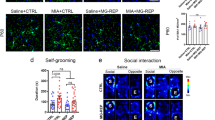
Abstract
A subset of children with autism spectrum disorder appear to show an improvement in their behavioural symptoms during the course of a fever, a sign of systemic inflammation1,2. Here we elucidate the molecular and neural mechanisms that underlie the beneficial effects of inflammation on social behaviour deficits in mice. We compared an environmental model of neurodevelopmental disorders in which mice were exposed to maternal immune activation (MIA) during embryogenesis3,4 with mouse models that are genetically deficient for contactin-associated protein-like 2 (Cntnap2)5, fragile X mental retardation-1 (Fmr1)6 or Sh3 and multiple ankyrin repeat domains 3 (Shank3)7. We establish that the social behaviour deficits in offspring exposed to MIA can be temporarily rescued by the inflammatory response elicited by the administration of lipopolysaccharide (LPS). This behavioural rescue was accompanied by a reduction in neuronal activity in the primary somatosensory cortex dysgranular zone (S1DZ), the hyperactivity of which was previously implicated in the manifestation of behavioural phenotypes associated with offspring exposed to MIA8. By contrast, we did not observe an LPS-induced rescue of social deficits in the monogenic models. We demonstrate that the differences in responsiveness to the LPS treatment between the MIA and the monogenic models emerge from differences in the levels of cytokine production. LPS treatment in monogenic mutant mice did not induce amounts of interleukin-17a (IL-17a) comparable to those induced in MIA offspring; bypassing this difference by directly delivering IL-17a into S1DZ was sufficient to promote sociability in monogenic mutant mice as well as in MIA offspring. Conversely, abrogating the expression of IL-17 receptor subunit a (IL-17Ra) in the neurons of the S1DZ eliminated the ability of LPS to reverse the sociability phenotypes in MIA offspring. Our data support a neuroimmune mechanism that underlies neurodevelopmental disorders in which the production of IL-17a during inflammation can ameliorate the expression of social behaviour deficits by directly affecting neuronal activity in the central nervous system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
29 January 2020
This article was amended to update Figs 1-4, which were originally converted erroneously from RGB to CMYK.
References
Curran, L. K. et al. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 120, e1386–e1392 (2007).
Grzadzinski, R., Lord, C., Sanders, S. J., Werling, D. & Bal, V. H. Children with autism spectrum disorder who improve with fever: insights from the Simons Simplex Collection. Autism Res. 11, 175–184 (2018).
Shi, L., Fatemi, S. H., Sidwell, R. W. & Patterson, P. H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 23, 297–302 (2003).
Smith, S. E. P., Li, J., Garbett, K., Mirnics, K. & Patterson, P. H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702 (2007).
Peñagarikano, O. et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147, 235–246 (2011).
The Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33 (1994).
Peça, J. et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472, 437–442 (2011).
Yim, Y. et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487 (2017).
Whitrow, M. Wagner-Jauregg and fever therapy. Med. Hist. 34, 294–310 (1990).
Lyall, K. et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 38, 81–102 (2017).
Crawley, J. N. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 14, 293–305 (2012).
Ey, E., Leblond, C. S. & Bourgeron, T. Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 4, 5–16 (2011).
Patterson, P. H. Maternal infection and immune involvement in autism. Trends Mol. Med. 17, 389–394 (2011).
Kozak, W., Conn, C. A. & Kluger, M. J. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am. J. Physiol. 266, R125–R135 (1994).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Malkova, N. V., Yu, C. Z., Hsiao, E. Y., Moore, M. J. & Patterson, P. H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616 (2012).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Zhao, Z. D. et al. A hypothalamic circuit that controls body temperature. Proc. Natl Acad. Sci. USA 114, 2042–2047 (2017).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Gogolla, N. et al. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181 (2009).
Selby, L., Zhang, C. & Sun, Q. Q. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci. Lett. 412, 227–232 (2007).
Orefice, L. L. et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell 178, 867–886 (2019).
Erickson, M. A. & Banks, W. A. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav. Immun. 25, 1637–1648 (2011).
Chen, C. et al. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542, 43–48 (2017).
Kim, S. et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 (2017).
Lammert, C. R. et al. Cutting edge: critical roles for microbiota-mediated regulation of the immune system in a prenatal immune activation model of autism. J. Immunol. 201, 845–850 (2018).
El Malki, K. et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J. Invest. Dermatol. 133, 441–451 (2013).
