Abstract
Efforts to develop gene therapies for hearing loss have been hampered by the lack of safe, efficient, and clinically relevant delivery modalities1,2. Here we demonstrate the safety and efficiency of Anc80L65, a rationally designed synthetic vector3, for transgene delivery to the mouse cochlea. Ex vivo transduction of mouse organotypic explants identified Anc80L65 from a set of other adeno-associated virus (AAV) vectors as a potent vector for the cochlear cell targets. Round window membrane injection resulted in highly efficient transduction of inner and outer hair cells in mice, a substantial improvement over conventional AAV vectors. Anc80L65 round window injection was well tolerated, as indicated by sensory cell function, hearing and vestibular function, and immunologic parameters. The ability of Anc80L65 to target outer hair cells at high rates, a requirement for restoration of complex auditory function, may enable future gene therapies for hearing and balance disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Fukui, H. & Raphael, Y. Gene therapy for the inner ear. Hear. Res. 297, 99–105 (2013).
Géléoc, G.S. & Holt, J.R. Sound strategies for hearing restoration. Science 344, 1241062 (2014).
Zinn, E. et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 12, 1056–1068 (2015).
Parker, M. & Bitner-Glindzicz, M. Genetic investigations in childhood deafness. Arch. Dis. Child. 100, 271–278 (2015).
Stamatiou, G.A. & Stankovic, K.M. A comprehensive network and pathway analysis of human deafness genes. Otol. Neurotol. 34, 961–970 (2013).
Hoffmann, T.J. et al. A large genome-wide association study of age-related hearing impairment using electronic health records. PLoS Genet. 12, e1006371 (2016).
Rubel, E.W., Furrer, S.A. & Stone, J.S. A brief history of hair cell regeneration research and speculations on the future. Hear. Res. 297, 42–51 (2013).
Groves, A.K. The challenge of hair cell regeneration. Exp. Biol. Med. (Maywood) 235, 434–446 (2010).
Akil, O. et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75, 283–293 (2012).
Askew, C. et al. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 7, 295ra108 (2015).
Chien, W.W. et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol. Ther. 24, 17–25 (2016).
Izumikawa, M. et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 11, 271–276 (2005).
Chien, W.W., Monzack, E.L., McDougald, D.S. & Cunningham, L.L. Gene therapy for sensorineural hearing loss. Ear Hear. 36, 1–7 (2015).
LeMasurier, M. & Gillespie, P.G. Hair-cell mechanotransduction and cochlear amplification. Neuron 48, 403–415 (2005).
Spicer, S.S., Thomopoulos, G.N. & Schulte, B.A. Structural evidence for ion transport and tectorial membrane maintenance in the gerbil limbus. Hear. Res. 143, 147–161 (2000).
Gavara, N., Manoussaki, D. & Chadwick, R.S. Auditory mechanics of the tectorial membrane and the cochlear spiral. Curr. Opin. Otolaryngol. Head Neck Surg. 19, 382–387 (2011).
Guinan, J.J. Jr., Salt, A. & Cheatham, M.A. Progress in cochlear physiology after Békésy. Hear. Res. 293, 12–20 (2012).
White, P.M., Doetzlhofer, A., Lee, Y.S., Groves, A.K. & Segil, N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984–987 (2006).
Bramhall, N.F., Shi, F., Arnold, K., Hochedlinger, K. & Edge, A.S. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports 2, 311–322 (2014).
May, L.A. et al. Inner ear supporting cells protect hair cells by secreting HSP70. J. Clin. Invest. 123, 3577–3587 (2013).
Mellado Lagarde, M.M. et al. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc. Natl. Acad. Sci. USA 111, 16919–16924 (2014).
Chien, W.W., McDougald, D.S., Roy, S., Fitzgerald, T.S. & Cunningham, L.L. Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope 125, 2557–2564 (2015).
Nguyen, S. et al. Outcomes review of modern hearing preservation technique in cochlear implant. Auris Nasus Larynx 43, 485–488 (2016).
Zou, B. et al. The application of genome editing in studying hearing loss. Hear. Res. 327, 102–108 (2015).
Zuris, J.A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2015).
Luebke, A.E., Rova, C., Von Doersten, P.G. & Poulsen, D.J. Adenoviral and AAV-mediated gene transfer to the inner ear: role of serotype, promoter, and viral load on in vivo and in vitro infection efficiencies. Adv. Otorhinolaryngol. 66, 87–98 (2009).
