Abstract
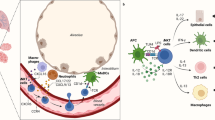
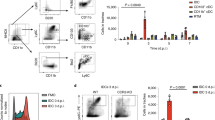
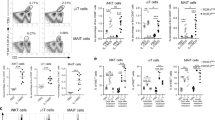
Aspergillus fumigatus is a saprophytic fungus that is ubiquitous in the environment and is commonly associated with allergic sensitization and severe asthma in humans. Although A. fumigatus is recognized by multiple microbial pattern-recognition receptors, we found that an A. fumigatus–derived glycosphingolipid, asperamide B, directly activates invariant natural killer T (iNKT) cells in vitro in a CD1d-restricted, MyD88-independent and dectin-1–independent fashion. Moreover, asperamide B, when loaded onto CD1d, directly stained, and was sufficient to activate, human and mouse iNKT cells. In vivo, asperamide B rapidly induced airway hyperreactivity, which is a cardinal feature of asthma, by activating pulmonary iNKT cells in an interleukin-33 (IL-33)-ST2–dependent fashion. Asperamide B is thus the first fungal glycolipid found to directly activate iNKT cells. These results extend the range of microorganisms that can be directly detected by iNKT cells to the kingdom of fungi and may explain how A. fumigatus can induce severe chronic respiratory diseases in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
27 September 2013
In the version of this article initially published online, the molecular mass of asperamide B was incorrectly given as 738, when it is actually 754. The error has been corrected for the print, PDF and HTML versions of this article.
References
Trompette, A. et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 (2009).
Barrett, N.A. et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 208, 593–604 (2011).
O'Connor, G.T. et al. Airborne fungi in the homes of children with asthma in low-income urban communities: The Inner-City Asthma Study. J. Allergy Clin. Immunol. 114, 599–606 (2004).
Shelton, B.G., Kirkland, K.H., Flanders, W.D. & Morris, G.K. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68, 1743–1753 (2002).
Steele, C. et al. The β-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1, e42 (2005).
Balloy, V. & Chignard, M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 11, 919–927 (2009).
Fairs, A. et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am. J. Respir. Crit. Care Med. 182, 1362–1368 (2010).
Menzies, D., Holmes, L., McCumesky, G., Prys-Picard, C. & Niven, R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy 66, 679–685 (2011).
Agarwal, R. Severe asthma with fungal sensitization. Curr. Allergy Asthma Rep. 11, 403–413 (2011).
Greenberger, P.A. Allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 110, 685–692 (2002).
Matangkasombut, P., Pichavant, M., Dekruyff, R.H. & Umetsu, D.T. Natural killer T cells and the regulation of asthma. Mucosal Immunol. 2, 383–392 (2009).
Iwamura, C. & Nakayama, T. Role of NKT cells in allergic asthma. Curr. Opin. Immunol. 22, 807–813 (2010).
Kurup, V.P., Mauze, S., Choi, H., Seymour, B.W.P. & Coffman, R.L. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. J. Immunol. 148, 3783–3788 (1992).
Grünig, G. et al. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 185, 1089–1099 (1997).
Kurup, V.P. & Grunig, G. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia 153, 165–177 (2002).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 (2005).
Zhang, Y. et al. New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids 42, 759–764 (2007).
Cohen, N.R. et al. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10, 437–450 (2011).
Bendelac, A., Savage, P.B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Kinjo, Y. et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7, 978–986 (2006).
Chang, Y.J. et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J. Clin. Invest. 121, 57–69 (2011).
Kinjo, Y. et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat. Immunol. 12, 966–974 (2011).
Godfrey, D.I. et al. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin. Immunol. 22, 61–67 (2010).
Brigl, M. et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208, 1163–1177 (2011).
Kawakami, K. et al. Monocyte chemoattractant protein-1–dependent increase of Vα14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J. Immunol. 167, 6525–6532 (2001).
Blackstock, R. & Murphy, J.W. Age-related resistance of C57BL/6 mice to Cryptococcus neoformans is dependent on maturation of NKT cells. Infect. Immun. 72, 5175–5180 (2004).
Sherif, R. & Segal, B.H. Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr. Opin. Pulm. Med. 16, 242–250 (2010).
Ramaprakash, H. et al. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am. J. Pathol. 179, 104–115 (2011).
Lilly, L.M. et al. The β-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 189, 3653–3660 (2012).
Murdock, B.J. et al. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect. Immun. 80, 1424–1436 (2012).
Kim, H.Y. et al. Innate lymphoid cells responding to IL-33 mediate airway-hyperreactivity independent of adaptive immunity. J. Allergy Clin. Immunol. 129, 216–227 (2012).
Arshad, M.I. et al. NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. Eur. J. Immunol. 41, 2341–2348 (2011).