Tusi, B. K. et al. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60 (2018).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Silverman, J. L., Yang, M., Lord, C. & Crawley, J. N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502 (2010).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates (Academic, 2001).
Hrvatin, S. et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 21, 120–129 (2018).
Halassa, M. M. et al. State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808–821 (2014).
Brunetti, P. M. et al. Design and fabrication of ultralight weight, adjustable multi-electrode probes for electrophysiological recordings in mice. J. Vis. Exp. 91, e51675 (2014).
Acknowledgements
We thank N. Soares, M. Garcia and Y. Liu for assistance with experiments, and B. Noro and M. Trombly for critical reading of the manuscript. This work was supported by the National Institute of Mental Health (1-R01-MH115037-01, to G.B.C.), Jeongho Kim Neurodevelopmental Research Fund (G.B.C. and J.R.H.), Hock E. Tan and K. Lisa Yang Center for Autism Research (G.B.C. and M.D.R.), P. Ha (G.B.C.), Simons Center for the Social Brain (G.B.C., J.R.H. and Y.S.Y.), the Simons Foundation Autism Research Initiative (G.B.C. and J.R.H.), the Champions of the Brain Weedon Fellowship (G.M.W.) and the National Science Foundation Graduate Research Fellowship (no. 1122374, to M.D.R.). We are deeply grateful to B. Picower, the JPB Foundation, the Picower Institute for Learning and Memory, Lore McGovern and the McGovern Institute for Brain Research for their mentorship and direct support of this work over the years.
Author information
Authors and Affiliations
Contributions
M.D.R., Y.S.Y., M.M.H., J.R.H. and G.B.C. designed the experiments and/or provided advice and technical expertise. M.D.R., Y.S.Y., R.D.W., H.K., C.R., G.M.W., M.A. and H.O.K. performed the experiments. A.W. provided Il-17rafl/fl mice. M.D.R., Y.S.Y., J.R.H. and G.B.C. wrote the manuscript with input from the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Thomas Blank, Craig M. Powell, Marco Prinz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
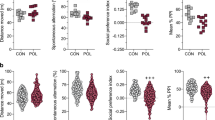
Extended Data Fig. 1 Cntnap2, Fmr1 and Shank3 mutant mice show variable sociability performance.
a, Sociability performance. MIA, n = 13; wild-type, n = 22; Cntnap2 mutant, n = 71; Fmr1 mutant, n = 165; Shank3 mutant, n = 50; from 30 independent experiments. b, Time spent investigating social (S) versus inanimate (I) objects for mice described in a. c, d, Total interaction time (c) and distance travelled (d) during the three-chambered sociability experiments described in a. All n values refer to the number of mice used. Statistics calculated by one-way ANOVA with Dunnett’s post-hoc test (a, c, d) or two-way ANOVA with Dunnett’s post-hoc test (b). Graphs are mean ± s.e.m.
Extended Data Fig. 2 Further behavioural analyses for sociability performance after treatment with LPS in PBS and MIA offspring, and monogenic mutant mice.
a–d, Time spent investigating social (S) versus inanimate (I) objects (a), total interaction time (b), time spent in social (S), centre (C) or inanimate (I) chamber (c), and distance travelled (d) for sociability experiments in Fig. 1c. PBS offspring + vehicle, n = 10; PBS offspring + LPS, n = 9; MIA offspring + vehicle, n = 10; MIA offspring + LPS, n = 12; wild type (WT) + vehicle, n = 8; wild type + LPS, n = 11; Cntnap2 mutant + vehicle, n = 11; Cntnap2 mutant + LPS, n = 11; Fmr1 mutant + vehicle, n = 11; Fmr1 mutant + LPS, n = 15; Shank3 mutant + vehicle, n = 8; Shank3 mutant + LPS, n = 10; from 3 independent experiments. All n values refer to the number of mice used. Statistics calculated by two-way ANOVA with Sidak’s (a) or Dunnett’s (c) post-hoc test, or two-way repeated-measures ANOVA with Sidak’s post-hoc test (b, d). Graphs are mean ± s.e.m.
