Abstract
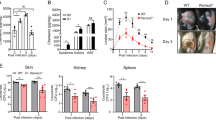
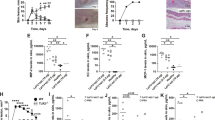
Staphylococcus aureus causes most infections of human skin and soft tissue and is a major infectious cause of mortality. Host defense mechanisms against S. aureus are incompletely understood. Interleukin 19 (IL-19), IL-20 and IL-24 signal through type I and type II IL-20 receptors and are associated with inflammatory skin diseases such as psoriasis and atopic dermatitis. We found here that those cytokines promoted cutaneous infection with S. aureus in mice by downregulating IL-1β- and IL-17A-dependent pathways. We noted similar effects of those cytokines in human keratinocytes after exposure to S. aureus, and antibody blockade of the IL-20 receptor improved outcomes in infected mice. Our findings identify an immunosuppressive role for IL-19, IL-20 and IL-24 during infection that could be therapeutically targeted to alter susceptibility to infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Miller, L.S. & Cho, J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11, 505–518 (2011).
Waters, A.E. et al. Multidrug-resistant Staphylococcus aureus in US meat and poultry. Clin. Infect. Dis. 52, 1227–1230 (2011).
Rizek, C.F. et al. Identification of Staphylococcus aureus carrying the mecA gene in ready-to-eat food products sold in Brazil. Foodborne Pathog. Dis. 8, 561–563 (2011).
Mazmanian, S.K., Round, J.L. & Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Tong, S.Y., Chen, L.F. & Fowler, V.G. Jr. Colonization, pathogenicity, host susceptibility, and therapeutics for Staphylococcus aureus: what is the clinical relevance? Semin. Immunopathol. 34, 185–200 (2012).
Myles, I.A. & Datta, S.K. Staphylococcus aureus: an introduction. Semin. Immunopathol. 34, 181–184 (2012).
Krishna, S. & Miller, L.S. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin. Immunopathol. 34, 261–280 (2012).
Cassat, J.E. & Skaar, E.P. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin. Immunopathol. 34, 215–235 (2012).
Gaidamakova, E.K. et al. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio- protective Mn2+-peptide complex from Deinococcus. Cell Host Microbe 12, 117–124 (2012).
McLoughlin, R.M. et al. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl. Acad. Sci. USA 103, 10408–10413 (2006).
Kim, H.K., Kim, H.Y., Schneewind, O. & Missiakas, D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 10, 3605–3612 (2011).
Rogers, D.E. & Melly, M.A. Speculations on the immunology of staphylococcal infections. Ann. NY Acad. Sci. 128, 274–284 (1965).
Schmaler, M., Jann, N.J., Ferracin, F. & Landmann, R. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J. Immunol. 186, 443–452 (2011).
Cho, J.S. et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010).
Miller, L.S. et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24, 79–91 (2006).
Rigby, K.M. & DeLeo, F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 34, 237–259 (2012).
Kunz, S. et al. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp. Dermatol. 15, 991–1004 (2006).
Commins, S., Steinke, J.W. & Borish, L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 121, 1108–1111 (2008).
Ouyang, W., Rutz, S., Crellin, N., Valdez, P. & Hymowitz, S. Regulation and function of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29, 71–109 (2011).
Logsdon, N.J., Deshpande, A., Harris, B.D., Rajashankar, K.R. & Walter, M.R. Structural basis for receptor sharing and activation by interleukin-20 receptor-2 (IL-20R2) binding cytokines. Proc. Natl. Acad. Sci. USA 109, 12704–12709 (2012).
Sa, S.M. et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J. Immunol. 178, 2229–2240 (2007).
Rømer, J. et al. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J. Invest. Dermatol. 121, 1306–1311 (2003).
He, M. & Liang, P. IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. J. Immunol. 184, 1793–1798 (2010).
Blumberg, H. et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 104, 9–19 (2001).
Nograles, K.E. & Krueger, J.G. Anti-cytokine therapies for psoriasis. Exp. Cell Res. 317, 1293–1300 (2011).
Tokura, Y., Mori, T. & Hino, R. Psoriasis and other Th17-mediated skin diseases. J. UOEH 32, 317–328 (2010).
Holland, D.B., Bojar, R.A., Farrar, M.D. & Holland, K.T. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 290, 149–155 (2009).
Ma, Y. et al. Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection. Microbes Infect. 13, 1099–1110 (2011).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Aujla, S.J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281 (2008).