Smithgall, M.D. et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Saenz, S.A. et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464, 1362–1366 (2010).
Neill, D.R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Chang, Y.-J. et al. Innate lymphoid cells mediate influenza-induced airway hyperreactivity independent of adaptive immunity. Nat. Immunol. 12, 631–638 (2011).
Price, A.E. et al. Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107, 11489–11494 (2010).
Halim, T.Y., Krauss, R.H., Sun, A.C. & Takei, F. Lung natural helper cells are a critical source of Th2 cell–type cytokines in protease allergen-induced airway inflammation. Immunity 36, 451–463 (2012).
Chan-Yeung, M. et al. Geographical variations in the prevalence of atopic sensitization in six study sites across Canada. Allergy 65, 1404–1413 (2010).
Broadfield, E. et al. Increase in the prevalence of allergen skin sensitization in successive birth cohorts. J. Allergy Clin. Immunol. 109, 969–974 (2002).
O'Driscoll, B.R. et al. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clin. Exp. Allergy 39, 1677–1683 (2009).
Meyer, E.H. et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl. Acad. Sci. USA 103, 2782–2787 (2006).
Agea, E. et al. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 202, 295–308 (2005).
Wingender, G. et al. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J. Exp. Med. 208, 1151–1162 (2011).
Brennan, P.J. et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12, 1202–1211 (2011).
Jyonouchi, S. et al. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J. Allergy Clin. Immunol. 128, 102–109 e13 (2011).
Huang, Y.J. et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 127, 372–381.e1–3 (2011).
Scanlon, S.T. et al. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J. Exp. Med. 208, 2113–2124 (2011).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Karp, C.L. Guilt by intimate association: what makes an allergen an allergen? J. Allergy Clin. Immunol. 125, 955–960 (2010).
Thomas, W.R., Hales, B.J. & Smith, W.A. Structural biology of allergens. Curr. Allergy Asthma Rep. 5, 388–393 (2005).
Martin, T.R., Gerard, N.P., Galli, S.J. & Drazen, J.M. Pulmonary responses to bronchoconstrictor agonists in the mouse. J. Appl. Physiol. 64, 2318–2323 (1988).
Liu, Y. et al. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J. Immunol. Methods 312, 34–39 (2006).
Lee, H.-H. et al. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. J. Immunol. 185, 5225–5235 (2010).
Acknowledgements
This work was supported in part by US National Institutes of Health (NIH) grants RO1AI026322, RC1HL099839 and R21AI083523 to D.T.U. and by the Bunning Food Allergy Project to D.T.U. L.A.A. was a graduate student in the Program in Immunology, Division of Medical Sciences, Harvard University. We would also like to acknowledge the NIH National Institute of Allergy and Infectious Diseases (NIAID) research reagent program that provided the A. fumigatus lysates used in this study. We thank the NIH NIAID Tetramer Facility for providing CD1d monomers and tetramers for this study, D.B. Moody for help with plate-bound CD1d assays and M. Grusby (Harvard School of Public Health), M. Taniguchi and T. Nakayama (Chiba University), S. Akira (Osaka University), R. Geha (Boston Children's Hospital) and A. McKenzie (Medical Research Council, Laboratory of Molecular Biology) for providing genetically modified mice.
Author information
Authors and Affiliations
Contributions
L.A.A. conceived and initiated the project, planned and performed experiments and wrote the manuscript. V.C. performed lipid isolation, identification and synthesis. Y.-J.C. planned and performed experiments, including the tetramer studies. H.Y.K. performed experiments and edited the manuscript. Y.-T.C. isolated and maintained iNKT cell lines. M.P. maintained iNKT cell lines, assisted with experiments and provided training in invasive plethysmography. R.H.D. helped plan experiments and edited the manuscript. P.B.S. planned lipid isolation, identification and synthesis and wrote the manuscript. D.T.U. conceived the idea for the project, planned experiments, shepherded the project along and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1517 kb)
Rights and permissions
About this article
Cite this article
Albacker, L., Chaudhary, V., Chang, YJ. et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med 19, 1297–1304 (2013). https://doi.org/10.1038/nm.3321
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3321
This article is cited by
-
Role of NKT cells in cancer immunotherapy—from bench to bed
Medical Oncology (2022)
-
Eosinophil extracellular traps drive asthma progression through neuro-immune signals
Nature Cell Biology (2021)
-
CPEB2-activated PDGFRα mRNA translation contributes to myofibroblast proliferation and pulmonary alveologenesis
Journal of Biomedical Science (2020)
-
The role of invariant T cells in inflammation of the skin and airways
Seminars in Immunopathology (2019)
-
The common γ-chain cytokine IL-7 promotes immunopathogenesis during fungal asthma
Mucosal Immunology (2018)