Extended Data Fig. 3 LPS-induced rescue of MIA behavioural phenotypes is transient, effective in aged mice and extends beyond three-chambered sociability.
a–e, Sociability measured 72 h after injection with vehicle (Veh) or LPS, in PBS and MIA offspring from Fig. 1c. Data expressed as per cent sociability (a), time spent investigating social (S) versus inanimate (I) objects (b), total interaction time (c), time spent in social (S), centre (C) or inanimate (I) chamber (d), and distance travelled (e) during three-chambered sociability experiments. PBS offspring + vehicle, n = 7; PBS offspring + LPS, n = 7; MIA offspring + vehicle, n = 8; MIA offspring + LPS, n = 6; from 2 independent experiments. f–j, Sociability measured before and 4 h after injection of vehicle or LPS in aged MIA mice (9–12 months old). Data are expressed as per cent sociability (f), time spent investigating social (S) versus inanimate (I) objects (g), total interaction time (h), time spent in social (S), centre (C) or inanimate (I) chamber (i), and distance travelled (j) during three-chambered sociability experiments. MIA offspring + vehicle, n = 6; MIA offspring + LPS, n = 7; from 2 independent experiments. k, Reciprocal social interactions measured after treatment with vehicle or LPS in PBS or MIA offspring. PBS offspring + vehicle, n = 9; PBS offspring + LPS, n = 9; MIA offspring + vehicle, n = 11; MIA offspring + LPS, n = 11; from 4 independent experiments. l, Marble burying index (percentage of buried marbles) measured before and 4 h after treatment with vehicle or LPS in PBS or MIA offspring. PBS offspring + vehicle, n = 12; PBS offspring + LPS, n = 12; MIA offspring + vehicle, n = 12; MIA offspring + LPS, n = 11; from 5 independent experiments. All n values refer to the number of mice used. Statistics calculated by two-way ANOVA with Sidak’s (a–c, e, g), Dunnett’s (d, i) or Tukey’s (k) post-hoc tests, or two-way repeated-measures ANOVA with Sidak’s post-hoc test (f, h, j, l). Graphs are mean ± s.e.m.
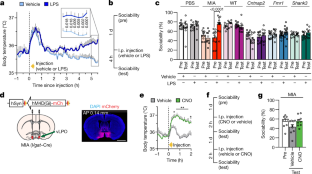
Extended Data Fig. 4 Acute increase in body temperature is insufficient to promote sociability.
a, Body temperature profile after injection of vehicle or LPS in MIA offspring. Vehicle, n = 10; LPS, n = 10; from 4 independent experiments. The initial spike in body temperature is due to handling stress. b–e, Data are expressed as time spent investigating social (S) versus inanimate (I) objects (b), total interaction time (c), time spent in social (S), centre (C) or inanimate (I) chamber (d), and distance travelled (e) during three-chambered sociability experiments described in Fig. 1g. n = 9 for all groups, from 2 independent experiments. f–j, Sociability performance in Vgat–Cre PBS and MIA offspring after treatment with vehicle, CNO or LPS. Data are expressed as per cent sociability (f), time spent investigating social (S) versus inanimate (I) objects (g), total interaction time (h), time spent in social (S), centre (C) or inanimate (I) chamber (i), and distance travelled (j) during three-chambered sociability experiments. PBS offspring, n = 11; MIA offspring, n = 7; from 2 independent experiments. All n values refer to the number of mice used. Statistics calculated by two-way repeated-measures ANOVA with Bonferroni’s (a) or Dunnett’s (f, h, j) post-hoc tests, two-way ANOVA with Sidak’s (b, g) or Dunnett’s (d, i) post-hoc tests, or one-way repeated-measures ANOVA with Tukey’s post-hoc test (c, e). Graphs are mean ± s.e.m.
Extended Data Fig. 5 Histological identification of the S1DZ.
a, Coronal section of the cortex counterstained with DAPI to highlight the abrupt reduction in cell density in layer 4, between the S1DZ and the S1BF, at AP −0.46 mm. n = 5, from 1 independent experiment. D, dorsal; V, ventral. b, Coronal section of the cortex imaged with differential interference contrast, further highlighting the reduced layer 4 in the S1DZ at AP −0.46 mm. n = 3, from 2 independent experiments. All n values refer to the number of mice used. White arrows indicate borders of S1DZ. Scale bars, 500 μm (a); 300 μm (b).
