Abstract
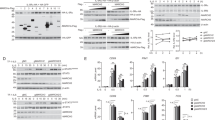
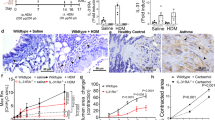
Individuals with chronic asthma show a progressive decline in lung function that is thought to be due to structural remodeling of the airways characterized by subepithelial fibrosis and smooth muscle hyperplasia. Here we show that the tumor necrosis factor (TNF) family member LIGHT is expressed on lung inflammatory cells after allergen exposure. Pharmacological inhibition of LIGHT using a fusion protein between the IgG Fc domain and lymphotoxin β receptor (LTβR) reduces lung fibrosis, smooth muscle hyperplasia and airway hyperresponsiveness in mouse models of chronic asthma, despite having little effect on airway eosinophilia. LIGHT-deficient mice also show a similar impairment in fibrosis and smooth muscle accumulation. Blockade of LIGHT suppresses expression of lung transforming growth factor-β (TGF-β) and interleukin-13 (IL-13), cytokines implicated in remodeling in humans, whereas exogenous administration of LIGHT to the airways induces fibrosis and smooth muscle hyperplasia, Thus, LIGHT may be targeted to prevent asthma-related airway remodeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Boulet, L.P. et al. Airway hyperresponsiveness, inflammation and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am. J. Respir. Crit. Care Med. 162, 1308–1313 (2000).
Chakir, J. et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17 and type I and type III collagen expression. J. Allergy Clin. Immunol. 111, 1293–1298 (2003).
Pepe, C. et al. Differences in airway remodeling between subjects with severe and moderate asthma. J. Allergy Clin. Immunol. 116, 544–549 (2005).
Wills-Karp, M. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 202, 175–190 (2004).
Halwani, R., Al-Muhsen, S., Al-Jahdali, H. & Hamid, Q. Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 44, 127–133 (2011).
Mauri, D.N. et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 8, 21–30 (1998).
Ware, C.F. Network communications: lymphotoxins, LIGHT and TNF. Annu. Rev. Immunol. 23, 787–819 (2005).
Salek-Ardakani, S. et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 198, 315–324 (2003).
Seshasayee, D. et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 117, 3868–3878 (2007).
Berry, M.A. et al. Evidence of a role of tumor necrosis factor α in refractory asthma. N. Engl. J. Med. 354, 697–708 (2006).
Hastie, A.T. et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J. Allergy Clin. Immunol. 125, 1028–1036.e13 (2010).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
De Trez, C. et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 180, 238–248 (2008).
Cho, J.Y. et al. Inhibition of airway remodeling in IL-5–deficient mice. J. Clin. Invest. 113, 551–560 (2004).
Southam, D.S., Ellis, R., Wattie, J. & Inman, M.D. Components of airway hyperresponsiveness and their associations with inflammation and remodeling in mice. J. Allergy Clin. Immunol. 119, 848–854 (2007).
Scheu, S. et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J. Exp. Med. 195, 1613–1624 (2002).
Doerner, A.M. & Zuraw, B.L. TGF-β1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1β but not abrogated by corticosteroids. Respir. Res. 10, 100 (2009).
Zasłona, Z. et al. Transcriptome profiling of primary murine monocytes, lung macrophages and lung dendritic cells reveals a distinct expression of genes involved in cell trafficking. Respir. Res. 10, 2 (2009).
Stevens, W.W., Kim, T.S., Pujanauski, L.M., Hao, X. & Braciale, T.J. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods 327, 63–74 (2007).
Bhavsar, P. et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax 63, 784–790 (2008).
Tetley, T.D. Macrophages and the pathogenesis of COPD. Chest 121, 156S–159S (2002).
Bogaert, P., Tournoy, K.G., Naessens, T. & Grooten, J. Where asthma and hypersensitivity pneumonitis meet and differ: noneosinophilic severe asthma. Am. J. Pathol. 174, 3–13 (2009).
