ROC(=O)OR is the prevailing reaction. This is a kinetically less favoured reaction compared to the initially dominant transesterification of carbonate groups with alcohols. This lower reactivity is particularly relevant as in this critical stage of the reaction at high conversions of functional groups the concentration of reacting groups is low. For the case of diethyl carbonate as a monomer, decomposition of –OC(=O)OEt end groups to carbon dioxide and ethylene can yield more reactive –OH groups. This pathway32 enables the decisively higher final product molecular weights observed with diethyl carbonate as a monomer. a, Model reaction of 5 g of 1,12-dodecanediol with diethyl carbonate (DEC) to investigate the decarboxylation of diethyl carbonate endgroups probing for the formation of CO2 and ethylene. b, Left, schematic experimental setup for the capture of CO2 in aqueous Ba(OH)2 (washing flask 1) and ethylene in CDCl3 (washing flask 2) from the condensed volatiles of the model reaction. Right, photographs showing the increasing turbidity of the Ba(OH)2 solution upon formation of BaCO3 (1), and a detail of the 1H NMR spectrum of the CDCl3 solution with the characteristic resonance for ethylene (2). c, Proposed decomposition mechanism of ethyl carbonate end groups during the polycondensation. B represents a base present in the reaction mixture that can facilitate the abstraction of the terminal proton.
Extended Data Fig. 3 Thermal and solid-state properties of polymers.
a, Comparison of the WAXS diffractograms of PC-18, PE-18,18, PC-48 and HDPE. Diffraction peaks correspond to the orthorhombic unit cell of polyethylene in all cases and all materials exhibit an HDPE-like crystallinity of about 80%. b, Comparison of the differential scanning calorimetry (DSC) traces of PC-18, PE-18,18, PC-48 and HDPE (top, heating curve; bottom, cooling curve). c, Thermogravimetric analysis traces of PC-18 and PE-18,18 in comparison to that of HDPE.
Extended Data Fig. 4 Mechanical properties of 3D-printed PC-18 and PE-18,18 tensile testing specimens.
a, 3D-printed PE-18,18 tensile testing specimen on the print bed. b, As a but for blue-coloured PC-18. c, As a but for carbon fibre (CF)-reinforced PC-18. d, 3D-printed PC-18 tensile testing specimens after removal from the print bed. e, 3D-printed PC-18 tensile testing specimens after tensile testing. f, Representative stress–strain curves of 3D-printed PC-18 and PE-18,18 tensile testing specimens.
Extended Data Fig. 5 Depolymerization of PC-18 and PE-18,18.
a, b, Degree of polymerization of PC-18 (a) and PE-18,18 (b) as a function of time using ethanol or methanol at different temperatures and with and without KOH; c, depolymerization of PC-18 in water. For a and b, to assure a facile and rapid depolymerization in the recycling of the polymers, different conditions (see keys) were screened for both PC-18 and PE-18,18 in small-scale pressure reactor experiments. 200 mg of polymer was stirred (500 rpm) with 5 ml of methanol or ethanol, respectively, at the temperature and for the duration listed. Optionally, 10 wt% KOH with respect to the mass of polymer was added as a basic depolymerization catalyst. The volatiles of the obtained reaction mixture were removed under reduced pressure and the degree of polymerization of the crude product was determined by 1H-NMR spectroscopy in tetrachloroethane-d2 at 383 K. Catalysis of the polyester depolymerization reaction by KOH lead to partial saponification of the 1,18-dimethyl octadecanedioate monomer. c, Depolymerization in water (50 wt% KOH) of PC-18 also proceeded completely to the monomer, the carbonate being released as CO2. The 1H NMR spectrum (383 K, 400 MHz, C2D2Cl4) shows the precipitated hydrolysis product 1,18-octadecane diol obtained by filtration of the reaction mixture.
Extended Data Fig. 6 Properties of recycled PC-18.
a, DSC traces of virgin and recycled PC-18. The melting temperature (Tm), crystallization temperature (Tc), melt enthalpy (ΔHm) and crystallization enthalpy (ΔHc) are given. b, GPC traces of virgin and recycled PC-18. The number- and weight-average molecular weight (Mw and Mn, respectively), and polydispersity (Đ = Mw/Mn) are given. c, 1H-NMR spectrum (C2D2Cl4, 383 K, 400 MHz) of recycled PC-18.
