Abstract
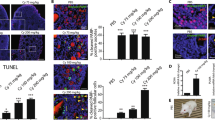
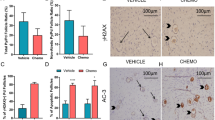
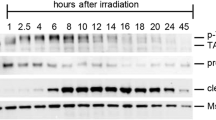
Germ cells are sensitive to genotoxins, and ovarian failure and infertility are major side effects of chemotherapy in young patients with cancer. Here we describe the c-Abl–TAp63 pathway activated by chemotherapeutic DNA-damaging drugs in model human cell lines and in mouse oocytes and its role in cell death. In cell lines, upon cisplatin treatment, c-Abl phosphorylates TAp63 on specific tyrosine residues. Such modifications affect p63 stability and induce a p63-dependent activation of proapoptotic promoters. Similarly, in oocytes, cisplatin rapidly promotes TAp63 accumulation and eventually cell death. Treatment with the c-Abl kinase inhibitor imatinib counteracts these cisplatin-induced effects. Taken together, these data support a model in which signals initiated by DNA double-strand breaks are detected by c-Abl, which, through its kinase activity, modulates the p63 transcriptional output. Moreover, they suggest a new use for imatinib, aimed at preserving oocytes of the follicle reserve during chemotherapeutic treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kastan, M.B. & Bartek, J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004).
Zhou, B.B. & Elledge, S.J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Harris, S.L. & Levine, A.J. The p53 pathway: positive and negative feedback loops. Oncogene 24, 2899–2908 (2005).
De Felici, M. et al. Experimental approaches to the study of primordial germ cell lineage and proliferation. Hum. Reprod. Update 10, 197–206 (2004).
Suh, E.K. et al. p63 protects the female germ line during meiotic arrest. Nature 444, 624–628 (2006).
Livera, G. et al. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 135, 3–12 (2008).
Yang, A., Kaghad, M., Caput, D. & McKeon, F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 18, 90–95 (2002).
Yang, A. et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998).
Katoh, M.A. et al. Female-specific dominant lethal effects in mice. Mutat. Res. 230, 205–217 (1990).
Blommaert, F.A. & Saris, C.P. Detection of platinum-DNA adducts by 32P-postlabelling. Nucleic Acids Res. 23, 1300–1306 (1995).
Gong, J.G. et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399, 806–809 (1999).
Liu, Z.G. et al. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature 384, 273–276 (1996).
Kharbanda, S. et al. c-Abl activation regulates induction of the SEK1/stress-activated protein kinase pathway in the cellular response to 1-β-D-arabinofuranosylcytosine. J. Biol. Chem. 270, 30278–30281 (1995).
Kharbanda, S. et al. Activation of MEK kinase 1 by the c-Abl protein tyrosine kinase in response to DNA damage. Mol. Cell. Biol. 20, 4979–4989 (2000).
Pandey, P. et al. Activation of p38 mitogen-activated protein kinase by c-Abl-dependent and -independent mechanisms. J. Biol. Chem. 271, 23775–23779 (1996).
Barilà, D. et al. Caspase-dependent cleavage of c-Abl contributes to apoptosis. Mol. Cell. Biol. 23, 2790–2799 (2003).
Machuy, N., Rajalingam, K. & Rudel, T. Requirement of caspase-mediated cleavage of c-Abl during stress-induced apoptosis. Cell Death Differ. 11, 290–300 (2004).
Agami, R., Blandino, G., Oren, M. & Shaul, Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399, 809–813 (1999).
Kharbanda, S., Yuan, Z.M., Weichselbaum, R. & Kufe, D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene 17, 3309–3318 (1998).
Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Melino, G. et al. Functional regulation of p73 and p63: development and cancer. Trends Biochem. Sci. 28, 663–670 (2003).
Tsai, K.K. & Yuan, Z.M. c-Abl stabilizes p73 by a phosphorylation-augmented interaction. Cancer Res. 63, 3418–3424 (2003).
