Abstract
The dentate gyrus (DG) is important for encoding contextual memories, but little is known about how a population of DG neurons comes to encode and support a particular memory. One possibility is that recruitment into an engram depends on a neuron’s excitability. Here, we manipulated excitability by overexpressing CREB in a random population of DG neurons and examined whether this biased their recruitment to an engram supporting a contextual fear memory. To directly assess whether neurons overexpressing CREB at the time of training became critical components of the engram, we examined memory expression while the activity of these neurons was silenced. Chemogenetically (hM4Di, an inhibitory DREADD receptor) or optogenetically (iC++, a light-activated chloride channel) silencing the small number of CREB-overexpressing DG neurons attenuated memory expression, whereas silencing a similar number of random neurons not overexpressing CREB at the time of training did not. As post-encoding reactivation of the activity patterns present during initial experience is thought to be important in memory consolidation, we investigated whether post-training silencing of neurons allocated to an engram disrupted subsequent memory expression. We found that silencing neurons 5 min (but not 24 h) following training disrupted memory expression. Together these results indicate that the rules of neuronal allocation to an engram originally described in the lateral amygdala are followed in different brain regions including DG, and moreover, that disrupting the post-training activity pattern of these neurons prevents memory consolidation.
Similar content being viewed by others
INTRODUCTION
Memories are thought to be represented in the brain as enduring physical changes in ensembles of neurons, known as the memory trace or engram (Semon, 1923; Schacter et al, 1978; Josselyn, 2010, 2015; Tonegawa et al, 2015). Evidence from electrophysiological and immediate-early gene imaging experiments suggests that only a small portion of neurons within a region encodes any one memory (Guzowski et al, 1999; Vazdarjanova et al, 2006; Reijmers et al, 2007). This seems particularly true in the dentate gyrus (DG) layer of the hippocampus, which exhibits strikingly sparse activity patterns during experiences (Chawla et al, 2005; Leutgeb et al, 2007). The DG is important in hippocampal dependent learning, including contextual fear memory (Hernández-Rabaza et al, 2008). Past theoretical work suggests that sparsity in this region may be part of a mechanism by which the DG supports hippocampal memory, in particular by generating unique patterns for distinct events (O’Reilly and McClelland, 1994; Treves et al, 2008). What remain unclear, however, are the specific factors and processes that determine a DG neuron’s engagement during an experience and its incorporation into DG components of an engram.
Research focusing on other brain regions show that a neuron’s participation in a given engram may be biased experimentally by manipulating its intrinsic excitability (Han et al, 2009; Zhou et al, 2009; Sano et al, 2014; Yiu et al, 2014). However, it is unknown whether the same rules of allocation apply to the DG. We (Han et al, 2007, 2009; Yiu et al, 2014) and others (Zhou et al, 2009; Gouty-Colomer et al, 2016), previously examined how neurons in the lateral nucleus of the amygdala (LA) are allocated to an engram supporting a discrete cue fear memory (in which an initially motivationally neutral auditory cue is paired with an aversive foot shock) (Davis, 1992; LeDoux, 2000; Maren, 2003). Increasing the function of the transcription factor CREB (Ca2+/cAMP response element-binding protein) in individual principal LA neurons increased the likelihood that a particular neuron would become part of a fear/threat memory engram (Han et al, 2007, 2009; Zhou et al, 2009). Furthermore, genetic ablation or suppression of LA neurons overexpressing CREB (and not an equal number of random LA neurons) impaired memory expression (Han et al, 2009; Zhou et al, 2009). Subsequent experiments showed that neurons with high CREB function were preferentially allocated to the LA fear memory engram owing to increased excitability (Zhou et al, 2009; Yiu et al, 2014). Specifically, increasing the excitability of a small population of LA neurons in the minutes before training using chemogenetics or optogenetics also resulted in preferential allocation of these more excitable neurons to the resulting engram (Yiu et al, 2014). Artificially increasing excitability in a subset of neurons immediately before training may exaggerate endogenous processes that mediate normal memory encoding. Thus, during natural engram formation, neurons that happen to be more excitable at the time of training are likely allocated to the resulting engram (Yiu et al, 2014; Gouty-Colomer et al, 2016). Using this principle, viral vectors have been designed to express constructs to first allocate neurons to an engram and subsequently manipulate the function of these same neurons (Hsiang et al, 2014; Sano et al, 2014).