Nathwani, A.C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011).
Maguire, A.M. et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
MacLaren, R.E. et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 383, 1129–1137 (2014).
Bryant, L.M. et al. Lessons learned from the clinical development and market authorization of Glybera. Hum. Gene Ther. Clin. Dev. 24, 55–64 (2013).
Liu, Y. et al. Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol. Ther. 12, 725–733 (2005).
Kilpatrick, L.A. et al. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther. 18, 569–578 (2011).
Kesser, B.W., Hashisaki, G.T., Fletcher, K., Eppard, H. & Holt, J.R. An in vitro model system to study gene therapy in the human inner ear. Gene Ther. 14, 1121–1131 (2007).
Dilwali, S., Landegger, L.D., Soares, V.Y., Deschler, D.G. & Stankovic, K.M. Secreted factors from human vestibular Schwannomas can cause cochlear damage. Sci. Rep. 5, 18599 (2015).
Liu, L. & Duff, K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J. Vis. Exp. http://dx.doi.org/10.3791/960 (2008).
Sergeyenko, Y., Lall, K., Liberman, M.C. & Kujawa, S.G. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J. Neurosci. 33, 13686–13694 (2013).
Horwitz, G.C., Risner-Janiczek, J.R., Jones, S.M. & Holt, J.R. HCN channels expressed in the inner ear are necessary for normal balance function. J. Neurosci. 31, 16814–16825 (2011).
Iancu, R., Mohapel, P., Brundin, P. & Paul, G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav. Brain Res. 162, 1–10 (2005).
Paes-Branco, D., Abreu-Villaça, Y., Manhães, A.C. & Filgueiras, C.C. Unilateral hemispherectomy at adulthood asymmetrically affects motor performance of male Swiss mice. Exp. Brain Res. 218, 465–476 (2012).
Acknowledgements
This work was supported by the Bertarelli Foundation grants (K.M.S., J.R.H.), the Jeff and Kimberly Barber Gene Therapy Research Fund (J.R.H.), the Patel Gene Therapy Fund (J.R.H.), Department of Defense Grant W81XWH-15-1-0472 (K.M.S.), the National Institutes of Health (NIH) 1R01DC015824 (K.M.S.), Nancy Sayles Day Foundation (K.M.S.), Lauer Tinnitus Research Center (K.M.S.), Giving/Grousbeck (L.H.V.), Foundation Fighting Blindness (L.H.V.), Ush2A Consortium (L.H.V.), and NIH 5DP1EY023177 (L.H.V.). The authors would like to thank H.-C. Lin and S. Narasimhan for help with the brain tissue staining protocol, G. Geleoc and C. Nist-Lund for assistance with vestibular tissue imaging, and R. Xiao, R. Shelke, Y. Lin, and T. Barungi for vector preparation.
Author information
Authors and Affiliations
Contributions
L.D.L., B.P., C.A., S.J.W., S.D.G., and A.G. conducted, analyzed, and reported the mouse gene transfer experiments described, and R.T. and A.F. performed and led the human vestibular transduction experiments. L.H.V. provided vector materials. K.M.S., J.R.H., and L.H.V. conceived the study, led experimental design and oversaw analysis. J.R.H., L.H.V., and L.D.L. drafted the manuscript with topical input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
L.H.V. holds founder equity in GenSight Biologics, is a consultant to a number of biotech and pharmaceutical companies, and is an inventor on gene therapy patents, including Anc80L65, which are licensed to various entities. L.H.V. also receives sponsored research from Lonza Houston and Selecta Biosciences, licensees of Anc80L65. L.H.V., K.M.S., and J.R.H. have filed a patent on the use of Anc80L65 in the cochlea.
Integrated supplementary information
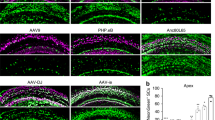
Supplementary Figure 1 Representative images of an in vitro comparison of several AAV serotypes regarding eGFP expression in cochlear explants of CBA/CaJ mice.
(A-F): Results after incubation at equal doses of 1010 genome containing (GC) particles for 48 hours. Scale bar = 200 μm.
(G-J): Mean indicated by horizontal bar. Each condition had minimally N=3 for 48h, and N=2 for 48h+5d unless otherwise noted.
