Abstract
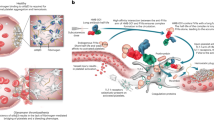
Hemophilia A is a bleeding disorder resulting from coagulation factor VIII (FVIII) deficiency. Exogenously provided FVIII effectively reduces bleeding complications in patients with severe hemophilia A. In approximately 30% of such patients, however, the 'foreignness' of the FVIII molecule causes them to develop inhibitory antibodies against FVIII (inhibitors), precluding FVIII treatment in this set of patients1,2,3. Moreover, the poor pharmacokinetics of FVIII, attributed to low subcutaneous bioavailability and a short half-life of 0.5 d, necessitates frequent intravenous injections3,4,5. To overcome these drawbacks, we generated a humanized bispecific antibody to factor IXa (FIXa) and factor X (FX), termed hBS23, that places these two factors into spatially appropriate positions and mimics the cofactor function of FVIII. hBS23 exerted coagulation activity in FVIII-deficient plasma, even in the presence of inhibitors, and showed in vivo hemostatic activity in a nonhuman primate model of acquired hemophilia A. Notably, hBS23 had high subcutaneous bioavailability and a 2-week half-life and would not be expected to elicit the development of FVIII-specific inhibitory antibodies, as its molecular structure, and hence antigenicity, differs from that of FVIII. A long-acting, subcutaneously injectable agent that is unaffected by the presence of inhibitors could markedly reduce the burden of care for the treatment of hemophilia A.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fischer, K., Lewandowski, D., Marijke van den Berg, H. & Janssen, M.P. Validity of assessing inhibitor development in haemophilia PUPs using registry data: the EUHASS project. Haemophilia 18, e241–e246 (2012).
Oldenburg, J., El-Maarri, O. & Schwaab, R. Inhibitor development in correlation to factor VIII genotypes. Haemophilia 8 (suppl. 2), 23–29 (2002).
Berntorp, E. & Shapiro, A.D. Modern haemophilia care. Lancet 379, 1447–1456 (2012).
Shi, Q., Kuether, E.L., Schroeder, J.A., Fahs, S.A. & Montgomery, R.R. Intravascular recovery of VWF and FVIII following intraperitoneal injection and differences from intravenous and subcutaneous injection in mice. Haemophilia 18, 639–646 (2012).
Björkman, S. et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood 119, 612–618 (2012).
Stonebraker, J.S., Bolton-Maggs, P.H., Soucie, J.M., Walker, I. & Brooker, M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia 16, 20–32 (2010).
Geraghty, S. et al. Practice patterns in haemophilia A therapy—global progress towards optimal care. Haemophilia 12, 75–81 (2006).
Manco-Johnson, M.J. et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 357, 535–544 (2007).
Young, G. et al. When should prophylaxis therapy in inhibitor patients be considered? Haemophilia 17, e849–e857 (2011).
Astermark, J. et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood 109, 546–551 (2007).
Leissinger, C. et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N. Engl. J. Med. 365, 1684–1692 (2011).
Hay, C.R. & DiMichele, D.M. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 119, 1335–1344 (2012).
Ragni, M.V. et al. Survey of current prophylaxis practices and bleeding characteristics of children with severe haemophilia A in US haemophilia treatment centres. Haemophilia 18, 63–68 (2012).
Fay, P.J. Activation of factor VIII and mechanisms of cofactor action. Blood Rev. 18, 1–15 (2004).
Lenting, P.J., Donath, M.J., van Mourik, J.A. & Mertens, K. Identification of a binding site for blood coagulation factor IXa on the light chain of human factor VIII. J. Biol. Chem. 269, 7150–7155 (1994).
Fay, P.J. & Koshibu, K. The A2 subunit of factor VIIIa modulates the active site of factor IXa. J. Biol. Chem. 273, 19049–19054 (1998).
Lapan, K.A. & Fay, P.J. Localization of a factor X interactive site in the A1 subunit of factor VIIIa. J. Biol. Chem. 272, 2082–2088 (1997).
Liu, Z. et al. A potent erythropoietin-mimicking human antibody interacts through a novel binding site. Blood 110, 2408–2413 (2007).
Mayorov, A.V. et al. Catalytic antibody degradation of ghrelin increases whole-body metabolic rate and reduces refeeding in fasting mice. Proc. Natl. Acad. Sci. USA 105, 17487–17492 (2008).
Bhaskar, V. et al. A fully human, allosteric monoclonal antibody that activates the insulin receptor and improves glycemic control. Diabetes 61, 1263–1271 (2012).
Beck, A., Wurch, T., Bailly, C. & Corvaia, N. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 10, 345–352 (2010).
Baeuerle, P.A., Kufer, P. & Bargou, R. BiTE: teaching antibodies to engage T-cells for cancer therapy. Curr. Opin. Mol. Ther. 11, 22–30 (2009).
Jackman, J. et al. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J. Biol. Chem. 285, 20850–20859 (2010).
Shen, B.W. et al. The tertiary structure and domain organization of coagulation factor VIII. Blood 111, 1240–1247 (2008).
Saphire, E.O. et al. Contrasting IgG structures reveal extreme asymmetry and flexibility. J. Mol. Biol. 319, 9–18 (2002).
Baker, M.P., Reynolds, H.M., Lumicisi, B. & Bryson, C.J. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself 1, 314–322 (2010).
Wang, W., Wang, E.Q. & Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 84, 548–558 (2008).
Martens, T. et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin. Cancer Res. 12, 6144–6152 (2006).
Shima, M., Matsumoto, T. & Ogiwara, K. New assays for monitoring haemophilia treatment. Haemophilia 14 (suppl. 3), 83–92 (2008).