Extended Data Fig. 6 Treatment of MIA offspring with LPS does not have a distinguishable effect on FOS expression in the other cortical regions analysed.
a, Full cortical depth of S1DZ FOS staining as shown in Fig. 2a, for PBS and MIA offspring after administration of vehicle or LPS. PBS offspring + vehicle, n = 8; PBS offspring + LPS, n = 9; MIA offspring + vehicle, n = 13; MIA offspring + LPS, n = 11; from 3 independent experiments. Scale bar, 200 μm. b, c, Representative images (b) and quantification (c) of FOS (green) and NeuN (red) colabelled cells within the S1DZ of PBS and MIA offspring. PBS offspring, n = 4; MIA offspring, n = 3; from 1 independent experiment. Scale bar, 50 μm. d, e, Representative images (d) and quantification (e) of FOS expression in a series of cortical regions and after injection of vehicle or LPS in PBS or MIA offspring. Sections are stained for FOS (green) and DAPI (blue). Scale bars, 200 μm. For S1BF, M2, M1 and AuD: PBS offspring + vehicle, n = 8; PBS offspring + LPS, n = 9; MIA offspring + vehicle, n = 13; MIA offspring + LPS, n = 11. For mPFC: PBS offspring + vehicle, n = 8; PBS offspring + LPS, n = 9; MIA offspring + vehicle, n = 12; MIA offspring + LPS, n = 11. For V1: PBS offspring + vehicle, n = 7; PBS offspring + LPS, n = 8; MIA offspring + vehicle, n = 12; MIA offspring + LPS, n = 9; from 4 independent experiments. All n values refer to the number of mice used. Statistics calculated by unpaired two-tailed t-test (c) or two-way ANOVA with Tukey’s post-hoc test (e). Graphs are mean ± s.e.m.
Extended Data Fig. 7 Further behavioural analyses of S1DZ optical-inhibition-mediated rescue of sociability in monogenic mutant mice.
a, Quantification of FOS-expressing cells in the S1DZ of monogenic mutant mice. Wild type, n = 6; Cntnap2 mutant, n = 21; Fmr1 mutant, n = 17; Shank3 mutant, n = 15; from 5 independent experiments. b, Correlation of FOS expression in the S1DZ with severity of sociability deficits across monogenic mutant mice. Cntnap mutant, n = 21; Fmr1 mutant, n = 17; Shank3 mutant, n = 15; from 4 independent experiments. Black solid lines represent regression line; grey lines indicate 90% confidence intervals. c, Individual data for experiments in Fig. 2f. d–f, Data are expressed as time spent investigating social (S) versus inanimate (I) objects (d), total interaction time (e), and distance travelled (f) during the three-chambered sociability experiments described in Fig. 2f. Wild type + EYFP, n = 7; wild type + NpHR, n = 8; Cntnap2 mutant, + EYFP, n = 11; Cntnap2 mutant + NpHR, n = 9; Fmr1 mutant + EYFP, n = 8; Fmr1 mutant + NpHR, n = 12; Shank3 mutant + EYFP, n = 8; Shank3 mutant + NpHR, n = 10; from 6 independent experiments. All n values refer to the number of mice used. Statistics calculated by one-way ANOVA with Dunnett’s post-hoc test (a), linear regression (b), one-way repeated-measures ANOVA with Dunnet’s post-hoc test (c), two-way ANOVA with Sidak’s post-hoc test (d) or two-way repeated-measures ANOVA with Sidak’s post-hoc test (e, f). Graphs are mean ± s.e.m.
Extended Data Fig. 8 Il17ra expression in the S1DZ of PBS and MIA offspring and further behavioural analyses of S1DZ IL-17a rescue of sociability in MIA offspring and monogenic mutant mice.
a, Representative images of Il17ra expression in the S1DZ of PBS and MIA offspring. Scale bar, 1 mm. b, Quantification of Il17ra expression within the S1DZ of MIA offspring according to cortical layer. n = 6, from 2 independent experiments. c, Quantification of overall Il17ra expression in the S1DZ of PBS and MIA offspring. PBS offspring, n = 8; MIA offspring, n = 6; from 2 independent experiments. d–h, Further behavioural analyses of experiments described in Fig. 3f. Time spent investigating social (S) versus inanimate (I) objects (e), total interaction time (f), time spent in social (S), centre (C) or inanimate (I) chamber (g), and distance travelled (h). PBS offspring + vehicle, n = 11; PBS offspring + IL-17a, n = 12; MIA offspring + vehicle, n = 14; MIA offspring + IL-17a, n = 10; wild type + vehicle, n = 11; wild type + IL-17a, n = 11; Cntnap2 mutant + vehicle, n = 8; Cntnap2 mutant + IL-17a, n = 10; Fmr1 mutant + vehicle, n = 9; Fmr1 mutant + IL-17a, n = 11; from 6 independent experiments. All n values refer to the number of mice used. Statistics calculated by unpaired two-tailed t-test (c), two-way ANOVA with Sidak’s (e) or Dunnett’s (g) post-hoc tests, or two-way repeated-measures ANOVA with Sidak’s post-hoc test (f, h). Graphs are mean ± s.e.m.