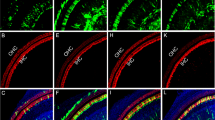
Supplementary Figure 2 eGFP expression scoring system.
eGFP expression in supporting and limbus cells following AAV exposure of cochlear explants was assessed using a qualitative scoring system ranging from 0 (D, H) (lowest expression) to 3 (A, E) (highest level of expression). Representative images illustrate the range of expression both in terms of intensity and number of transduced cells with “0” representing no noted expression (D, H), “1” select number of cells expressing dimly (C, G), “2” low to moderate levels of expression in significant numbers of cells per microscopic field (B, F), and “3” high percentage of cells expressing eGFP at levels ranging from moderate to high (A, E). Scale bar for all images as in A (for A-D) and E (for E-H) = 20 μm.
Supplementary Figure 3 eGFP expression in limbus, supporting cells, and spiral ganglion neurons (SGNs) in cochlear explants of C57BL/6 mice.
Expression in limbus and supporting cells was assessed using an eGFP scoring system detailed in Supplementary Fig. 2. SGN transduction was evaluated by eGFP-positive cell counts per microscopic field. Mean indicated by horizontal bar. Each condition had minimally N=3 for the 48h, and N=2 for the 48h+5d unless otherwise noted.
Supplementary Figure 4 eGFP expression in limbus, supporting cells, and spiral ganglion neurons (SGNs) in cochlear explants of CBA/CaJ mice.
Expression in limbus and supporting cells was assessed using an eGFP scoring system detailed in Supplementary Fig. 2. SGN transduction was evaluated by eGFP-positive cell counts per microscopic field. Mean indicated by horizontal bar. Each condition had minimally N=3 for the 48h, and N=2 for the 48h+5d unless otherwise noted.
Supplementary Figure 5 Bilateral cochlear transduction from base to apex in mouse cochleas with Anc80.
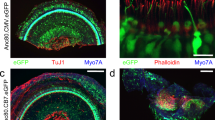
Mice injected at P1 were sacrificed and cochleas were evaluated for eGFP transgene expression 30 days following injection on histological section stained for TuJ1 (red) and Myo7A (blue). Efficient Anc80 transduction was observed in the injected cochlea extending up to apex (A/F), however also in the contralateral uninjected ear (B-E=from apex to base). (G) Close-up image of eGFP-positive and TuJ1-positive spiral ganglion neurons (SGNs). (H) Reconstructed 3D image for SGN evaluation of Anc80 transduction. Scale bars 100 μm (A-E) and 20 μm (F/G).
Supplementary Figure 6 Expression in the central nervous system and Anti-AAV Neutralizing Antibody (NAB) titers.
(A) Axial section of a mouse brain after unilateral cochlear injection with Anc80. (B) Predominant expression in the cerebellum, in particular Purkinje cells (white arrowheads). Scale bars 1 mm (A) and 300 μm (B). (C) Anti-AAV Neutralizing Antibody (NAB) titers in serum and Cerebrospinal Fluid (CSF) in uninjected (n=4 for CSF, n=2 for serum) and Anc80 RWM-injected animals (n=6 for CSF, n=2 for serum). Titers reflect the dilution of serum or CSF at which 50% inhibition of transduction was observed in the NAB assay. Due to sample volume limitations, the limit of sensitivity for serum NAB was 1/4 and 1/52.5 for CSF.
Supplementary Figure 7 Vestibular function following Anc80 cochlear transduction.
Mice were injected at P1 with Anc80.CMV.eGFP via the RWM and evaluated for expression and balance function on the rotarod device. (A) Expression of eGFP (green) in the vestibular tissue is detected via confocal microscopy with immunofluorescent staining for Myo7A (red). (B) Rotarod data revealed no difference between injected and uninjected controls. The mean time until the mice fell off the device +/- s.d. is plotted. N=3 animals, 5 trials each (injected) and 2 animals 5 trials each (control). Scale bar = 50 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1248 kb)
Rights and permissions
About this article
Cite this article
Landegger, L., Pan, B., Askew, C. et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol 35, 280–284 (2017). https://doi.org/10.1038/nbt.3781
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3781
This article is cited by
-
Distributional comparison of different AAV vectors after unilateral cochlear administration
Gene Therapy (2024)
-
Adeno-associated virus as a delivery vector for gene therapy of human diseases
Signal Transduction and Targeted Therapy (2024)
-
Mutational spectrum in patients with dominant non-syndromic hearing loss in Austria
European Archives of Oto-Rhino-Laryngology (2024)
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
SIRT3/GLUT4 signaling activation by metformin protect against cisplatin-induced ototoxicity in vitro
Archives of Toxicology (2023)