Shetty, S., Bhave, M. & Ghosh, K. Acquired hemophilia A: diagnosis, aetiology, clinical spectrum and treatment options. Autoimmun. Rev. 10, 311–316 (2011).
Lin, Y.S. et al. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 288, 371–378 (1999).
Benincosa, L.J. et al. Pharmacokinetics and pharmacodynamics of a humanized monoclonal antibody to factor IX in cynomolgus monkeys. J. Pharmacol. Exp. Ther. 292, 810–816 (2000).
Deng, R. et al. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs 3, 61–66 (2011).
Lillicrap, D. Improvements in factor concentrates. Curr. Opin. Hematol. 17, 393–397 (2010).
Powell, J.S. et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood 119, 3031–3037 (2012).
High, K.A. Gene therapy for haemophilia: a long and winding road. J. Thromb. Haemost. 9 (suppl. 1), 2–11 (2011).
Harding, F.A., Stickler, M.M., Razo, J. & DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2, 256–265 (2010).
Merchant, A.M. et al. An efficient route to human bispecific IgG. Nat. Biotechnol. 16, 677–681 (1998).
Igawa, T. et al. Engineering the variable region of therapeutic IgG antibodies. MAbs 3, 243–252 (2011).
Igawa, T. et al. Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng. Des. Sel. 23, 385–392 (2010).
Bloom, J.W., Madanat, M.S., Marriott, D., Wong, T. & Chan, S.Y. Intrachain disulfide bond in the core hinge region of human IgG4. Protein Sci. 6, 407–415 (1997).
Okuda, M. & Yamamoto, Y. Usefulness of synthetic phospholipid in measurement of activated partial thromboplastin time: a new preparation procedure to reduce batch difference. Clin. Lab. Haematol. 26, 215–223 (2004).
Jones, P.T., Dear, P.H., Foote, J., Neuberger, M.S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
Healey, J.F., Lubin, I.M. & Lollar, P. The cDNA and derived amino acid sequence of porcine factor VIII. Blood 88, 4209–4214 (1996).
Yonemura, H. et al. Efficient production of recombinant human factor VIII by co-expression of the heavy and light chains. Protein Eng. 6, 669–674 (1993).
Hemker, H.C. et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 33, 4–15 (2003).
Acknowledgements
We thank T. Matsuura, T. Houjo, K. Kanisawa, R. Takemoto, T. Koike and M. Hiranuma for carrying out the in vivo experiments and M. Fujii, Y. Nakata, H. Ishida and F. Isomura for antibody generation and preparation. We also thank S. Ohtsu for carrying out in vitro experiments.
Author information
Authors and Affiliations
Contributions
T. Kitazawa and T. Igawa led the pharmacological studies and the optimization of the bispecific antibody, respectively, in the program and wrote the manuscript. Z.S. designed the lead chimeric bispecific antibody and hBS23. T. Kojima led the lead identification. H.T. provided ideas on bispecific antibody engineering. T. Suzuki, H.A., T.M., S.I., M.K.-S. and T. Iida generated FVIII-, FIXa- and FX-specific antibodies. T. Soeda, Y.O.-N., A.H., M.F., C.M., E.T., T. Toyoda and A.U. performed the in vitro experiments. K.E. and S.S. performed the affinity analyses. Y. Kikuchi, T.W., M.W. and M.G. purified the bispecific antibody and the coagulation factor. A.M. and K.Y. performed the in vivo pharmacological study. K. Haraya and T. Tachibana performed the pharmacokinetic study. H.S. and Y. Kawabe provided direction and guidance for the various functional areas. M.S. and A.Y. provided advice on the program from the viewpoints of their medical expertise in hemophilia. K. Hattori provided the hypothesis and directed and organized the program.
Corresponding author
Ethics declarations
Competing interests
Declaration: T. Kitazawa, T. Igawa, Z.S., A.M., T. Kojima., T. Soeda, K.Y., Y.O.-N., H.S., T. Suzuki, T.M., C.M. and K. Hattori are employees of Chugai Pharmaceutical and inventors on the patents, patent applications or both relating to the bispecific antibodies to FIXa and FX, of which all rights have been assigned to the company. H.T., H.A., S.I., M.K.-S., T. Iida, A.H., K.E., M.F., E.T., Y. Kikuchi, T.W., M.W., M.G., T. Toyoda, A.U., S.S., K. Haraya, T. Tachibana and Y. Kawabe are employees of Chugai Pharmaceutical. M.S. receives consulting honoraria and research support from Chugai Pharmaceutical. A.Y. previously received research support from Chugai Pharmaceutical.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 306 kb)
Rights and permissions
About this article
Cite this article
Kitazawa, T., Igawa, T., Sampei, Z. et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med 18, 1570–1574 (2012). https://doi.org/10.1038/nm.2942
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2942
This article is cited by
-
Emicizumab-mediated hemostatic function assessed by thrombin generation assay in an in vitro model of factor VIII-depleted thrombophilia plasma
International Journal of Hematology (2024)
-
Application of systems biology to identify pharmacological mechanisms of thrombotic microangiopathy evoked by combined activated prothrombin complex concentrate and emicizumab
Scientific Reports (2023)
-
Construction of IgG–Fab2 bispecific antibody via intein-mediated protein trans-splicing reaction
Scientific Reports (2023)
-
Two pediatric cases of severe hemophilia A in which emicizumab prophylaxis failed to prevent traumatic extra-articular hemorrhage
International Journal of Hematology (2023)
-
Non-viral and viral delivery systems for hemophilia A therapy: recent development and prospects
Annals of Hematology (2023)