Extended Data Fig. 7 Depolymerization of PC-18 in presence of polyolefins and PET.
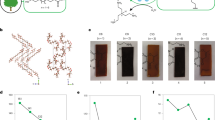
To simulate a potential waste stream, methanolysis of coloured PC-18 was conducted in the presence of polyolefin samples (HDPE, PP) and PET. a, Blue-coloured PC-18 and specimens of HDPE and PP with their respective weights before depolymerization. b, In contrast to the depolymerized and dissolved PC-18, the polyolefin samples remained unchanged during the solvolysis process and could be separated from the monomer solution formed from PC-18, with the blue colourant deposited on their surfaces. c, 1,18-octadecane diol crystallized from the MeOH monomer solution. d, GC analysis of 1,18-octadecane diol recovered (FID, flame ionization detector; tr, retention time). e, Specimens of blue-coloured PC-18 and PET with their respective weights before depolymerization. f, Depolymerization mixture after 24 h at 150 °C in MeOH. The PC-18 specimen depolymerized to a monomer solution while the PET specimen retained its shape and could be separated. The blue colourant was deposited on the surface of the stirring bar. g, 1,18-Octadecane diol crystallized from the MeOH monomer solution. h, 1H NMR spectrum (383 K, 400 MHz, C2D2Cl4) of 1,18-octadecane diol recovered by depolymerization in the presence of PET. The absence of the characteristic resonances for PET (dashed blue spectrum, shown for comparison) underline the successful separation.
Extended Data Fig. 8 Depolymerization of PE-18,18 from a blend with HDPE.
To simulate potential contamination of a HDPE waste stream, blends of HDPE with PE-18,18 were generated by melt compounding. a, Tensile properties of 80/20 wt% and 95/5 wt% HDPE/PE-18,18 blends. These show no adverse impact of the presence of PE-18,18 compared to the neat HDPE. b, Photographs of a HDPE/PE-18,18 80/20 wt% blend specimen before and after depolymerization by methanolysis (120 °C, 10 wt% KOH, 24 h). c, Infrared spectra and d, 1H-NMR spectra (C2D2Cl4, 383 K, 400 MHz) of the original HDPE/PE-18,18 80/20 wt% blend and of the residue from depolymerization. The absence of the carbonyl band and of the characteristic ester 1H resonances demonstrates the removal of the PE-18,18 component from the blend.
Extended Data Fig. 9 Properties of recycled PE-18,18.
a, DSC traces of virgin and recycled PE-18,18. b, GPC traces of virgin and recycled PE-18,18. c, 1H-NMR spectrum (C2D2Cl4, 383 K, 400 MHz) of recycled PE-18,18.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, including monomer preparation and characterization, additional polymer characterization (NMR and IR spectra, DSC traces, elemental analysis, crystallinity values, surface tension), details of depolymerization procedures, polymer endgroup analysis, water stability at ambient conditions, setup for filament fabrication, 3D printing parameters, estimation of environmental impact of monomer sourcing. It also includes 55 Supplementary Figures and 6 Supplementary Tables.
Rights and permissions
About this article
Cite this article
Häußler, M., Eck, M., Rothauer, D. et al. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021). https://doi.org/10.1038/s41586-020-03149-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-03149-9
This article is cited by
-
To curb plastic pollution, industry and academia must unite
Nature (2024)
-
Circular olefin copolymers made de novo from ethylene and α-olefins
Nature Communications (2024)
-
Proton-triggered topological transformation in superbase-mediated selective polymerization enables access to ultrahigh-molar-mass cyclic polymers
Nature Chemistry (2024)
-
Closed-loop recycling of sulfur-rich polymers with tunable properties spanning thermoplastics, elastomers, and vitrimers
Nature Communications (2024)
-
Preparation of Chemically Recyclable Poly(ether-alt-ester) by the Ring Opening Polymerization of Cyclic Monomers Synthesized by Coupling Glycolide and Epoxides
Chinese Journal of Polymer Science (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.