Abstract
Studies over the last couple of decades have provided exciting new insights into mechanisms underlying the pathogenesis of preeclampsia. In addition, several novel and innovative molecules and ideas for management of the syndrome have also come forth. While our basic understanding of the initiating events of preeclampsia continues to be placental ischemia/hypoxia stimulating the release of a variety of factors from the placenta that act on the cardiovascular and renal systems, the number of candidate pathways for intervention continues to increase. Recent studies have identified apelin and its receptor, APJ, as an important contributor to the regulation of cardiovascular and fluid balance that is found to be disrupted in preeclampsia. Likewise, continued studies have revealed a critical role for the complement arm of the innate immune system in placental ischemia induced hypertension and in preeclampsia. Finally, the recent increase in animal models for studying hypertensive disorders of pregnancy has provided opportunities to evaluate the potential role for physical activity and exercise in a more mechanistic fashion. While the exact quantitative importance of the various endothelial and humoral factors that mediate vasoconstriction and elevation of arterial pressure during preeclampsia remains unclear, significant progress has been made. Thus, the goal of this review is to discuss recent efforts towards identifying therapies for hypertension during pregnancy that derive from work exploring the apelinergic system, the complement system as well as the role that exercise and physical activity may play to that end.
Similar content being viewed by others
Introduction
Preeclampsia and related hypertensive disorders of pregnancy are estimated to affect up to 10% of all pregnancies in the United States.1, 2 This may be due to increases in the number of higher-order pregnancies, the age at onset of pregnancy and rate of obesity.3 Hypertensive disorders of pregnancy are classified into one of several categories: preeclampsia–eclampsia, chronic hypertension of any cause that pre-dates pregnancy or is evident in early pregnancy before 20 weeks gestation, chronic hypertension with superimposed preeclampsia and gestational hypertension.
Until recently proteinuria was requisite for the diagnosis of preeclampsia, although due to the heterogeneous and systemic nature of disease, the American College of Obstetrics and Gynecology (ACOG) recently broadened the diagnosis of preeclampsia.4 As such, the diagnosis of preeclampsia and gestational hypertension remains dependent on new onset blood pressure ⩾140 mm Hg systolic or ⩾90 mm Hg diastolic after 20 weeks of gestation with or without proteinuria (⩾300 mg protein in a 24 h urine collection or urine spot protein/creatinine ratio ⩾0.3). In the absence of proteinuria, preeclampsia is now established by new onset hypertension with signs or symptoms of systemic disease including thrombocytopenia, impaired liver function, renal insufficiency or cerebral or visual disturbances. Gestational hypertension is distinguished from preeclampsia by absence of proteinuria or other symptoms. While hypertensive disorders of pregnancy are often characterized by fetal growth restriction, they are also a leading cause of medically indicated preterm birth. In addition, preeclampsia may also be distinguished as early onset vs late onset preeclampsia5, 6 based on evidence that the two entities have distinct pathophysiologies.7, 8 This distinction is often identified as early-onset preeclampsia prompting delivery <34 weeks or late onset with delivery ⩾34 weeks gestation.
Although the mechanisms of the pathophysiology of preeclampsia remains unclear, placental ischemia/hypoxia is widely regarded as a key factor.9, 10 Perhaps the foremost hypothesis regarding the initiating event in PE is that reduced placental perfusion leads to widespread dysfunction of the maternal vascular endothelium. Inadequate trophoblast invasion leading to incomplete remodeling of the uterine spiral arteries is considered to be a primary cause of the placental ischemia.10 Thus, the poorly perfused and hypoxic placenta is thought to synthesize and release increased amounts of factors such as soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng).11, 12, 13, 14 Other molecules that play a role in the hypertension associated with preeclampsia have been identified from both clinical and experimental studies and these include the complement factors C3 and C5, Angiotensin receptor type 1 auto antibodies (AT1-AA), apelin, adipocytokines, interluekin-6 and anandamide.11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
Currently, the primary clinical course of action for patients is the management of symptoms until delivery is indicated. Since delivery is frequently indicated pre-term, preeclampsia is often associated with a variety of sequelae known to be associated with early and small for gestational age births such as endothelial dysfunction, hypertension, and type 2 diabetes mellitus in the mother and/or the child.23, 24, 25, 26 Evidence has also accumulated indicating women that endure preeclampsia during pregnancy are also at a high risk for hypertension and other metabolic abnormalities in later life.24, 27, 28 Thus, development of treatments to prevent or attenuate symptoms of preeclampsia and other hypertensive disorders of pregnancy are likely to have clear benefits to public health. With that in mind the present review seeks to explore recent work in several areas to bring updates to the standard of care for women with preeclampsia and other related hypertensive disorders of pregnancy.
Apelinergic system
While the apelin molecule and its G-protein-coupled APJ receptor were recognized as a ligand-receptor pair in the late 1990’s,29, 30 they have quickly become recognized as important factors with regard to cardiovascular31 and metabolic diseases32 and more recently cancer33 and pregnancy.34 The apelinergic system is present in variety of organs such as heart, kidney and placenta all of which contribute in various roles to cardiovascular disorders of pregnancy.35 Apelin is observed to have a short half-life and its direct effects on the cardiovascular system seem to be transient.36 An interesting feature of the apelin system is the many similarities with other known cardiovascular effector systems such as angiotensin and endothelin. Angiotensin converting enzyme type 2 is one such molecule that acts as a negative regulator that inactivates some angiotensin molecules also is an enzyme that works to inactivate some apelin molecules as well.37 While APJ signaling via apelin stimulates NO production and ascular smooth muscle relaxation in most endothelium-intact vessels, similar to endothelin it has constrictor effects when applied to endothelium-denuded vessels. Apelin has also been observed to inhibit angiotensin II-induced elevated cytosolic calcium and vasoconstriction which in turn contributes to an overall vasodilatory action.38 Although the direct effects of apelinergic stimulation favor vasodilation in a transient sense, the indirect effects of APJ agonism via secondary pathways remains unclear.
Only recently have investigators turned their attention toward the role of apelin in hypertension during pregnancy and thus far the studies regarding APJ and apelin during preeclampsia have not been entirely clear. There have been reports suggesting increased protein expression of apelin in the late gestation placenta from women with preeclampsia via immunohistochemistry.39 There have also been reports of decreased mRNA and protein expression by way of RT-PCR and western blot/immunohistochemistry in the term placenta of patients presenting with preeclampsia when compared to uncomplicated control preganncies.40 In addition a recent report indicated placental apelin was decreased via radioimmunoassay.41 In contrast, reports on circulating apelin in preeclampsia primarily report an increase40, 42, 43 while one group observed a decrease.44
While there are precious few studies to date that have investigated the role of apelin in preeclampsia there is a dearth of studies in animal models or preeclampsia. Preliminary studies from my laboratory using the reduced uterine perfusion pressure (RUPP) model of preeclampsia suggest APJ receptor expression is increased in the placenta via western blot (unpublished observations). In light of its vasodilatory and other potentially beneficial properties for treating hypertensive disorders of pregnancy, it is clear more investigations of the apelinergic system are needed for the therapeutic potential of this system to be fully recognized.
Endocannabinoids
With recent changes in drug laws regarding cannabis in the United States there has been increased interest in studying the role of cannabinoids and endocannabinoids during pregnancy. Cannabinoid (CB1) receptor expression has been observed in numerous tissues including the myocardium, kidney, placenta and nerve fibers innervating resistance vessels.45, 46 In addition, recent studies have shown plasma levels of the endocannibinoid anandamide are altered in clinical hypertension as well as in animals models of hypertension.21, 47 Anandamide is thought to have a variety of effects on reproductive tissues ranging from fertilization to placentation and modulation of blood flow but exact mechanisms remains unclear in some cases.46, 47 Hence, clear evidence is accumulating that endogenous or exogenous cannabinoids may play a role in hypertension during pregnancy.
A recent study by Alban et al., reported increased placental expression of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD), an enzyme involved in the generation of anandamide although no change was observed in the expression of the CB1. The authors also examine NOS activity in response to anandamide in placental explants and found increased NOS activity in the explants from preeclamptic patients compared to those from normal pregnancies. In another recent clinical study, Molvarec et al., evaluated serum concentrations of sFlt-1, placental growth factor and anandamide. The authors also found that patients with preeclampsia had reduced concentrations of anandamide and consistent with previous studies reported increased sFlt-1 and decreased placental growth factor (PlGF) concentrations when compared with healthy pregnant controls. Additional analysis by the authors revealed there was no relationship observed between sFlt-1 or PlGF and anandamide concentrations in either the healthy pregnant or preeclampsia patients studied. Another recent study utilizing an transgenic mouse model of preeclampsia, in which a female rat carrying the human angiotensinogen gene is mated with a male rat carrying the human renin gene (TgA). This crossing results in a pregnant dam that develops many features of preeclampsia seen in human patients.48 The authors found that anandamide reduced angiotensin II mediated contraction of uterine arteries in a CB1 receptor independent manner. Taken together these studies clearly indicate there may be important relationships between anandamide and hypertension in preeclampsia that might be exploited to reduce blood pressure although it is clear that much more work is needed in this area.
Immune system
While the immune system is increasingly recognized as important in the pathophysiology of preeclampsia, the delicate balance of positive and negative regulators of the immune system requisite for successful pregnancy makes it an especially challenging system for intervention. Both the adaptive and innate immune systems are modified in pregnancy compared to the non-pregnant state and many of these changes are exacerbated in preeclampsia.49, 50 Changes to the adaptive immune system include increased CD4+ T cells, increased Th17 cells and decreased Treg cells.51 During preeclampsia neutrophil activation is elevated52, 53 and infiltration in the systemic vasculature is increased compared to normal pregnancy. Preeclamptic women also exhibit a heightened inflammatory state and greater number of neutrophils (PMNs) in the vasculature compared to normal pregnancy. Recent work by Regal and colleagues demonstrated PMNs are critical to placental ischemia-induced hypertension using the reduced uterine perfusion pressure (RUPP) model in the rat.54 PMNs were depleted with repeated injections of polyclonal rabbit anti-rat neutrophil antibody (antiPMN) on gestation days (GD) 13, 14,16 and 18. PMN depletion with this regimen decreased blood pressure in the RUPP group but did not have any effect on circulating vascular endothelial growth factor (VEGF) levels, RUPP-induced fetal demise nor the generation of complement activation product C3a.
Advances in measurement of complement activation products in clinical studies have clearly demonstrated that complement activation is increased in pregnancies complicated with preeclampsia compared to uncomplicated pregnancies. Previous studies have shown increased C3a/C3 ratio and terminal lytic pathway activation (sC5b-9) in preeclampsia compared to uncomplicated pregnancies.55 They also reported increased sC5b-9 was associated with fetal growth restriction. These measures all support the conclusion that excessive complement activation had occurred in the preeclamptic pregnancy leading to a depletion of C3 in the plasma—C3 synthesis was outpaced by C3 activation. Soto et al.56 compared complement activation products C3a, C4a and C5a in preeclampsia compared to small for gestational age fetuses and found increased C5a was associated with preeclampsia but not small for gestational age fetuses. Both studies reported complement activation products in the last half of pregnancy and during the manifestation of symptoms.
In an effort to identify whether alterations in complement activation early in gestation were predictive and/or potentially causal in preeclampsia, Lynch et al.57, measured complement activation products and followed patients through pregnancy. They reported increased Factor Bb suggesting excessive alternative pathway activation early in pregnancy, and this increase was associated with preeclampsia development later in pregnancy, while no predictive value was observed for C3a or sC5b-9. Additional studies evaluated hypertensive disease of pregnancy, preterm birth (<37 week), premature rupture of the membranes, intrauterine fetal loss, and fetal growth restriction. This analysis revealed women in the highest quartile of C3a were three times more likely to have an adverse pregnancy outcome such as hypertension, preterm birth and premature rupture of membranes.58
Increased complement activation products C5a and sC5b-9 have also been observed in plasma and urine indicating activation of the terminal complement components in patients with severe preeclampsia.59 Urinary excretion of sC5b-9 was increased markedly in severe preeclampsia but minimal or absent in healthy controls or pregnancies with chronic hypertension. Additionally, urinary detection of sC5b-9 correlated positively with increased soluble VEGFR-1 and decreased placental growth factor (PlGF) and VEGF.60 An exciting report from a recent case study indicated pregnancy could be safely extended in a woman with severe preeclampsia following treatment with the anti-C5 antibody eculizumab61 further suggesting complement is a viable therapeutic target.
Animal studies of hypertensive disorders of pregnancy are important to critically test hypotheses regarding pathophysiology and putative treatments that are not possible in humans, and to inform critical studies in humans. Many studies have utilized murine models that share hemichorial placentation with humans. Humans and rats have deeper trophoblast invasion in placental development, than mice where placentation is more superficial.62
Other models of preeclampsia such as the reduced uterine perfusion pressure (RUPP) model which mechanically reduces blood flow to the uteroplacental unit ~40%, focus on events in the later part of pregnancy to investigate the consequences of placental ischemia once it has developed. Alternatively, other models use pharmacologic or viral means to generate a hypertensive state in latter pregnancy. In these models, preeclampsia symptoms are initiated either by surgical intervention to cause placental ischemia or by infusion of mediators to mimic the clinical characteristics observed in women with preeclampsia.63, 64 Placental ischemia in rat and mouse results in elaboration of many factors including but not limited to AT1-AA, TNF-alpha, endothelin, increased sFlt-1, decreased VEGF and increased reactive oxygen species. Studies by Lillegard et al.19, 20 using the RUPP model of placental ischemia in the rat were the first to mechanistically link complement activation, particularly C3a and C5a, with the hypertension caused by placental ischemia. Inhibiting activation of complement using a soluble version of endogenous CR1 (sCR1) significantly attenuated hypertension. Additional studies using the C5a receptor antagonist PMX 53 and the C3a receptor antagonist SB290157 illustrated C3a and C5a are the important products of complement activation responsible for the RUPP hypertension. While only the C5a antagonist PMX 53 prevented endothelial dysfunction in mesenteric arterioles that is often observed following placental ischemia suggesting it appears that both C3a and C5a contribute to increased blood pressure in this model. Interpretation of these studies regarding C3a involvement is dependent on the specificity of the C3a antagonist SB290157 at the dosage used (5 mg kg−1) and acknowledging that SB290157 has been criticized for its partial agonist activity and effects on neutrophils. Hence, the exact involvement of C3a in RUPP hypertension should be taken cautiously.65 What continues to remain unclear at this point is whether complement activation and increased C3a/C5a production is linked to other recognized mediators of high blood pressure in placental ischemia induced hypertension such as endothelin, sFlt-1 or TNF-α, or whether complement activation acts in concert with one or more other factors in hypertension.
In concert, the clinical data support the notion that excessive complement activation is associated with adverse outcomes of pregnancy such as preeclampsia. The observations that increased complement activation may presage the presentation of symptoms suggest both a causal role in addition to a role in perpetuating the syndrome as gestation progresses.
Exercise and physical activity
Exercise training in the non-pregnant state is widely recognized as a useful method to both prevent and help mitigate high blood pressure.15, 66, 67, 68 Likewise, exercise training has also been shown to benefit patients seeking to reduce body mass.15 Epidemiological studies of prenatal exercise and/or continued/initiated during pregnancy report a lowered incidence of morbidities that are frequently associated with obesity such as gestational diabetes, gestational hypertension, fetal growth restriction and preeclampsia.69, 70, 71 With respect to hypertensive disorders of pregnancy, retrospective reports have consistently suggested physically active women had improved pregnancy outcomes and reduced incidence of preeclampsia.72, 73 Further, an increased NO production and decreased reactive oxygen species has recently been observed in placental tissue samples from women who were exercising during pregnancy when compared to the sedentary/low-activity controls.74 Indeed, recent clinical studies evaluating the effects of physical activity before and during pregnancy on the incidence and severity of preeclampsia have been promising.70, 72, 75, 76, 77, 78, 79 Clinical studies indicate exercise during pregnancy promotes placental growth80, 81 and maternal angiogenic balance.76 Several reports also show exercise during pregnancy may positively influence fetal growth and later developmental milestones.82, 83, 84 In addition to putative feto-placental effects of exercise, recent evidence suggests 10 weeks of exercise during pregnancy lowers diastolic blood pressure in women with a predisposition to pregnancy-induced hypertension77 and decrease cardiovascular risk profile.83 Other studies have recently shown that exercise during pregnancy may promote a pro-angiogenic state by increasing placental growth factor (PlGF) in pregnant women.76
Despite past evidence that exercise may work to minimize the chances of preeclampsia, few hypothesis-driven mechanistic and molecular experiments have been reported. The recent development of numerous animal models that mimic clinical features of preeclampsia has led to recent work evaluating the effects of exercise before and during pregnancy on blood pressure and other characteristics of the syndrome.85, 86, 87 We have recently observed exercise before and during gestation creates a pro-angiogenic state in rodents.85, 88 Further, we and others have previously observed exercise before and during gestation mitigates hypertension in several experimental models85, 86, 87 of preeclampsia.
The beneficial role of physical activity before and during pregnancy on blood pressure during hypertensive pregnancy has also been recently reported in two different animal models of preeclampsia.87, 89 Using a transgenic mouse model of super-imposed preeclampsia that employs female mice over-expressing human angiotensinogen mated with male mice over-expressing human renin, Falcao and colleagues reported reductions in blood pressure, proteinuria and cardiac hypertrophy following a voluntary wheel running regimen that spanned 4 weeks prior to and throughout gestation.87 The same group performed additional studies in the same model and reported exercise induced alteration in various molecules in the renin–angiotensin system that all favored decreased blood pressure.86
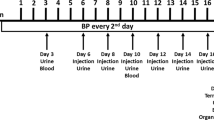
Studies from my laboratory have utilized a similar model of voluntary wheel running that encompassed the six weeks prior to mating and then the balance of gestation after the confirmation of breeding. Our voluntary wheel running paradigm elicits metabolic adaptations in the skeletal muscle (i.e. increased gastrocnemius ATP synthase and PGC1-α expression) of pregnant rats that are consistent with known training adaptations to exercise.90 We also observed exercise induced increases in cytoprotective heat shock proteins (HSP) 27, 60 and 90.90 An important point to note is that the studies of Falcao,87 Genest 86 and Gilbert,85, 90 all employ voluntary running activity and to avoid the stress that has previously been associate with treadmill running as an activity intervention.91 With respect to the role of exercise as an intervention for hypertension in pregnancy, our studies have employed the RUPP model of preeclampsia and show that exercise before and during pregnancy decreases blood pressure and improves the angiogenic profile of the rat dams.85 Another intriguing insight gleaned from these studies is that exercise decreases the RUPP-induced fetal demise routinely observed by us and others in this model.92, 93, 94, 95 More recent studies have begun to evaluate the effects of exercise during pregnancy only and preliminary findings suggest the shorter period of exercise training does not have the same effects (unpublished observations). Similar to reports from studies in women, we have recently reported that exercise before and during gestation improved placental efficiency (that is, the ratio of fetal weight to placental weight)85, 88 in both normal pregnancy and in RUPP dams, which suggests an improvement of placental diffusion capacity. Others have reported various effects on placental growth, and this may be due to the variations in timing, intensity, and duration of exercise between these studies.74, 76, 80, 81, 82, 86, 87, 96, 97, 98 Our working hypothesis for the role of exercise as a means to lower blood pressure in the RUPP model is summarized in Figure 1. Viewed in concert, the data available from clinical and experimental studies strongly suggest that exercise may hold therapeutic potential in hypertensive pregnancies despite the traditional approach that has contraindicated exercise in these pregnancies.99
Is AMPK the link between exercise and reduced blood pressure?
Activation of skeletal muscle during exercise is reported to stimulate the heterotrimeric serine/threonine protein kinase AMPK (AMP-activated protein kinase) and downstream pathways, in a workload dependent manner100, 101 and the role of AMPK in acute and chronic training adaptations following exercise has been well-studied.100, 102, 103 Importantly, activation of AMPK in exercising skeletal muscle reportedly mediates production of pro-angiogenic factors, such as VEGF, through mRNA stabilization.103, 104 Indeed, Zwetsloot et al., have reported a central role for AMPK in VEGF expression post-exercise in transgenic model of muscle-specific AMPK knockdown. Organs with high metabolic activity (skeletal muscle, heart, brain, placenta) express a specialized γ2-isoform of AMPK-γ subunit,105 which is thought to tightly regulate AMPK activation because of increased sensitivity to AMP levels.105, 106 Viewed together, administration of AMPK activators or AMP analogues during pregnancy may stimulate expression of VEGF through the stimulation of AMPK and in turn work towards ameliorating adverse symptoms of preeclampsia.
Epidemiological evidence supports the notion that Metformin (1,1-dimethylbiguanide) reduces blood pressure in addition to its other favorable metabolic actions in diabetics.107 In addition, numerous studies have followed patients treated with metformin through pregnancy and found that it does not have any adverse effects on the mother or the fetus.108, 109 Moreover, a recent study by Brownfoot and colleagues indicates metformin reduced soluble VEGFR-1 and soluble endoglin expression in cell culture and placental villous explants from women with preeclampsia. The authors also reported that metformin reduced mRNA expression of TNF-α and vascular adhesion molecule 1 both markers of endothelial dysfunction. Further, conditioned media from preeclamptic villi treated with metformin showed improved vasodilation compared media from untreated villous cultures.110
The AMPK-activator AICAR (5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside) is reported to mitigate hypertension in several experimental animal models complicated by high blood pressure.111, 112, 113, 114 A study from my laboratory investigate the role of AMPK-activator AICAR in the RUPP model, we administered AICAR for 5 days following the surgical restriction of uteroplacental blood flow. First, AICAR treatment (50 mg kg−1 b.i.d) ameliorated the RUPP-induced hypertension, angiogenic imbalance, and endothelial dysfunction.115 Interestingly, AICAR administration improved circulating free VEGF and decreased sFlt-1 in the RUPP and had no effect in the normal pregnant, which suggested an intriguing effect on sFlt-1 secretion under ischemic conditions. Notably, placental and renal markers of oxidative stress and antioxidative capacity were also mitigated with AICAR treatment in the RUPP, and had no effect in the normal pregnant dams. It is important to note that no detrimental effects were observed in the fetal or placental weights when normal pregnant or RUPP dams that received the AICAR treatment. Similar to our observations with exercise before and during pregnancy, AICAR also mitigated RUPP-induced fetal demise suggesting a beneficial effect on the fetal environment in the RUPP dams.115 Together, these findings suggest that AICAR treatment may have several beneficial effects on the hypertension and endothelial dysfunction in pregnancies complicated by placental ischemia.
Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the exact mechanisms responsible for the pathogenesis of preeclampsia remain unknown. The initiating event in preeclampsia is likely due to reduced uteroplacental perfusion as a result of abnormal cytotrophoblast invasion of spiral arterioles. This leads to chronic placental ischemia and elaboration of factors that lead to widespread activation/dysfunction of the maternal vascular endothelium. Recent studies show that maternal vascular function can be improved and blood pressure lowered by inhibiting complement activation, using anti-complement antibodies and removal of sVEGFR-1 via plasmapheresis. In addition, stimulating pro-angiogenic factors such as VEGF can be done by exercise training and with the administration of AICAR. Some of these management strategies such as exercise and AICAR have also been shown to improve fetal outcomes in the RUPP model as well. While clear progress is being made towards improving the management of preeclampsia and hypertensive diseases of pregnancy further studies involving these pathways will hopefully bring forth the possibility of preventing preeclampsia and improving the long term health of mothers and babies alike.
References
Roberts JM, Pearson G, Cutler J, Lindheimer M . Summary of the NHLBI Working Group on research on hypertension during pregnancy. Hypertension 2003; 41: 437–445.
Conde-Agudelo A, Villar J, Lindheimer M . World Health Organization systematic review of screening tests for preeclampsia. Obstet Gynecol 2004; 104: 1367–1391.
Taebi M, Sadat Z, Saberi F, Kalahroudi MA . Early pregnancy waist-to-hip ratio and risk of preeclampsia: a Prospective Cohort Study. Hypertens Res 2015; 38: 80–83.
American College of Obstetricians and Gynecologists' Task Force on. Hypertension in Pregnancy. Hypertension in Pregnancy: Executive Summary. Obstet Gynecol 2013; 122: 1122–1131.
Moore MP, Redman CW . Preeclampsia. Am J Obstet Gynecol 1987; 156: 257–259.
Paruk F, Moodley J . Maternal and neonatal outcome in early- and late-onset pre-eclampsia. Semin Neonatol 2000; 5: 197–207.
Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ . Placental pathology suggesting that preeclampsia is more than one disease. Am J Obstet Gynecol 2014; 210: 66–67.
Pinheiro CC, Rayol P, Gozzani L, Reis LM, Zampieri G, Dias CB, Woronik V . The relationship of angiogenic factors to maternal and neonatal manifestations of early-onset and late-onset preeclampsia. Prenat Diagn 2014; 34: 1084–1092.
Fisher SJ, Roberts JM Defects in Placentation and Placental Perfusion. In: Lindheimer, MD, Roberts, JM, Cunningham FG (eds), Chesley's Hypertensive Disorders of Pregnancy, Appleton & Lange, 2nd edn 1999, pp 377–394..
Conrad KP, Benyo DF . Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 1997; 37: 240–249.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA . Excess placental soluble Fms-like tyrosine kinase 1 (SFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111: 649–658.
Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A . Uteroplacental Ischemia Results in Proteinuric Hypertension and Elevated SFLT-1. Kidney Int 2007; 71: 977–984.
Gilbert JS, Babcock SA, Granger JP . Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 2007; 50: 1142–1147.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA . Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12: 642–649.
Mori T, Shinohara K, Wakatsuki A, Watanabe K, Fujimaki A . Adipocytokines and endothelial function in preeclamptic women. Hypertens Res 2010; 33: 250–254.
Regal JF, Gilbert JS, Burwick RM . The complement system and adverse pregnancy outcomes. Mol Immunol 2015; 67: 56–70.
Regal JF, Strehlke ME, Peterson JM, Wing CR, Parker JE, Nieto NF, Bemis LT, Gilbert JS, Fleming SD . Role of IgM and angiotensin II type I receptor autoantibodies in local complement activation in placental ischemia-induced hypertension in the rat. Mol Immunol 2016; 78: 38–47.
Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y . Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008; 14: 855–862.
Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF . Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol 2013; 56: 91–97.
Lillegard KE, Loeks-Johnson AC, Opacich JW, Peterson JM, Bauer AJ, Elmquist BJ, Regal RR, Gilbert JS, Regal JF . Differential effects of complement activation products C3a and C5a on cardiovascular function in hypertensive pregnant rats. J Pharmacol Exp Ther 2014; 351: 344–351.
Molvarec A, Fugedi G, Szabo E, Stenczer B, Walentin S, Rigo J Jr . Decreased circulating anandamide levels in preeclampsia. Hypertens Res 2015; 38: 413–418.
Luizon MR, Belo VA, Palei AC, Amaral LM, Lacchini R, Sandrim VC, Duarte G, Cavalli RC, Tanus-Santos JE . Effects of NAMPT polymorphisms and haplotypes on circulating visfatin/NAMPT levels in hypertensive disorders of pregnancy. Hypertens Res 2015; 38: 361–366.
Gilbert JS, Bauer AJ, Gilbert SA, Banek CT . The opposing roles of anti-angiogenic factors in cancer and preeclampsia. Front Biosci (Elite Ed) 2012; 4: 2752–2769.
Gilbert JS, Nijland MJ, Knoblich P . Placental ischemia and cardiovascular dysfunction in preeclampsia and beyond: making the connections. Exp Rev Cardiovasc Ther 2008; 6: 1367–1377.
Magnussen EBM, Vatten LJM, Smith GD, Romundstad PRP . Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol 2009; 114: 961–970.
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ . Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008; 156: 918–930.
Ostlund E, Al-Nashi M, Hamad RR, Larsson A, Eriksson M, Bremme K, Kahan T . Normalized endothelial function but sustained cardiovascular risk profile 11 years following a pregnancy complicated by preeclampsia. Hypertens Res 2013; 36: 1081–1087.
Gingery A, Bahe EL, Gilbert JS . Placental Ischemia and Breast Cancer Risk After Preeclampsia: Tying the Knot. Expert Review of Anticancer Therapy. 2009; 9: 671–681.
O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T . A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993; 136: 355–360.
Yang P, Maguire JJ, Davenport AP . Apelin, elabela/toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci 2015; 36: 560–567.
Narayanan S, Harris DL, Maitra R, Runyon SP . Regulation of the apelinergic system and its potential in cardiovascular disease: peptides and small molecules as tools for discovery. J Med Chem 2015; 58: 7913–7927.
Bertrand C, Valet P, Castan-Laurell I . Apelin and energy metabolism. Front Physiol 2015; 6: 115-.
Yang Y, Lv SY, Ye W, Zhang L . Apelin/APJ system and cancer. Clin Chim Acta 2016; 457: 112–116.
Briana DD, Malamitsi-Puchner A . Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci 2009; 16: 921–937.
Pope GR, Roberts EM, Lolait SJ, O'Carroll AM . Central and peripheral apelin receptor distribution in the mouse: species differences with rat. Peptides 2012; 33: 139–148.
Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD, Newby DE . Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 2010; 121: 1818–1827.
Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, McLean B, Llorens-Cortes C, Hazra S, Murray AG, Vederas JC, Oudit GY . Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension 2016; 68: 365–377.
Hus-Citharel A, Bouby N, Frugiere A, Bodineau L, Gasc JM, Llorens-Cortes C . Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int 2008; 74: 486–494.
Cobellis L, De FM, Mastrogiacomo A, Giraldi D, Dattilo D, Scaffa C, Colacurci N, De LA . Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol 2007; 22: 1–8.
Inuzuka H, Nishizawa H, Inagaki A, Suzuki M, Ota S, Miyamura H, Miyazaki J, Sekiya T, Kurahashi H, Udagawa Y . Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy 2013; 32: 410–421.
Yamaleyeva LM, Chappell MC, Brosnihan KB, Anton L, Caudell DL, Shi S, McGee C, Pirro N, Gallagher PE, Taylor RN, Merrill DC, Mertz HL . Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab 2015; 309: E852–E860.
Kucur M, Tuten A, Oncul M, Acikgoz AS, Yuksel MA, Imamoglu M, Balci, Ekmekci O, Yilmaz N, Madazli R . Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy 2014; 33: 467–475.
Simsek Y, Celik O, Yilmaz E, Karaer A, Dogan C, Aydin S, Ozer A . Serum levels of apelin, salusin-alpha and salusin-beta in normal pregnancy and preeclampsia. J Matern Fetal Neonatal Med 2012; 25: 1705–1708.
Bortoff KD, Qiu C, Runyon S, Williams MA, Maitra R . Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens Pregnancy 2012; 31: 398–404.
Toczek M, Schlicker E, Grzeda E, Malinowska B . Enhanced function of inhibitory presynaptic cannabinoid CB1 receptors on sympathetic nerves of DOCA-salt hypertensive rats. Life Sci 2015; 138: 78–85.
Aban C, Leguizamon GF, Cella M, Damiano A, Franchi AM, Farina MG . Differential expression of endocannabinoid system in normal and preeclamptic placentas: effects on nitric oxide synthesis. Placenta 2013; 34: 67–74.
Zubrzycki M, Liebold A, Janecka A, Zubrzycka M . A New face of endocannabinoids in pharmacotherapy. Part I: protective role of endocannabinoids in hypertension and myocardial infarction. J Physiol Pharmacol 2014; 65: 171–181.
Pulgar VM, Yamaleyeva LM, Varagic J, McGee CM, Bader M, Dechend R, Howlett AC, Brosnihan KB . Increased angiotensin II contraction of the uterine artery at early gestation in a transgenic model of hypertensive pregnancy is reduced by inhibition of endocannabinoid hydrolysis. Hypertension 2014; 64: 619–625.
Saito S, Nakashima A, Shima T, Ito M . Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010; 63: 601–610.
Laresgoiti-Servitje E . A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol 2013; 94: 247–257.
Alijotas-Reig J, Llurba E, Gris JM . Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta 2014; 35: 241–248.
Gupta AK, Gebhardt S, Hillermann R, Holzgreve W, Hahn S . Analysis of plasma elastase levels in early and late onset preeclampsia. Arch Gynecol Obstet 2006; 273: 239–242.
Gandley RE, Rohland J, Zhou Y, Shibata E, Harger GF, Rajakumar A, Kagan VE, Markovic N, Hubel CA . Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertension 2008; 52: 387–393.
Regal JF, Lillegard KE, Bauer AJ, Elmquist BJ, Loeks-Johnson AC, Gilbert JS . Neutrophil depletion attenuates placental ischemia-induced hypertension in the rat. PLoS ONE 2015; 10: e0132063.
Derzsy Z, Prohaszka Z, Rigo J Jr, Fust G, Molvarec A . Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol 2010; 47: 1500–1506.
Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, Kim YM, Hong JS, