Key Points
-
The skin barrier is essential to protect animals from the external environment. The proper establishment of the skin barrier during embryogenesis and its maintenance during adult homeostasis is crucial for survival.
-
Different modes of asymmetric stem cell (SC) division contribute to the development and the homeostasis of the skin barrier.
-
Different populations of SCs reside in the skin epidermis and contribute to the homeostasis of the different epidermal compartments, such as the interfollicular epidermis, hair follicles and sebaceous glands.
-
Slow-cycling hair follicle SCs form early during skin embryogenesis and are responsible for formation of the sebaceous glands, completion of hair follicle morphogenesis and efficient wound repair of epidermis.
-
In the adult, hair follicle SCs are required for wound repair and for the normal cyclic bouts of hair growth that occur throughout life.
-
The molecular mechanisms that govern embryonic development of the epidermis are reused during postnatal life to regulate harmoniously the balance between SC activation and differentiation, ensuring the homeostasis of the different compartments of the skin epidermis.
Abstract
The skin epidermis and its array of appendages undergo ongoing renewal by a process called homeostasis. Stem cells in the epidermis have a crucial role in maintaining tissue homeostasis by providing new cells to replace those that are constantly lost during tissue turnover or following injury. Different resident skin stem cell pools contribute to the maintenance and repair of the various epidermal tissues of the skin, including interfollicular epidermis, hair follicles and sebaceous glands. Interestingly, the basic mechanisms and signalling pathways that orchestrate epithelial morphogenesis in the skin are reused during adult life to regulate skin homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blanpain, C., Horsley, V. & Fuchs, E. Epithelial stem cells: turning over new leaves. Cell 128, 445–458 (2007).
Blanpain, C. & Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22, 339–373 (2006).
Koster, M. I. & Roop, D. R. Mechanisms regulating epithelial stratification. Annu. Rev. Cell Dev. Biol. 23, 93–113 (2007).
Fuchs, E. & Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033–1042 (1980).
Candi, E., Schmidt, R. & Melino, G. The cornified envelope: a model of cell death in the skin. Nature Rev. Mol. Cell Biol. 6, 328–340 (2005).
Senoo, M., Pinto, F., Crum, C. P. & McKeon, F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129, 523–536 (2007).
Truong, A. B., Kretz, M., Ridky, T. W., Kimmel, R. & Khavari, P. A. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 20, 3185–3197 (2006).
Mills, A. A. et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999).
Yang, A. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999).
Koster, M. I., Kim, S., Mills, A. A., DeMayo, F. J. & Roop, D. R. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 18, 126–131 (2004).
Blanpain, C., Lowry, W. E., Pasolli, H. A. & Fuchs, E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 20, 3022–3035 (2006).
Rangarajan, A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001).
Watt, F. M., Estrach, S. & Ambler, C. A. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr. Opin. Cell Biol. 20, 171–179 (2008).
Moriyama, M. et al. Multiple roles of Notch signaling in the regulation of epidermal development. Dev. Cell 14, 594–604 (2008).
Wang, X., Pasolli, H. A., Williams, T. & Fuchs, E. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J. Cell Biol. 183, 37–48 (2008).
Yi, R. et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nature Genet. 38, 356–362 (2006).
Yi, R., Poy, M. N., Stoffel, M. & Fuchs, E. A skin microRNA promotes differentiation by repressing 'stemness'. Nature 452, 225–229 (2008).
Andl, T. et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr. Biol. 16, 1041–1049 (2006).
Lena, A. M. et al. miR-203 represses 'stemness' by repressing ΔNp63. Cell Death Differ. 15, 1187–1195 (2008).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Sen, G. L., Webster, D. E., Barragan, D. I., Chang, H. Y. & Khavari, P. A. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 22, 1865–1870 (2008).
Frye, M., Fisher, A. G. & Watt, F. M. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS ONE 2, e763 (2007).
Watt, F. M., Frye, M. & Benitah, S. A. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nature Rev. Cancer 8, 234–242 (2008).
Smart, I. H. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br. J. Dermatol. 82, 276–282 (1970).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005). Describes how the temporal regulation of spindle pole orientation controls epidermal stratification during embryonic development.