Here, we examined whether these principles guiding neuronal allocation to an engram hold for DG neurons. We used viral vectors to increase the excitability of a small number of DG neurons using CREB overexpression. If these neurons are preferentially allocated to a DG engram, then silencing their activity (via chemogenetic or optogenetic techniques) during a memory test should disrupt memory expression.
MATERIALS AND METHODS
Mice
Adult (<12 weeks of age) male and female F1 hybrid (C57BL/6NTac × 129S6/SvEvTac) mice were used. Mice were bred at the Hospital for Sick Children and group-housed (4/cage) on a 12 h light/dark cycle with food and water available ad libitum. All procedures were conducted in accordance with Hospital for Sick Children Animal Care and Use Committee policies and conformed to both the Canadian Council on Animal Care (CCAC) and National Institutes of Health (NIH) Guidelines on the Care and Use of Laboratory Animals.
HSV Vectors
Wild-type full-length CREB (provided by Dr Satoshi Kida, Tokyo University of Agriculture), hM4Di (a Gi-DREADD, provided by Dr Bryan Roth, University of North Carolina) and iC++ (an engineered chloride-conducting channelrhodopsin, provided by Dr Karl Deisseroth, Stanford University) cDNAs were subcloned into an HSV vector backbone that co-expresses green fluorescent protein (GFP) as a fluorescent reporter, HSV-p1005 (Russo et al, 2009).
Surgery
Mice were pre-treated with atropine sulfate (0.1 mg/kg, ip), anesthetized (chloral hydrate, 400 mg/kg, ip) and placed in a stereotaxic frame. Viral vectors were bilaterally microinjected (1.5–2.0 μl/side, 0.1 μl/min) into the DG through glass micropipettes connected via polyethylene tubing to a microsyringe (Hamilton, Reno, NV). Micropipettes remained in place for 10 min after microinjection to ensure vector diffusion. For optogenetic experiments, optical fibers (constructed in-house) were implanted just dorsal to DG and secured with dental cement. Mice were treated with analgesia (ketoprofen, 5 mg/kg, sc) following surgery.
Verifying Location of Vector Microinjection and Extent of Viral Infection
Following behavioral testing, mice were transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde. Brains were fixed overnight (4 °C) and transferred to sucrose solution, and coronal brain slices (50 μm) were collected across the anterior-posterior extent of the hippocampus. Mice were included in subsequent data analysis only if robust bilateral GFP expression was observed in DG of dorsal hippocampus in at least five consecutive brain sections (across anterior-posterior axis). To determine the percent of GFP-infected cells within our target region (DG of dorsal hippocampus), we traced the target region (−1.46 to −3.08 mm AP, corresponding to plates 43–56 in Paxinos and Franklin (2001)) across 15 serial sections in random brains (vCREB-hM4Di, n=5; hM4Di, n=5), and then assessed the number of GFP+ neurons within this region of interest (ImageJ software, NIH).
Immunohistochemistry
To examine CREB protein levels, coronal brain slices were incubated with blocking solution (0.1% BSA, 2% NGS, 0.3% Triton X-100) for 2 h at room temperature, then with anti-CREB primary mouse antibody (1 : 1000, Upstate Cell Signaling Solutions, NY) and GFP antibody (1 : 1000, Invitrogen, Carlsbad, CA) for 24 h. Sections were washed, then incubated with anti-mouse Alexa 568/488 secondary antibody (1 : 500, Invitrogen) for 2 h at room temperature (Yiu et al, 2014). Sections were washed, mounted on slides, and coverslipped using PermaFluor mounting medium. Nuclei were counterstained with DAPI (Vectashield, Vector Labs, Burlingame, CA). Sections from mice microinjected with vCREB-iC++ or iC++ were amplified with TSA (tyramide signal amplification). Images were obtained using a confocal laser scanning microscope (LSM 710; Zeiss).
