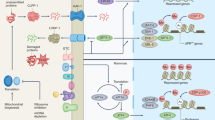
Abstract
The reactive oxygen species that are generated by mitochondrial respiration, including hydrogen peroxide (H2O2), are potent inducers of oxidative damage and mediators of ageing. It is not clear, however, whether oxidative stress is the result of a genetic programme or the by-product of physiological processes. Recent findings demonstrate that a fraction of mitochondrial H2O2, produced by a specialized enzyme as a signalling molecule in the pathway of apoptosis, induces intracellular oxidative stress and accelerates ageing. We propose that genes that control H2O2 production are selected determinants of lifespan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frisard, M. & Ravussin, E. Energy metabolism and oxidative stress: impact on the metabolic syndrome and the ageing process. Endocrine 29, 27–32 (2006).
Harman, D. Ageing: phenomena and theories. Ann. NY Acad. Sci. 854, 1–7 (1998).
Balaban, R. S., Nemoto, S., & Finkel, T. Mitochondria, oxidants, and ageing. Cell 120, 483–495 (2005).
Chance, B., Sies, H. & Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 59, 527–605 (1979).
Bayne, A. C., Mockett, R. J., Orr, W. C. & Sohal, R. S. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and ageing in transgenic Drosophila. Biochem. J. 391, 277–284 (2005).
Valko, M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 (2007).
Gius, D. & Spitz, D. R. Redox signaling in cancer biology. Antioxid. Redox Signal. 8, 1249–1252 (2006).
Stone, J. R. & Yang, S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270 (2006).
Choi, H. et al. Structural basis of the redox switch in the OxyR transcription factor. Cell 105, 103–113 (2001).
Vivancos, A. P., Castillo, E. A., Jones, N., Ayte, J. & Hidalgo, E. Activation of the redox sensor Pap1 by hydrogen peroxide requires modulation of the intracellular oxidant concentration. Mol. Microbiol. 52, 1427–1435 (2004).
Tavakoli, N., Kluge, C., Golldack, D., Mimura, T. & Dietz, K. J. Reversible redox control of plant vacuolar H+-ATPase activity is related to disulfide bridge formation in subunit E as well as subunit A. Plant. J. 28, 51–59 (2001).
Davis, D. A. et al. HIV-2 protease is inactivated after oxidation at the dimer interface and activity can be partly restored with methionine sulphoxide reductase. Biochem. J. 346, 305–311 (2000).
Atmane, N., Dairou, J., Paul, A., Dupret, J. M. & Rodrigues-Lima, F. Redox regulation of the human xenobiotic metabolizing enzyme arylamine N-acetyltransferase 1 (NAT1). Reversible inactivation by hydrogen peroxide. J. Biol. Chem. 278, 35086–35092 (2003).
Poliak, A. et al. Inhibition of indoleamine 2,3 dioxygenase activity by H2O2 . Arch. Biochem. Biophys. 450, 9–19 (2006).
Song, H., Bao, S., Ramanadham, S. & Turk, J. Effects of biological oxidants on the catalytic activity and structure of group VIA phospholipase A2. Biochemistry 45, 6392–6406 (2006).
Bossis, G. & Melchior, F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21, 349–357 (2006).
Lee, S. R., Kwon, K. S., Kim, S. R. & Rhee, S. G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 273, 15366–15372 (1998).
Yu, C. K., Li, S. & Whorton, A. R. redox regulation of PTEN by S-nitrosothiols. Mol. Pharmacol. 68, 847–854 (2005).
Rhee, S. G., Yang, K. S., Kang, S. W., Woo, H. A. & Chang, T. S. Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 7, 619–626 (2005).
Woo, H. A. et al. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 278, 47361–47364 (2003).
Caplan, J. F., Filipenko, N. R., Fitzpatrick, S. L. & Waisman, D. M. Regulation of annexin A2 by reversible glutathionylation. J. Biol. Chem. 279, 7740–7750 (2004).
Ahn, S. G. & Thiele, D. J. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 17, 516–528 (2003).
Bulteau, A. L., Ikeda-Saito, M. & Szweda, L. I. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry 42, 14846–14855 (2003).