Clayton, E. et al. A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 (2007). Combines lineage tracing experiments and mathematical modelling and suggests that epidermal tail homeostasis does not require the existence of TA cells.
Knoblich, J. A. Mechanisms of asymmetric stem cell division. Cell 132, 583–597 (2008).
Gönczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nature Rev. Mol. Cell Biol. 9, 355–366 (2008).
Ro, S. & Rannala, B. Evidence from the stop-EGFP mouse supports a niche-sharing model of epidermal proliferative units. Exp. Dermatol. 14, 838–843 (2005).
Kolodka, T. M., Garlick, J. A. & Taichman, L. B. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc. Natl Acad. Sci. USA 95, 4356–4361 (1998).
Ghazizadeh, S. & Taichman, L. B. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20, 1215–1222 (2001).
Mackenzie, I. C. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 109, 377–383 (1997).
Ro, S. & Rannala, B. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO Rep. 5, 914–920 (2004).
Rochat, A., Kobayashi, K. & Barrandon, Y. Location of stem cells of human hair follicles by clonal analysis. Cell 76, 1063–1073 (1994). The first paper to demonstrate that HF bulge SCs present a greater clonogenic potential and can reconstitute the IFE on transplantation.
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004). This work, together with reference 52, show that the progeny of a single cultured bulge SC can differentiate in all cell lineages of the skin epidermis.
Trempus, C. S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003).
Li, A., Simmons, P. J. & Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl Acad. Sci. USA 95, 3902–3907 (1998).
Jones, P. H. & Watt, F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724 (1993). The first study to isolate and functionally characterize SCs and TA cells of the human skin epidermis.
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990). The first study to suggest that HF SCs are slow-cycling cells that reside in the bulge region.
Braun, K. M. et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130, 5241–5255 (2003).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004). Introduced a transgenic mouse model to fluorescently tag, isolate and functionally characterize slow-cycling cells in mice.
Wilson, C. et al. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation 55, 127–136 (1994).
Waghmare, S. K. et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 27, 1309–1320 (2008).
Jaks, V. et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genet. 40, 1291–1299 (2008).
Sotiropoulou, P. A., Candi, A. & Blanpain, C. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells 26, 2964–2973 (2008).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Nowak, J. A., Polak, L., Pasolli, H. A. & Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43 (2008). Shows that the slow-cycling bulge SCs are specified during embryogenesis, in which they function to make the SG, complete HF morphogenesis and efficiently repair epidermal wounds.
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nature Biotech. 22, 411–417 (2004).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 11, 1351–1354 (2005).
Levy, V., Lindon, C., Harfe, B. D. & Morgan, B. A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 9, 855–861 (2005).
Levy, V., Lindon, C., Zheng, Y., Harfe, B. D. & Morgan, B. A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 21, 1358–1366 (2007). References 48–51 show that during homeostasis, the IFE is maintained independently of HF SCs, but that during wound repair, HF cells contribute to the epidermis.
Claudinot, S., Nicolas, M., Oshima, H., Rochat, A. & Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl Acad. Sci. USA 102, 14677–14682 (2005).
Vidal, V. P. et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351 (2005).
Ito, M., Kizawa, K., Hamada, K. & Cotsarelis, G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72, 548–557 (2004).
Legue, E. & Nicolas, J. F. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 132, 4143–4154 (2005).
Rheinwald, J. G. & Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343 (1975).
Gallico, G. G. 3rd, O'Connor, N. E., Compton, C. C., Kehinde, O. & Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 311, 448–451 (1984).
Kobayashi, K., Rochat, A. & Barrandon, Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc. Natl Acad. Sci. USA 90, 7391–7395 (1993).
Barrandon, Y. & Green, H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc. Natl Acad. Sci. USA 82, 5390–5394 (1985).
Horsley, V. et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126, 597–609 (2006).
Waikel, R. L., Kawachi, Y., Waikel, P. A., Wang, X. J. & Roop, D. R. Deregulated expression of c-Myc depletes epidermal stem cells. Nature Genet. 28, 165–168 (2001).
Arnold, I. & Watt, F. M. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11, 558–568 (2001).
Nijhof, J. G. et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133, 3027–3037 (2006).