Goldberg, Z. et al. Tyrosine phosphorylation of Mdm2 by c-Abl: implications for p53 regulation. EMBO J. 21, 3715–3727 (2002).
Mantovani, F. et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol. Cell 14, 625–636 (2004).
Strano, S. et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol. Cell 18, 447–459 (2005).
Levy, D., Adamovich, Y., Reuven, N. & Shaul, Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol. Cell 29, 350–361 (2008).
Yuan, Z.M. et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399, 814–817 (1999).
Sanchez-Prieto, R., Sanchez-Arevalo, V.J., Servitja, J.M. & Gutkind, J.S. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene 21, 974–979 (2002).
Rossi, M. et al. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 24, 836–848 (2005).
Melino, G., Knight, R.A. & Cesareni, G. Degradation of p63 by Itch. Cell Cycle 5, 1735–1739 (2006).
Yoshida, K. et al. JNK phosphorylation of 14–3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat. Cell Biol. 7, 278–285 (2005).
Raina, D. et al. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J. 25, 3774–3783 (2006).
Chen, Z. et al. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene 18, 173–180 (1999).
Jones, E.V., Dickman, M.J. & Whitmarsh, A.J. Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase. Biochem. J. 405, 617–623 (2007).
Barilà, D. et al. A nuclear tyrosine phosphorylation circuit: c-Jun as an activator and substrate of c-Abl and JNK. EMBO J. 19, 273–281 (2000).
Acknowledgements
We thank G. Mazzeo for critical suggestions and support. We thank G. Superti-Furga for critical reading of the manuscript. We thank R. Sacco for help in preparing some figures. We thank S. Bernardini, M. Angelini, A.M. Lena, A. Gamboi-Miraglia, M. Ranalli and G. Palmieri for technical advice. This work was supported by Associazione Italiana per la Ricerca sul Cancro to S.G. and G.C., and the EU integrated projects Interaction Proteome to G.C. and EPISTEM to G.M.
Author information
Authors and Affiliations
Contributions
S.G. designed, performed and analyzed the majority of the experiments and wrote the paper. L.D.T. helped with experiments in cells. S.C. did quantitative RT-PCR experiments. S.M.C. performed BrdU labeling experiments. F.G.K. did ovary culturing and TUNEL staining. C.D.B. collected adult ovaries. M.M. did mouse injections. E.C. provided support and the SaosTAp63 cell line. M.D.F. provided a key contribution for the project and edited the manuscript. G.M. provided a key contribution for the development of project and pointed out the in vivo strategy. G.C. supervised the project and wrote most of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.G. and G.C. submitted a patent for the use of c-Abl inhibitors to protect oocytes from the toxic effects induced by chemotherapy to the Italian Patent Office, with patent number RM2008A000684 on 19 December 2008.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5 and Supplementary Methods (PDF 457 kb)
Rights and permissions
About this article
Cite this article
Gonfloni, S., Di Tella, L., Caldarola, S. et al. Inhibition of the c-Abl–TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 15, 1179–1185 (2009). https://doi.org/10.1038/nm.2033
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2033
This article is cited by
-
The Dominant Mechanism of Cyclophosphamide-Induced Damage to Ovarian Reserve: Premature Activation or Apoptosis of Primordial Follicles?
Reproductive Sciences (2024)
-
Menstrual blood-derived endometrial stem cell, a unique and promising alternative in the stem cell-based therapy for chemotherapy-induced premature ovarian insufficiency
Stem Cell Research & Therapy (2023)
-
Heterozygous TP63 pathogenic variants in isolated primary ovarian insufficiency
Journal of Assisted Reproduction and Genetics (2023)
-
Summary of the ISFP congress, Brussels, 10–12 November, 2022
Journal of Assisted Reproduction and Genetics (2023)
-
Thyroid hormone triiodothyronine does not protect ovarian reserve from DNA damage induced by X-ray and cisplatin
Journal of Assisted Reproduction and Genetics (2023)