Abstract
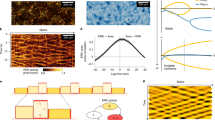
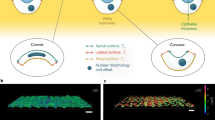
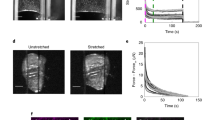
The processes by which an organism develops its shape and heals wounds involve expansion of a monolayer sheet of cells. The mechanism underpinning this epithelial expansion remains obscure, despite the fact that its failure is known to contribute to several diseases, including carcinomas, which account for about 90% of all human cancers. Here, using the micropatterned epithelial monolayer as a model system, we report the discovery of a mechanical wave that propagates slowly to span the monolayer, traverses intercellular junctions in a cooperative manner and builds up differentials of mechanical stress. Essential features of this wave generation and propagation are captured by a minimal model based on sequential fronts of cytoskeletal reinforcement and fluidization. These findings establish a mechanism of long-range cell guidance, symmetry breaking and pattern formation during monolayer expansion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Du Roure, O. et al. Force mapping in epithelial cell migration. Proc. Natl Acad. Sci. USA 102, 2390–2395 (2005).
Trepat, X. & Fredberg, J. J. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 21, 638–646 (2011).
Leckband, D. E., le Duc, Q., Wang, N. & de Rooij, J. Mechanotransduction at cadherin-mediated adhesions. Curr. Opin. Cell Biol. 23, 523–530 (2011).
Trepat, X. et al. Physical forces during collective cell migration. Nature Phys. 5, 426–430 (2009).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nature Mater. 10, 469–475 (2011).
Nikolic, D. L., Boettiger, A. N., Bar-Sagi, D., Carbeck, J. D. & Shvartsman, S. Y. Role of boundary conditions in an experimental model of epithelial wound healing. Am. J. Physiol. Cell Physiol. 291, C68–C75 (2006).
Poujade, M. et al. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl Acad. Sci. USA 104, 15988–15993 (2007).
Matsubayashi, Y., Razzell, W. & Martin, P. ‘White wave’ analysis of epithelial scratch wound healing reveals how cells mobilise back from the leading edge in a myosin-II-dependent fashion. J. Cell Sci. 124, 1017–1021 (2011).
Maruthamuthu, V., Sabass, B., Schwarz, U. S. & Gardel, M. L. Cell-ECM traction force modulates endogenous tension at cell–cell contacts. Proc. Natl Acad. Sci. USA 108, 4708–4713 (2011).
Liu, Z. et al. Mechanical tugging force regulates the size of cell–cell junctions. Proc. Natl Acad. Sci. USA 107, 9944–9949 (2010).
Blanchard, G. B. et al. Tissue tectonics: Morphogenetic strain rates, cell shape change and intercalation. Nature Methods 6, 458–464 (2009).
Chen, X. & Brodland, G. W. Multi-scale finite element modeling allows the mechanics of amphibian neurulation to be elucidated. Phys. Biol. 5, 015003 (2008).
Gros, J., Feistel, K., Viebahn, C., Blum, M. & Tabin, C. J. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 324, 941–944 (2009).
Wan, L. Q. et al. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc. Natl Acad. Sci. USA 108, 12295–12300 (2010).
Brangwynne, C., Huang, S., Parker, K. K., Ingber, D. E. & Ostuni, E. Symmetry breaking in cultured mammalian cells. In Vitro Cell Dev. Biol. Anim. 36, 563–565 (2000).
Bois, J. S., Julicher, F. & Grill, S. W. Pattern formation in active fluids. Phys. Rev. Lett. 106, 028103 (2011).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Carmona-Fontaine, C. et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456, 957–961 (2008).
Weber, G. F., Bjerke, M. A. & Desimone, D. W. A mechanoresponsive Cadherin-Keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22, 104–115 (2012).
Angelini, T. E. et al. From the cover: Glass-like dynamics of collective cell migration. Proc. Natl Acad. Sci. USA 108, 4714–4719 (2011).
Garrahan, J. P. Dynamic heterogeneity comes to life. Proc. Natl Acad. Sci. USA 108, 4701–4702 (2011).
Riveline, D. et al. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 (2001).
Trepat, X. et al. Universal physical responses to stretch in the living cell. Nature 447, 592–595 (2007).
Bursac, P. et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nature Mater. 4, 557–561 (2005).
Eyckmans, J., Boudou, T., Yu, X. & Chen, C. S. A Hitchhiker’s guide to mechanobiology. Dev. Cell 21, 35–47 (2011).
Mammoto, T. & Ingber, D. E. Mechanical control of tissue and organ development. Development 137, 1407–1420 (2010).
Shraiman, B. I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl Acad. Sci. USA 102, 3318–3323 (2005).
Montell, D. J. Morphogenetic cell movements: Diversity from modular mechanical properties. Science 322, 1502–1505 (2008).
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009).
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Johnson, C. P., Tang, H-Y., Carag, C., Speicher, D. W. & Discher, D. E. Forced unfolding of proteins within cells. Science 317, 663–666 (2007).
Zhang, H. et al. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471, 99–103 (2011).
Ostuni, E., Kane, R., Chen, C. S., Ingber, D. E. & Whitesides, G. M. Patterning mammalian cells using elastomeric membranes. Langmuir 16, 7811–7819 (2000).
Kandow, C. E., Georges, P. C., Janmey, P. A. & Beningo, K. A. in Methods in Cell Biology Vol. 83 (eds Wang Yu-Li, E. & Discher, Dennis) 29–46 (Academic, 2007).
Yeung, T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34 (2005).
Acknowledgements
We thank M. Bintanel for technical assistance, S. Garcia and A. Carreras for help with polyacrylamide gels and micropatterning, the Nanotechnology Platform from Barcelona Science Park, J. J. Munoz for help with the numerical implementation of the model, and P. Roca-Cusachs, D.G. Miguez, D. Navajas, R. Farre and J. Alcaraz for discussions. This research was supported by the Spanish Ministry for Science and Innovation (BFU2009-07595 and FPU fellowship XS), the European Research Council (Grant Agreement 242993) and the National Institutes of Health (R01HL102373, R01HL107561).
Author information
Authors and Affiliations
Contributions
X.S-P. and X.T. designed experiments; X.S-P. performed all experiments. E.B. and X.S-P. performed gene expression experiments; X.S-P., R.V., V.C. and X.T. analysed data; D.T.T. contributed software; V.C. built the computer model and performed simulations; X.S-P., V.C., J.J.F. and X.T. wrote the manuscript; all authors discussed and interpreted results and commented on the manuscript; X.T. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Movie descriptions (PDF 334 kb)
Supplementary Information
Supplementary Information (PDF 1646 kb)
Supplementary Information
Supplementary Information (MOV 1100 kb)
Supplementary Information
Supplementary Information (MOV 3989 kb)
Supplementary Information
Supplementary Information (MOV 2770 kb)
Supplementary Information
Supplementary Information (MOV 5755 kb)
Supplementary Information
Supplementary Information (MOV 4318 kb)
Supplementary Information
Supplementary Information (MOV 5846 kb)
Supplementary Information
Supplementary Information (MOV 3559 kb)
Supplementary Information
Supplementary Information (MOV 93 kb)
Supplementary Information
Supplementary Information (MOV 82 kb)
Supplementary Information
Supplementary Information (MOV 90 kb)
Supplementary Information
Supplementary Information (MOV 93 kb)
Rights and permissions
About this article
Cite this article
Serra-Picamal, X., Conte, V., Vincent, R. et al. Mechanical waves during tissue expansion. Nature Phys 8, 628–634 (2012). https://doi.org/10.1038/nphys2355
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys2355
This article is cited by
-
Role of viscoelasticity in the appearance of low-Reynolds turbulence: considerations for modelling
Journal of Biological Engineering (2024)
-
Biophysical control of plasticity and patterning in regeneration and cancer
Cellular and Molecular Life Sciences (2024)
-
Mechanical waves help zebrafish regrow their tails
Nature Physics (2023)
-
Mechanical stress driven by rigidity sensing governs epithelial stability
Nature Physics (2023)
-
Mechanical waves identify the amputation position during wound healing in the amputated zebrafish tailfin
Nature Physics (2023)