Jensen, U. B. et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell Sci. 121, 609–617 (2008).
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999).
Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G. & Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533–545 (2001).
Andl, T., Reddy, S. T., Gaddapara, T. & Millar, S. E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653 (2002).
Lo Celso, C., Prowse, D. M. & Watt, F. M. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131, 1787–1799 (2004).
Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95, 605–614 (1998). The first study to point to the key role of Wnt signalling in HF specification and in inducing HF derived tumours.
Silva-Vargas, V. et al. β-catenin and hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell 9, 121–131 (2005).
Zhang, Y. et al. Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135, 2161–2172 (2008).
Sick, S., Reinker, S., Timmer, J. & Schlake, T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314, 1447–1450 (2006).
Lowry, W. E. et al. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19, 1596–1611 (2005).
Van Mater, D., Kolligs, F. T., Dlugosz, A. A. & Fearon, E. R. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 17, 1219–1224 (2003).
Merrill, B. J., Gat, U., DasGupta, R. & Fuchs, E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15, 1688–1705 (2001).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Nguyen, H., Rendl, M. & Fuchs, E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127, 171–183 (2006).
Plikus, M. V. et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344 (2008).
Botchkarev, V. A. et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nature Cell Biol. 1, 158–164 (1999).
Jamora, C., DasGupta, R., Kocieniewski, P. & Fuchs, E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422, 317–322 (2003).
Muller-Rover, S. et al. E- and P-cadherin expression during murine hair follicle morphogenesis and cycling. Exp. Dermatol. 8, 237–246 (1999).
Kobielak, K., Stokes, N., de la Cruz, J., Polak, L. & Fuchs, E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl Acad. Sci. USA 104, 10063–10068 (2007).
Kobielak, K., Pasolli, H. A., Alonso, L., Polak, L. & Fuchs, E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell Biol. 163, 609–623 (2003).
Andl, T. et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131, 2257–2268 (2004).
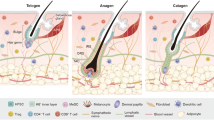
Paus, R. & Foitzik, K. In search of the “hair cycle clock”: a guided tour. Differentiation 72, 489–511 (2004).
Horsley, V., Aliprantis, A. O., Polak, L., Glimcher, L. H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310 (2008).
Rendl, M., Lewis, L. & Fuchs, E. Molecular dissection of mesenchymal–epithelial interactions in the hair follicle. PLoS Biol. 3, e331 (2005).
Rendl, M., Polak, L. & Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 22, 543–557 (2008).
Botchkarev, V. A. et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 15, 2205–2214 (2001).
Zhang, J. et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells 24, 2826–2839 (2006).
St-Jacques, B. et al. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 8, 1058–1068 (1998).
Oro, A. E. & Higgins, K. Hair cycle regulation of Hedgehog signal reception. Dev. Biol. 255, 238–248 (2003).
Pan, Y. et al. γ-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev. Cell 7, 731–743 (2004).
Zhou, P., Byrne, C., Jacobs, J. & Fuchs, E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9, 700–713 (1995).
Kaufman, C. K. et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 17, 2108–2122 (2003).
Malanchi, I. et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature 452, 650–653 (2008).
Chan, E. F., Gat, U., McNiff, J. M. & Fuchs, E. A common human skin tumour is caused by activating mutations in β-catenin. Nature Genet. 21, 410–413 (1999).
Hoseong Yang, S. et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β-catenin signaling. Nature Genet. 40, 1130–1135 (2008).
Rhee, H., Polak, L. & Fuchs, E. Lhx2 maintains stem cell character in hair follicles. Science 312, 1946–1949 (2006).
Osorio, K. M. et al. Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Development 135, 1059–1068 (2008).
Morrison, S. J. & Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068–1074 (2006).
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001). The first study to suggest that the bulge region contains multipotent epidermal stem cells.
Hardy, M. H. The secret life of the hair follicle. Trends Genet. 8, 55–61 (1992).