Kao, C.Y. et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J. Immunol. 173, 3482–3491 (2004).
Cai, Y. et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Nagalakshmi, M.L., Murphy, E., McClanahan, T. & de Waal Malefyt, R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int. Immunopharmacol. 4, 577–592 (2004).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Sutton, C.E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Liu, F.L. et al. Interleukin (IL)-23 p19 expression induced by IL-1β in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-κB and AP-1 dependent pathway. Rheumatology (Oxford) 46, 1266–1273 (2007).
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Tsukada, J., Yoshida, Y., Kominato, Y. & Auron, P.E. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54, 6–19 (2011).
Tsukada, J., Saito, K., Waterman, W.R., Webb, A.C. & Auron, P.E. Transcription factors NF-IL6 and CREB recognize a common essential site in the human prointerleukin 1β gene. Mol. Cell Biol. 14, 7285–7297 (1994).
Otkjaer, K. et al. IL-20 gene expression is induced by IL-1β through mitogen-activated protein kinase and NF-κB-dependent mechanisms. J. Invest. Dermatol. 127, 1326–1336 (2007).
Tohyama, M. et al. IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur. J. Immunol. 39, 2779–2788 (2009).
Azuma, Y.T., Nakajima, H. & Takeuchi, T. IL-19 as a potential therapeutic in autoimmune and inflammatory diseases. Curr. Pharm. Des. 17, 3776–3780 (2011).
Azuma, Y.T. et al. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm. Bowel Dis. 16, 1017–1028 (2010).
Alanärä, T., Karstila, K., Moilanen, T., Silvennoinen, O. & Isomaki, P. Expression of IL-10 family cytokines in rheumatoid arthritis: elevated levels of IL-19 in the joints. Scand. J. Rheumatol. 39, 118–126 (2010).
Sakurai, N. et al. Expression of IL-19 and its receptors in RA: potential role for synovial hyperplasia formation. Rheumatology (Oxford) 47, 815–820 (2008).
Kragstrup, T.W. et al. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 41, 16–23 (2008).
Chan, J.R. et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 203, 2577–2587 (2006).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012).
Valdez, P.A. et al. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity 36, 668–679 (2012).
Straccia, M. et al. Pro-inflammatory gene expression and neurotoxic effects of activated microglia are attenuated by absence of CCAAT/enhancer binding protein β. J. Neuroinflammation 8, 156 (2011).
Acknowledgements
We thank Y. Iwakura (University of Tokyo) for Il17a−/− mice; J. O'Shea (National Institute of Arthritis, Musculoskeletal and Skin Diseases) for Stat3WT/ΔV463 mice; F. DeLeo (Rocky Mountain Labs, National Institute of Allergy and Infectious Diseases) for the clinical isolate of MRSA USA300 (LAC strain); A. Costanzo and J. Jameson (The Scripps Research Institute) for PAM 2-12 cells; A. Coxon (National Cancer Institute) for foreskin samples; and F. DeLeo and J. O'Shea for critical reading of the manuscript. Supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (US National Institutes of Health).
Author information
Authors and Affiliations
Contributions
I.A.M. designed, did and analyzed the experiments, and wrote the manuscript; N.M.F., P.A.V. and P.J.V. assisted with experiments; S.N. and Y.B. assisted with acquisition and analysis of skin cell flow cytometry; W.O. provided Il20rb−/− and Il22−/− mice; S.K.D. oversaw design and analysis of the experiments and wrote the manuscript; and all authors critically read the manuscript.
Corresponding author
Ethics declarations
Competing interests
W.O. is an employee of Genentech.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 946 kb)
Rights and permissions
About this article
Cite this article
Myles, I., Fontecilla, N., Valdez, P. et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol 14, 804–811 (2013). https://doi.org/10.1038/ni.2637
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2637
This article is cited by
-
Inhibition of IL-17 ameliorates keratinocyte-borne cytokine responses in an in vitro model for house-dust-mite triggered atopic dermatitis
Scientific Reports (2023)
-
Evaluation of cutaneous immune response in a controlled human in vivo model of mosquito bites
Nature Communications (2022)
-
Implication of T Helper Cytokines in Contact Dermatitis and Atopic Dermatitis
Current Treatment Options in Allergy (2020)
-
Murine astrocytes produce IL-24 and are susceptible to the immunosuppressive effects of this cytokine
Journal of Neuroinflammation (2019)
-
Ubc9 overexpression and SUMO1 deficiency blunt inflammation after intestinal ischemia/reperfusion
Laboratory Investigation (2018)