Khalil, N., Whitman, C., Zuo, L., Danielpour, D. & Greenberg, A. Regulation of alveolar macrophage transforming growth factor-β secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J. Clin. Invest. 92, 1812–1818 (1993).
Ochi, H. et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat. Med. 12, 627–635 (2006).
Berger, P. et al. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 17, 2139–2141 (2003).
Gandhi, R., Anderson, D.E. & Weiner, H.L. Cutting Edge: Immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-β–dependent manner. J. Immunol. 178, 4017–4021 (2007).
Burton, O.T. et al. Roles for TGF-β and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. J. Immunol. 185, 2754–2762 (2010).
Kaminska, M. et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J. Allergy Clin. Immunol. 124, 45–51.e4 (2009).
Woodruff, P.G. et al. T-helper type 2–driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 180, 388–395 (2009).
Booth, B.W., Sandifer, T., Martin, E.L. & Martin, L.D. IL-13–induced proliferation of airway epithelial cells: mediation by intracellular growth factor mobilization and ADAM17. Respir. Res. 8, 51 (2007).
Saito, A., Okazaki, H., Sugawara, I., Yamamoto, K. & Takizawa, H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int. Arch. Allergy Immunol. 132, 168–176 (2003).
Zhou, X. et al. Interleukin-13 augments transforming growth factor-β1–induced tissue inhibitor of metalloproteinase-1 expression in primary human airway fibroblasts. Am. J. Physiol. Cell Physiol. 288, C435–C442 (2005).
Espinosa, K., Bosse, Y., Stankova, J. & Rola-Pleszczynski, M. CysLT1 receptor upregulation by TGF-β and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J. Allergy Clin. Immunol. 111, 1032–1040 (2003).
Heo, S.K. et al. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J. Leukoc. Biol. 79, 330–338 (2006).
Luzina, I.G. et al. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum. 48, 2262–2274 (2003).
Lee, W.H. et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler. Thromb. Vasc. Biol. 21, 2004–2010 (2001).
Kelly, E.A. & Jarjour, N.N. Role of matrix metalloproteinases in asthma. Curr. Opin. Pulm. Med. 9, 28–33 (2003).
Lim, D.H. et al. Reduced peribronchial fibrosis in allergen-challenged MMP-9–deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L265–L271 (2006).
Fattouh, R. et al. Transforming growth factor-β regulates house dust mite–induced allergic airway inflammation but not airway remodeling. Am. J. Respir. Crit. Care Med. 177, 593–603 (2008).
Le, A.V. et al. Inhibition of allergen-induced airway remodeling in Smad 3–deficient mice. J. Immunol. 178, 7310–7316 (2007).
McMillan, S.J., Xanthou, G. & Lloyd, C.M. Manipulation of allergen-induced airway remodeling by treatment with anti–TGF-β antibody: effect on the Smad signaling pathway. J. Immunol. 174, 5774–5780 (2005).
Tomlinson, K.L., Davies, G.C., Sutton, D.J. & Palframan, R.T. Neutralisation of interleukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite. PLoS One 5, e13136 (2010).
Blease, K. et al. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 166, 5219–5224 (2001).
Yang, G. et al. Anti–IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 28, 224–232 (2004).
Flood-Page, P. et al. Anti–IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Invest. 112, 1029–1036 (2003).
Humbles, A.A. et al. A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004).
Fattouh, R. et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite–induced airway disease. Am. J. Respir. Crit. Care Med. 283, 179–188 (2011).
Wei, C.Y., Chou, Y.H., Ho, F.M., Hsieh, S.L. & Lin, W.W. Signaling pathways of LIGHT induced macrophage migration and vascular smooth muscle cell proliferation. J. Cell. Physiol. 209, 735–743 (2006).
Hikichi, Y. et al. LIGHT, a member of the TNF superfamily, induces morphological changes and delays proliferation in the human rhabdomyosarcoma cell line RD. Biochem. Biophys. Res. Commun. 289, 670–677 (2001).