Acknowledgements
We thank our many colleagues in the field who contributed to our understanding of skin homeostasis. We apologize to those whose papers are not cited owing to space constraints. We thank W. Lowry, J. Nowak, Y. Hsu, P. Chi, M. Hack, G. Guasch, R. Yi, G. Lapouge and A. Vankeymeulen for their thoughtful comments and critical reading of the manuscript. C.B. is supported by the Human Frontiers in Science Program Organization (HFSPO), the Belgian Fund for Scientific Research (FRS/FNRS), Wallonia Region, the Schlumberger Foundation, and the European Research Council. E.F. is an investigator of the Howard Hughes Medical Institute and receives funding from the National Institutes of Health and the Starr Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
The miRNA Registry
FURTHER INFORMATION
Glossary
- Hair follicle
-
A skin appendage that produces a hair.
- Gastrulation
-
A crucial stage during embryonic development that results in the formation of the three embryonic layers — ectoderm, endoderm and mesoderm — from which all tissues and organs arise.
- Dermis
-
The mesenchymal part of the skin that is rich in collagen fibres and contains fibroblasts, blood vessels and immune cells.
- Intermediate filament
-
(IF). A cytoskeletal filament that is 10 nm in diameter and exists in skin epithelium as a heteropolymer that is assembled from ∼10,000 individual keratin proteins of two distinct types. The IF network provides the epidermal cells with resistance against mechanical stress.
- Desmosome
-
A robust cell–cell junction, which is composed of adhesive desmosomal transmembrane cadherins that associate intracellularly with two proteins — plakoglobin, which is a relative of β-catenin, and desmoplakin. These, in turn, link the structure to keratin intermediate filaments.
- Cornified
-
The transformation of cells into a horny-like material, such as horn and nails.
- SC niche
-
The specialized microenvironment that is necessary to support the maintenance and differentiation of stem cells (SCs).
- Mitotic spindle
-
The molecular apparatus that allows the segregation of chromosomes to daughter cells during cell division and is composed mainly of microtubules and proteins.
- Astral microtubule
-
A microtubule that is nucleated at the spindle pole and grows outwards towards the cell cortex; it is involved in mitotic spindle positioning.
- Interfollicular epidermis
-
The skin epidermis located between the emergence of the periodically spaced hair follicles.
- Sebaceous gland
-
Attached to a hair follicle, this gland produces sebum, an oily substance that is made of lipids and cellular debris of sebaceous cells. The sebum helps to protect and waterproof the hair and the skin surface.
- Hair shaft
-
The structure that is composed of terminally differentiated keratinocytes and emerges from the skin surface as a hair.
- Inner root sheath
-
The inner channel of the hair shaft.
- Bulge
-
A specialized portion of the hair follicle that contains multipotent stem cells that give rise to all hair follicle cell lineages as well as sebocytes and cells of the interfollicular epidermis during physio-pathological conditions.
- Hair germ
-
An outgrowth emanating from the bulge that forms during the early stage of hair follicle regeneration and corresponds to early committed progenies of bulge stem cells.
- Dermal papilla
-
The dermal part of the hair follicle, which consists of a small cluster of specialized fibroblast cells that have an instructive role to epithelial cells during hair follicle specification and regeneration.
- Outer root sheath
-
The external layer of the hair follicle that is contiguous with the basal layer of the interfollicular epidermis.
- Upper isthmus
-
The part of the hair follicle between the top of the bulge and the infundibulum, the uppermost part of the hair follicle that contacts the interfollicular epidermis.
Rights and permissions
About this article
Cite this article
Blanpain, C., Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10, 207–217 (2009). https://doi.org/10.1038/nrm2636
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2636
This article is cited by
-
Stem Cells and Regenerative Strategies for Wound Healing: Therapeutic and Clinical Implications
Current Pharmacology Reports (2024)
-
Molecular hydrogen promotes wound healing by inducing early epidermal stem cell proliferation and extracellular matrix deposition
Inflammation and Regeneration (2023)
-
Niche formation and function in developing tissue: studies from the Drosophila ovary
Cell Communication and Signaling (2023)
-
Injury-induced interleukin-1 alpha promotes Lgr5 hair follicle stem cells de novo regeneration and proliferation via regulating regenerative microenvironment in mice
Inflammation and Regeneration (2023)
-
Use of mouse primary epidermal organoids for USA300 infection modeling and drug screening
Cell Death & Disease (2023)