Extended Data Fig. 9 IL-17a is necessary for LPS-induced behavioural rescue and reduction of FOS expression in MIA offspring.
a–e, Further behavioural analyses of experiments described in Fig. 4a. Time spent investigating social (S) versus inanimate (I) objects (b), total interaction time (c), time spent in social (S), centre (C) or inanimate (I) chamber (d), and distance travelled (e). PBS offspring + vehicle + isotype, n = 9; PBS offspring + LPS + isotype, n = 11; MIA offspring + vehicle + isotype, n = 10; MIA offspring + LPS + isotype, n = 10; MIA offspring + LPS + anti-IL-17a, n = 10; from 7 independent experiments. f, Quantification of FOS-expressing cells in the S1DZ and CeA after injection of vehicle or LPS in MIA offspring pre-treated intracerebroventricularly with isotype-control antibody or blocking antibody against IL-17a (anti-IL-17a). PBS offspring + vehicle + isotype, n = 14; MIA offspring + vehicle + isotype, n = 9; MIA offspring + LPS + isotype, n = 10; MIA offspring + LPS + anti-IL-17a, n = 10; from 4 independent experiments. All n values refer to the number of mice used. Statistics calculated by two-way ANOVA with Sidak’s (b) or Dunnett’s (d) post-hoc tests, two-way repeated-measures ANOVA with Sidak’s post-hoc tests (c, e) or one-way ANOVA with Dunnett’s post-hoc test (f). Graphs are mean ± s.e.m.
Extended Data Fig. 10 Further analyses of the necessity of IL-17a for the LPS-induced reduction of firing rate in the S1DZ, and the necessity of S1DZ IL-17Ra expression for the LPS-induced rescue of sociability deficits in MIA offspring.
a–c, Further analyses for experiments described in Fig. 4b–d. a, Example of a head-fixed mouse on the running wheel used during single-unit recording. b, Representative image of a tetrode placement in the S1DZ. Scale bar, 500 μm. c, Firing rate for individual cells before and 4 h after injection of vehicle or LPS in PBS and MIA offspring pre-treated with isotype-control antibody or blocking antibody against IL-17a (anti-IL-17a). d–h, Further analyses for experiments described in Fig. 4e, f. Time spent investigating social (S) versus inanimate (I) objects (e), total interaction time (f), time spent in social (S), centre (C) or inanimate (I) chambers (g), and distance travelled (h). Il-17rafl/fl;EYFP, n = 9; Il-17rafl/fl;EGFP:nCre, n = 10; from 5 independent experiments. i, j, Representative images (i) and corresponding quantification (j) of Il17ra and Gapdh amplicon following PCR using cDNA derived from cells isolated from the cortical region centred on S1DZ of Il-17rafl/fl;EYFP and Il-17rafl/fl;EGFP:nCre mice. n = 3 for both groups; from 1 experiment. All n values refer to the number of mice used. Statistics calculated by two-way ANOVA with Sidak’s post-hoc test (e, g), two-way repeated-measures ANOVA with Sidak’s post-hoc tests, (f, h) or unpaired two-tailed t-test (j). Graphs are mean ± s.e.m.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Reed, M.D., Yim, Y.S., Wimmer, R.D. et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577, 249–253 (2020). https://doi.org/10.1038/s41586-019-1843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1843-6
This article is cited by
-
Shank3 deficiency elicits autistic-like behaviors by activating p38α in hypothalamic AgRP neurons
Molecular Autism (2024)
-
A zinc finger transcription factor enables social behaviors while controlling transposable elements and immune response in prefrontal cortex
Translational Psychiatry (2024)
-
Prenatal and postnatal neuroimmune interactions in neurodevelopmental disorders
Nature Immunology (2024)
-
Contactin-associated protein-like 2 (CNTNAP2) mutations impair the essential α-secretase cleavages, leading to autism-like phenotypes
Signal Transduction and Targeted Therapy (2024)
-
IL-6 Enhances the Activation of PI3K-AKT/mTOR-GSK-3β by Upregulating GRPR in Hippocampal Neurons of Autistic Mice
Journal of Neuroimmune Pharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.