Nulton-Persoson, A. C., Starke, D. W., Mieval, J. J. & Szweda, L. I. Reversible inactivation of α-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry 42, 4235–4242 (2003).
Taylor, E. R. et al. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 278, 19603–19610 (2003).
Mueller, S. Sensitive and nonenzymatic measurement of hydrogen peroxide in biological systems. Free Radic. Biol. Med. 29, 410–415 (2000).
Seaver, L. C. & Imlay, J. A. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183, 7182–7189 (2001).
Polle, A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulation as a step towards flux analysis. Plant Physiol. 126, 445–462 (2001).
Finkel, T. Redox-dependent signal transduction. FEBS Lett. 476, 52–54 (2000).
Alexandrova, A. Y., Kopnin, P. B., Vasiliev, J. M. & Kopnin, B. P. ROS up-regulation mediates Ras-induced changes of cell morphology and motility. Exp. Cell Res. 312, 2066–2073 (2006).
Vafa, O. et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell 9, 1031–1044 (2002).
Schimmel, M., Bauer, G. Proapoptotic and redox state-related signaling of reactive oxygen species generated by transformed fibroblasts. Oncogene 21, 5886–5896 (2002).
Giles, G. I. The redox regulation of thiol dependent signaling pathways in cancer. Curr. Pharm. Des. 12, 4427–4443 (2006).
North, S., Moenner, M. & Bikfalvi, A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 218, 1–14 (2005).
Meng, T. C., Buckley D.A, Galic, S., Tiganis, T. & Tonks, N. K. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 279, 37716–37725 (2004).
Cho, S.H. et al. Redox regulation of PTEN and protein tyrosine phosphatases in H2O2-mediated cell signaling. FEBS Lett. 560, 7–13 (2004).
Caselli, A. et al. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2 . J. Biol. Chem. 273, 32554–32560 (1998).
Chiarugi, P. et al. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 161, 933–944 (2003).
Meng, T. C., Fukada, T. & Tonks, N. K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 (2002).
Sohn, J. & Rudolph, J. Catalytic and chemical competence of regulation of CDC25 phosphatase by oxidation/reduction. Biochemistry 42, 10060–10070 (2003).
Nakashima, I. et al. Redox control of catalytic activities of membrane-associated protein tyrosine kinases. Arch. Biochem. Biophys. 434, 3–10 (2005).
Paravicini, T. M. & Touyz, R. M. Redox signaling in hypertension. Cardiovasc. Res. 71, 247–258 (2006).
Passos, J. F. & Von Zglinicki, T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic. Res. 40, 1277–1283 (2006).
Kowaltowski, A. J., Castilho, R. F. & Vercesi, A. E. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 495, 12–15 (2001).
Trinei, M. et al. A p53–p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene 21, 3872–3878 (2002).
Pinton, P. et al. Protein kinase Cβ and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315, 659–663 (2007).
Migliaccio, E. et al. The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313 (1999).
Giorgio, M. et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 (2005).
Bloch, C.A. & Ausubel, F.M. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J. Bacteriol. 168, 795–798 (1986).
Orr, W. C. & Sohal, R. S. Effects of Cu-Zn superoxide dismutase overexpression of life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 301, 34–40 (1993).
Amstad, P., Moret, R. & Cerutti, P. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J. Biol. Chem. 269, 1606–1609 (1994).
Wallace, D. C. Animal models for mitochondrial disease. Methods Mol. Biol. 197, 3–54 (2002).
Keaney, M., Matthijssens, F., Sharpe, M., Vanfleteren, J. & Gems, D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard ageing in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 37, 239–250 (2004).
Melov, S. Therapeutics against mitochondrial oxidative stress in animal models of ageing. Ann. NY Acad. Sci. 959, 330–340 (2002).
Peng, J., Stevenson, F. F., Doctrow, S. R. & Andersen, J. K. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J. Biol. Chem. 280, 29194–29198 (2005).