Clozapine-N-Oxide (CNO)
Clozapine-N-oxide (CNO, Toronto Research Chemicals) was prepared in a stock solution (10 mg/ml in DMSO), and then diluted in saline before being injected at a dose of 5 mg/kg ip, 30 min before testing.
Context Fear Training and Testing
Training
Mice underwent contextual fear conditioning 3 days after viral microinjection. Fear conditioning chambers (31 × 24 × 21 cm; MED Associates, St Albans, VT), consisted of two stainless steel and two clear acrylic walls, with a stainless steel shock-grid floor (bars 3.2 mm diameter, spaced 7.9 mm apart). Mice were placed in the conditioning chamber and, 2 min later received an unsignaled shock (2 s, 0.5 or 0.6 mA intensity). Mice remained in the chamber for an additional 60 s before being returned to their homecage.
Testing
Memory was tested 24 h after training (except where noted). Mice were placed in the training context and the amount of time spent freezing, defined as an immobilized crouching with an absence of any movement except respiration (Blanchard and Blanchard, 1969; Bolles and Fanselow, 1982), was recorded. An automated frame-by-frame analysis of movement recorded by cameras was used to generate freezing scores (Freezeframe software; Actimetrics) for chemogenetic experiments and two observers unaware of treatment conditions manually generated freezing scores in optogenetic experiments.
Specific Methods
Experiment 1: Chemogenetically silencing DG neurons that overexpressed CREB during training during memory test
Mice microinjected with vCREB-hM4Di or hM4Di vector underwent contextual fear conditioning (0.5 mA shock). To silence hM4Di-expressing neurons, mice were systemically administered CNO or vehicle (VEH) 30 min before testing.
Experiment 2: Optogenetically silencing DG neurons that overexpressed CREB during training during memory test
Mice microinjected with vCREB-iC++ or iC++ vector were trained as above (0.6 mA shock). Mice were tested under two conditions; blue light ON (10 mW, square pulse, 473 nm, for 3 min) to silence neurons with iC++ (Berndt et al, 2016) or blue light OFF (for 3 min). The order of light ON/OFF was counterbalanced across mice.
Experiment 3: Optogenetically silencing DG neurons that overexpressed CREB during 5 min after training
Mice microinjected with vCREB-iC++ or iC++ vector were trained in a context fear paradigm (0.6 mA shock). Following training, mice were immediately returned to their homecage. Five minutes later, mice received blue light stimulation (10 mW, square pulse, 473 nm, 1 min duration) to silence infected neurons. Mice were tested 24 h later with blue light OFF for 3 min followed by ON for 3 min (as above). We included three control groups; one control group was treated similarly but did not receive blue light stimulation after training, a second received the same blue light stimulation, but 24 h (rather than 5 min) following training. A final group of mice was microinjected with iC++ alone (no CREB) vector and received blue light 5 min following training. Mice were tested as above, 24 h following post-training stimulation.
Data Analysis
The amount of time spent freezing when replaced in the context was analyzed in Experiment 1 using a two-way ANOVA with between-groups factors Vector (vCREB-hM4Di; hM4Di) and Treatment (VEH; CNO). Experiment 2 data were analyzed using a two-way repeated measures ANOVA with between-groups factor Vector (vCREB-iC++; iC++) and within-group factor Light (ON; OFF). Experiment 3 data were analyzed using a two-way repeated measures ANOVA with between-groups factor Condition (vCREB-iC++, post-training blue light; vCREB-iC++ no post-training blue light; iC++ post-training blue light) and within-group factor Light-at-test (ON; OFF). A one-way ANOVA within repeated measure Light-at-test (ON; OFF) was used for the 24 h post-training blue light delay group. Significant main effects or interactions were further analyzed using Newman–Keuls post hoc tests.
RESULTS
Chemogenetic Silencing of DG Neurons that were Expressing CREB at the Time of Training Attenuates Expression of Contextual Fear Memory whereas Silencing a Similar Number of Random DG Neurons Does Not
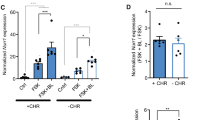
To inhibit the activity of vCREB-expressing neurons during the memory test, we first used an inhibitory DREADD (designer receptors exclusively activated by designer drugs). hM4Di is a modified G protein-coupled receptor that does not respond to endogenous ligands, but instead responds selectively to the synthetic ligand CNO (Armbruster et al, 2007; Nichols and Roth, 2009). Microinjecting a vector expressing both CREB and hM4Di permits infected neurons to be excited by CREB overexpression during training and these same neurons to be silenced by hM4Di specifically before the memory test (Hsiang et al, 2014; Sano et al, 2014). This allowed us to directly examine whether the infected neurons were necessary for memory expression. Microinjection of vCREB-hM4Di or hM4Di bilaterally into the DG of the dorsal hippocampus produced strong, localized transgene expression limited to this region of interest (Figure 1a). Consistent with previous reports (Sekeres et al, 2012), microinjections of HSV vectors produced robust, localized infection limited to dentate granule cells, and not glia or interneurons (Figure 1a and b). These vectors produced sparse infection that did not differ between vectors (F1,8=0.03, p>0.05). CREB overexpression was localized to vCREB-hM4Di-infected cells (Figure 1c).
CREB overexpression in DG neurons training preferentially biases their allocation to an engram supporting contextual fear memory (chemogenetic studies). (a) HSV vector microinjection produces strong localized infection of DG principal neurons. Example image from mouse brain 4 days post microinjection. (b) vCREB-hM4Di and hM4Di vectors infected a similar number of DG neurons. vhM4Di (n=15 sections from 5 mice), vCREB-hM4Di (n=15 sections from 5 mice). (c) DG neurons expressing hM4Di show endogenous levels of CREB protein, whereas DG neurons expressing vCREB-hM4Di show high levels of CREB. GFP (green, GFP, infected neuron), CREB (red, CREB protein expression). (d) Pre-test chemogenetic silencing of neurons that overexpressed CREB during training inhibits subsequent memory expression (CNO in mice expressing vCREB-hM4Di). Silencing a similar number of random neurons (expressing hM4Di without vCREB) failed to disrupt memory expression. These results indicate that the neurons overexpressing CREB are preferentially allocated to an engram. hM4Di and VEH (n=20); hM4Di and CNO (n=22); vCREB-hM4Di and VEH (n=23); vCREB-hM4Di and CNO (n=24). Data presented are mean±SEM. n.s., not statistically different, **p<0.01.
Silencing of neurons overexpressing CREB by administering CNO before the memory test disrupted memory expression, whereas silencing a similar number of neurons not overexpressing CREB at the time of training had no effect on memory expression (significant Vector (vCREB-hM4Di; hM4Di) × Treatment (VEH; CNO) interaction (F1,85=6.93, p<0.05), main effect of Treatment (F1,85=6.40, p<0.05), but not Vector (F1,85=0.06, p>0.05). Post hoc tests showed that silencing neurons expressing hM4Di alone did not decrease freezing (p=0.94), whereas silencing neurons expressing vCREB-hM4Di decreased freezing (p<0.01)). These data suggest that neurons overexpressing CREB at the time of training became key components of the engram supporting the memory for contextual fear, as silencing these neurons, and not an equal number of random neurons, impaired subsequent memory expression.
iC++ Silencing of DG Neurons Expressing CREB Attenuates Contextual Fear Memory
We used optogenetics to confirm that silencing neurons that overexpressed CREB during training disrupts memory expression. This technique allowed us to examine the effects of silencing specific neurons within the same memory test. To silence neurons, we used iC++, a blue light-gated chloride channel (rather than a chloride pump such as halorhodopsin or a proton pump such as archeorhodopsin), which allows for high chloride selectivity and conductivity (Berndt et al, 2016). We microinjected mice with vCREB-iC++ or iC++ vector before training. Mice were tested under two conditions: light ON (3 min) and light OFF (3 min). Only mice microinjected with vCREB-iC++ froze less when blue light was applied (to silence infected neurons), regardless of light order presentation (Figure 2b and c) (ON/OFF order, significant Vector by Light interaction (F1,22=8.79, p<0.05), main effects of Vector (F1,22=5.49, p<0.05) and Light (F1,22=8.26, p<0.05). OFF/ON order, main effect of Light (F1,17=12.46, p<0.01). Post hoc tests revealed that light decreased freezing only in mice with vCREB-iC++, regardless of light presentation order). Importantly, silencing a similar number of random neurons (mice with iC++) did not disrupt freezing, regardless of light presentation order. Therefore, silencing neurons that overexpressed CREB during training impaired memory expression, whereas silencing a similar number of random neurons not overexpressing CREB had no effect on memory expression. Together with the chemogenetic studies, these results indicate that neurons overexpressing CREB at the time of training are preferentially allocated to the contextual fear memory engram.
CREB overexpression in DG neurons preferentially biases their allocation to an engram supporting contextual fear memory. Optogenetically silencing their activity during a memory test selectively impairs memory expression. (a) Microinjection of vCREB-hM4Di produces strong localized transgene expression in DG principal neurons. (b) Blue light (BL+) silencing decreases freezing in mice with vCREB-iC++ vector but not in mice expressing iC++ vector alone, regardless of order of light presentation during test (BL+, BL−) (c). (b) vCREB-iC++ (n=14), iC++ (n=10), (c) vCREB-iC++ (n=9), iC++ (n=10). Data presented are mean±SEM. n.s., not statistically different, **p<0.01, ***p<0.001.
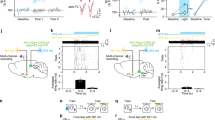
iC++ Silencing of CREB-Overexpressing DG Neurons Shortly After Training Inhibits Contextual Fear Memory Formation
Memory consolidation is thought to involve post-encoding reactivation of the activity patterns present during initial experience (activity replay) (Girardeau et al, 2009; Dupret et al, 2010). The frequency of activity replay decreases after the learning experience; replay is most frequent in the minutes following an experience (Tatsuno et al, 2006) but may persist for 18–24 h (Kudrimoti et al, 1999). Above, we showed that DG neurons overexpressing CREB are preferentially allocated to an engram supporting contextual fear memory and that their activity during the test is necessary for memory expression. We next asked whether post-training processing involving these neurons allocated to an engram is also necessary for recovery of the memory trace at a future time. Mice microinjected with vCREB-iC++ received blue light stimulation (for 1 min) 5 min after training (as above). Control mice were treated similarly but did not receive light stimulation after training. Memory was tested 24 h later, as above. Silencing the activity of neurons expressing CREB 5 min after training was sufficient to impair subsequent memory expression, even during the initial light OFF phase of the memory test. In contrast, control mice (microinjected with vCREB-iC++ vector) that did not receive post-training light stimulation showed intact memory during the light OFF epoch of the test, although they remained sensitive to light-induced silencing during the test ((Condition: vCREB-iC++ and post-training light; vCREB-iC++ and no light; iC++ and post-training light) and Light-at-test (ON; OFF) significant interaction (F1,19=7.30, p<0.01), and main effect of Condition (F1,19=53.48, p<0.001), Light-at-test (F1,19=34.89, p<0.001)) (Figure 3a). An additional group of mice was microinjected with vCREB-iC++ vector and trained as above, but received blue-light stimulation 24 h (rather than 5 min) after training. Blue-light silencing of vCREB-iC++-infected neurons did not affect subsequent memory expression: during the test, mice showed normal freezing during light OFF condition, but decreased freezing during the light ON condition ((F1,9=22.54, p<0.01)) (Figure 3b). Together, these data indicate that silencing neurons allocated to the engram at critical times after training impairs subsequent memory expression as if the memory was not successfully consolidated.
Post-training inhibition of DG neurons allocated to an engram disrupts subsequent contextual fear memory expression. (a) Silencing of neurons expressing vCREB-iC++ 5 min following training decreases subsequent freezing at test, even in the absence of blue light during the test. However, silencing neurons expressing iC++ alone (5 min post training) had no effect on subsequent memory test. vCREB-iC++ and post-training light (n =11), vCREB-iC++ and no light (n=5), iC++ and post-training light (n=6). (b) Silencing neurons expressing vCREB-iC++ 24 h following training does not impair freezing during the test in the absence of light. vCREB-iC++ and 24 h post-training light (n=10). Data presented are mean±SEM. n.s., not statistically different, *p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
Here, we examined the process of neuronal allocation to an engram supporting a contextual fear memory by manipulating excitability of a small random population of DG neurons. To increase neuronal excitability and bias their recruitment into an engram supporting contextual fear memory, we overexpressed CREB in these neurons before training. The finding that inhibiting this small population of neurons overexpressing CREB—and importantly not a similar number of random neurons—impaired subsequent expression of that memory indicates that these neurons were preferentially allocated to the DG component of an engram supporting contextual fear memory. Inhibiting these neurons shortly after training impaired subsequent memory expression, indicating that ongoing activity within this population of neurons is necessary for subsequent memory retrieval.
Neuronal Allocation to a Fear Memory Engram in the DG is Based on Excitability
Neuronal allocation to an engram based on relative CREB function or excitability at the time of training was initially described in the LA for auditory fear memory (Han et al, 2007, 2009; Zhou et al, 2009). Since that time, a similar allocation process was shown in several other brain regions supporting many types of memory, including in the LA for encoding of cocaine-conditioned place preference memories (Hsiang et al, 2014), in the LA for aversive memories (Zhou et al, 2009), in the insular cortex for encoding conditioned taste aversion (Sano et al, 2014), and in the piriform cortex during a shock avoidance paradigm (Choi et al, 2011). Here, we present evidence of neuronal recruitment to an engram in the DG supporting a contextual memory based on CREB-mediated excitability. Together these results indicate that neuronal allocation to an engram based on excitability may be a mechanism used across multiple brain regions and types of memory.
How might excitability-based engram allocation contribute to the computational functions performed by the DG? The DG is thought to distinguish unique input patterns, even when these input patterns are highly similar to those previously experienced (Yassa and Stark, 2011; Rolls, 2012). The precise algorithm by which the DG implements such a pattern separation function is unknown. One possibility raised by the present experiments is that for each experience, a random set of DG neurons is ‘selected’ based on the randomness of intrinsic fluctuations in the cells’ excitability. If the neurons that happen to be more excitable at one time differ from those that are more excitable at another, these experiences will engage different dentate neurons and may thereby be distinguishable to downstream processes. Context-related neuron population codes in the hippocampus also shift over time (Rubin and Umanath, 2015), and the cycling of individual neurons’ excitability may provide a mechanism by which different experiences are time-stamped and distinguished. In this way, two experiences that occur with close temporal proximity may be co-allocated to overlapping populations and linked.
Inhibition of a DG Fear Engram Shortly After Training Disrupts Memory Consolidation
The above results identify rules that mediate the allocation of DG neurons to an engram supporting a contextual memory. DG neurons with increased excitability are preferentially recruited to an engram, as selectively disrupting the activity of these neurons (and not an equivalent number of random DG neurons) before testing disrupts expression of that contextual memory. This result and other similar studies (Liu et al, 2012; Ramirez et al, 2013) identify neurons allocated to an engram. However, post-learning processes, presumably in neurons allocated to the engram, are thought to be important for successful memory consolidation (Marr, 1971; Buzsáki, 1989; Wilson and McNaughton, 1994; Girardeau et al, 2009; Ego-Stengel and Wilson, 2010; Carr et al, 2011). In vivo recording studies show that the precise patterns of event-induced neuronal activity may be subsequently replayed offline, during sleep (Skaggs and McNaughton, 1996; Ji and Wilson, 2007), or quiet wakeful periods (Carr et al, 2011). Disrupting these replay events impairs spatial memory (Ego-Stengel and Wilson, 2010; Jadhav et al, 2012). However, the frequency of consolidation-related replay declines with time after an experience, such that within roughly 24 h, replay can no longer be observed using current recording techniques (Kudrimoti et al, 1999; Tatsuno et al, 2006). Here, we examined the mnemonic effects on disrupting activity in allocated neurons after training. We found that disrupting the activity of allocated neurons, and not a similar number of non-allocated neurons, 5 min, but not 24 h following training, disrupted memory expression. These findings suggest that inhibition of DG components of an engram supporting a contextual memory shortly after learning disrupts the stability of the encoded memory by preventing reactivation. The results are consistent with those from studies of the LA, where disrupting activity of engram neurons during post-training periods can disrupt an appetitive conditioned memory (Hsiang et al, 2014). Together, these results suggest that interfering with activity during offline periods after training, when memory trace reactivation may be taking place, impairs memory consolidation.
FUNDING AND DISCLOSURE
This work was supported by grants from the Canadian Institutes of Health Research (CIHR; MOP-74650 to SAJ, FDN143227 to PWF), Natural Science and Engineering Council of Canada (NSERC to SAJ and PWF), Brain Canada (SAJ), Brain & Behavior Foundation (SAJ), and The Hospital for Sick Children’s Restracomp Fellowships (EEK, AJR). The authors declare no conflict of interest.
References
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168.
Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ et al (2016). Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci USA 113: 822–829.
Blanchard RJ, Blanchard DC (1969). Crouching as an index of fear. J Comp Physiol Psychol 67: 370–375.
Bolles RC, Fanselow MS (1982). Endorphins and behavior. Annu Rev Psychol 33: 87–101.
Buzsáki G (1989). Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31: 551–570.
Carr MF, Jadhav SP, Frank LM (2011). Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 14: 147–153.
Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK et al (2005). Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentate by brief spatial experience. Hippocampus 15: 579–586.
Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R (2011). Driving opposing behaviors with ensembles of piriform neurons. Cell 146: 1004–1015.
Davis M (1992). The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15: 353–375.
Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J (2010). The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13: 995–1002.
Ego-Stengel V, Wilson MA (2010). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20: 1–10.
Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12: 1222–1223.
Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S et al (2016). Arc expression identifies the lateral amygdala fear memory trace. Mol Psychiatry 21: 364–375.
Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999). Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci 2: 1120–1124.
Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA et al (2007). Neuronal competition and selection during memory formation. Science 316: 457–460.
Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A et al (2009). Selective erasure of a fear memory. Science 323: 1492–1496.
Hernández-Rabaza V, Hontecillas-Prieto L, Velázquez-Sánchez C, Ferragud A, Pérez-Villaba A, Arcusa A et al (2008). The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem 90: 553–559.
Hsiang WL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N et al (2014). Manipulating a “cocaine engram” in mice. J Neurosci 34: 14115–14127.
Jadhav SP, Kemere C, German PW, Frank LM (2012). Awake hippocampal sharp-wave ripples support spatial memory. Science 336: 1454–1458.
Ji D, Wilson MA (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–107.
Josselyn SA (2010). Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci 35: 221–228.
Josselyn SA, Köhler S, Frankland PW (2015). Finding the engram. Nat Rev Neurosci 16: 521–534.
Kudrimoti HS, Barnes CA, McNaughton BL (1999). Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci 19: 4090–4101.
LeDoux JE (2000). Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184.
Leutgeb JK, Leutgeb S, Moser MB, Moser EI (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315: 961–966.
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K et al (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484: 381–385.
Maren S (2003). The amygdala, synaptic plasticity, and fear memory. Ann N Y Acad Sci 985: 106–113.
Marr D (1971). Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci 262: 23–81.
Nichols CD, Roth BL (2009). Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front Mol Neurosci 2: 1–10.
Paxinos G, Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: San Diego, CA, USA.
O’Reilly RC, McClelland JL (1994). Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 4: 661–682.
Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL et al (2013). Creating a false memory in the hippocampus. Science 341: 387–391.
Reijmers LG, Perkins BL, Matsuo N, Mayford M (2007). Localization of a stable neural correlate of associate memory. Science 317: 1230–1233.
Rolls ET (2012). Advantages of dilution in the connectivity of attractor networks in the brain. Biologically Inspired Cognitive Architectures 1: 44–54.
Rubin DC, Umanath S (2015). Event memory: a theory of memory for laboratory, autobiographical, and fictional events. Psychol Rev 122: 1–23.
Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V et al (2009). Nuclear factor κB signaling regulates neuronal morphology and cocaine reward. J Neurosci 29: 3529–3537.
Sano Y, Shobe JL, Zhou M, Huang S, Shuman T, Cai DJ et al (2014). CREB regulates memory allocation in the insular cortex. Curr Biol 24: 2833–2837.
Schacter DL, Eich JE, Tulving E (1978). Richard Semon’s theory of memory. J Verbal Learning Verbal Behav 17: 721–743.
Sekeres MJ, Mercaldo V, Richards B, Sargin D, Mahadevan V, Woodin MA et al (2012). Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci 32: 17857–17868.
Semon R (1923) Mnemic Psychology. G. Allen & Unwin Limited: London.
Skaggs WE, McNaughton BL (1996). Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271: 1870–1873.
Tatsuno M, Lipa P, McNaughton BL (2006). Methodological considerations on the use of template matching to study long-lasting memory trace replay. J Neurosci 26: 10727–10742.
Tonegawa S, Liu X, Ramirez S, Redondo R (2015). Memory engram cells have come of age. Neuron 87: 918–931.
Treves A, Tashiro A, Witter MP, Moser EI (2008). What is the mammalian dentate gyrus good for? Neuroscience 154: 1155–1172.
Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S et al (2006). Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol 498: 317–329.
Wilson MA, McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679.
Yassa MA, Stark CEL (2011). Pattern separation in the hippocampus. Trends Neurosci 24: 515–525.
Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL et al (2014). Neurons are recruited to a memory based on relative neuronal excitability immediately before training. Neuron 83: 722–735.
Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J et al (2009). CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 12: 1438–1443.
Acknowledgements
We would like to thank Mika Yamamoto, Daisy Lin, Sherwin Nicholson, and Antonietta (Toni) Decristofaro for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S., Kramer, E., Mercaldo, V. et al. Neuronal Allocation to a Hippocampal Engram. Neuropsychopharmacol 41, 2987–2993 (2016). https://doi.org/10.1038/npp.2016.73
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.73
This article is cited by
-
Reactivation of encoding ensembles in the prelimbic cortex supports temporal associations
Neuropsychopharmacology (2024)
-
Peroxisome proliferator-activated receptor-α activation facilitates contextual fear extinction and modulates intrinsic excitability of dentate gyrus neurons
Translational Psychiatry (2023)
-
Locus coeruleus input-modulated reactivation of dentate gyrus opioid-withdrawal engrams promotes extinction
Neuropsychopharmacology (2023)
-
Formation and fate of an engram in the lateral amygdala supporting a rewarding memory in mice
Neuropsychopharmacology (2023)
-
Functional network of contextual and temporal memory has increased amygdala centrality and connectivity with the retrosplenial cortex, thalamus, and hippocampus
Scientific Reports (2023)