Boussaud, V., Soler, P., Moreau, J., Goodwin, R.G. & Hance, A.J. Expression of three members of the TNF-R family of receptors (4–1BB, lymphotoxin-β receptor and Fas) in human lung. Eur. Respir. J. 12, 926–931 (1998).
DiGiovanni, F.A. et al. Concurrent dual allergen exposure and its effects on airway hyperresponsiveness, inflammation and remodeling in mice. Dis. Model Mech. 2, 275–282 (2009).
Johnson, J.R. et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169, 378–385 (2004).
Jeffery, P.K. et al. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am. Rev. Respir. Dis. 145, 890–899 (1992).
Doherty, T.A., Soroosh, P., Broide, D.H. & Croft, M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L229–L235 (2009).
Yang, M. et al. Inhibition of arginase I activity by RNA interference attenuates IL-13–induced airways hyperresponsiveness. J. Immunol. 177, 5595–5603 (2006).
Kitani, A. et al. Treatment of experimental (trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-β1 plasmid: TGF-β1–mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor β2 chain downregulation. J. Exp. Med. 192, 41–52 (2000).
López, E. et al. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J. Immunol. 176, 1943–1950 (2006).
Cho, J.Y. et al. Immunostimulatory DNA inhibits transforming growth factor-β expression and airway remodeling. Am. J. Respir. Cell Mol. Biol. 30, 651–661 (2004).
Haeberle, H.A. et al. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J. Virol. 75, 878–890 (2001).
Summers-DeLuca, L.E. et al. Expression of lymphotoxin-αβ on antigen-specific T cells is required for DC function. J. Exp. Med. 204, 1071–1081 (2007).
Acknowledgements
We thank A. Meilan, Y. Adams, X. Tang and M. Macauley for technical assistance and the UCSD histology core for lung section processing and staining. Antibody to LTβ BBF6 was kindly provided by J. Browning, Biogen Idec. This work was supported by US National Institutes of Health (NIH) grant AI070535 to M.C., a fellowship from the American Academy of Asthma, Allergy and Immunology, UCSD Clinical and Translational Research Institute and NIH grant 1K08AI080938-01A1 award to T.A.D. and NIH grants R37AI068033 and AI067890 to C.F.W. This is manuscript number 1285 of the La Jolla Institute for Allergy and Immunology.
Author information
Authors and Affiliations
Contributions
T.A.D. and P.S. contributed to animal antigen administration, surgery, data collection, analysis and manuscript writing for all studies; S.F. and J.Y.C. contributed to immunostaining and data analysis; N.K. contributed to remodeling and cytokine data collection and analysis; P.R. contributed to airway hyper-responsiveness testing and analysis; P.S.N. produced plasmids, LTβR-F and antibody to LTβR and contributed to experimental design; H.C. contributed to cytokine data collection; S.S. and K.P. developed mutant mice; B.L.Z. contributed to experimental design; C.F.W. contributed to experimental design and reagent production; D.H.B. contributed to experimental design and remodeling data collection; M.C. contributed to experimental design, data analysis and manuscript writing for all studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 2642 kb)
Rights and permissions
About this article
Cite this article
Doherty, T., Soroosh, P., Khorram, N. et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med 17, 596–603 (2011). https://doi.org/10.1038/nm.2356
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2356
This article is cited by
-
Exploring immune related gene signatures and mechanisms linking non alcoholic fatty liver disease to atrial fibrillation through transcriptome data analysis
Scientific Reports (2023)
-
The new preparation method for paraffin-embedded samples applying scanning electron microscopy revealed characteristic features in asthma-induced mice
Scientific Reports (2022)
-
The LIGHT switch: mechanisms of fibroblast pathology in eosinophilic esophagitis
Mucosal Immunology (2022)
-
LIGHT deficiency attenuates acute kidney disease development in an in vivo experimental renal ischemia and reperfusion injury model
Cell Death Discovery (2022)
-
GITRL on dendritic cells aggravates house dust mite-induced airway inflammation and airway hyperresponsiveness by modulating CD4+ T cell differentiation
Respiratory Research (2021)