Landis, G. N. & Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 126, 365–379 (2005).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Francia, P. et al. Deletion of p66Shc gene protects against age-related endothelial dysfunction. Circulation 110, 2889–2895 (2004).
Menini, S. et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55, 1642–1650 (2006).
Napoli, C. et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc. Natl Acad. Sci. USA 18, 2112–2116 (2003).
Rota, M. et al. Diabetes promotes cardiac stem cell ageing and heart failure, which are prevented by deletion of the p66Shc gene. Circ. Res. 99, 42–52 (2006).
Holzenberger M. The GH/IGF-I axis and longevity. Eur. J. Endocrinol. 151 (Suppl. 1), S23–S27 (2004).
Sedensky, M. M. & Morgan, P. G. Mitochondrial respiration and reactive oxygen species in mitochondrial ageing mutants. Exp. Gerontol. 41, 237–245 (2006).
Martin, G. M. Somatic mutagenesis and antimutagenesis in ageing research. Mutat. Res. 350, 35–41 (1996).
Trifunovich, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian ageing. Science 309, 481–484 (2005).
Forster, M. J., Morris, P. & Sohal, R. S. Genotype and age influence the effect of caloric intake on mortality. FASEB J. 17, 690–692 (2003).
Bonkowski, M. S., Rocha, J. S., Masternak, M. M., Al Regaiey, K. A. & Bartke, A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl Acad. Sci. USA 103, 7901–7905 (2006).
Magwere, T. et al. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J. Gerontol. A. Biol. Sci. Med. Sci. 61, 36–47 (2004).
Lopez-Lluch, G. et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl Acad. Sci. USA 103, 1768–1773 (2006).
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005).
Weindruch, R. & Walford R. L. The Retardation of Ageing and Disease by Dietary Restriction (Charles C. Thomas, Springfield, Illinois, 1988).
Masaro, E. J., Yu, B. P. & Bertrand, H. A. Action of food restriction in delaying the ageing process. Proc. Natl Acad. Sci. USA 79, 4239–4241 (1982).
McCarter, R. J. & Palmer, J. Energy metabolism and ageing: a lifelong study of Fischer 344 rats. Am. J. Physiol. 263, E448–E452 (1992).
Gredilla, R., Lopez-Torres, M. & Barja, G. Effect of time of restriction on the decrease in mitochondrial H2O2 production and oxidative DNA damage in the heart of food-restricted rats. Microsc. Res. Tech. 59, 273–277 (2002).
Hagopian, K. et al. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 288, E674–E684 (2005).
Agarwal, S., Sharma, S., Agrawal, V. & Roy, N. Caloric restriction augments ROS defence in S. cerevisiae, by a Sir2p independent mechanism. Free Radic. Res. 39, 55–62 (2005).
Lin, S. J. et al. Calorie restriction extends Saccharomyces cerevisiae life span by increasing respiration. Nature 418, 344–348 (2002).
Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine (Oxford University Press, 1998).
Sohal, R. S. & Allen, R. G. Oxidative stress as a causal factor in differentiation and ageing: a unifying hypothesis. Exp. Gerontol. 25, 499–522 (1990).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Giorgio, M., Trinei, M., Migliaccio, E. et al. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals?. Nat Rev Mol Cell Biol 8, 722–728 (2007). https://doi.org/10.1038/nrm2240
Issue Date:
DOI: https://doi.org/10.1038/nrm2240
This article is cited by
-
Fluoride-Induced Mitochondrial Dysfunction and Approaches for Its Intervention
Biological Trace Element Research (2024)
-
Near-infrared-IIb emitting single-atom catalyst for imaging-guided therapy of blood-brain barrier breakdown after traumatic brain injury
Nature Communications (2023)
-
Polydopamine-Based Colorimetric Superwettable Biosensor for Highly Sensitive Detection of Hydrogen Peroxide and Glucose
Journal of Analysis and Testing (2023)
-
Application of oxygen vacancy defects in enhanced anti-cancer nanomedicine
Science China Chemistry (2023)
-
A selective fluorescent turn-on probe for imaging and sensing of hydrogen peroxide in living cells
Analytical and Bioanalytical Chemistry (2023)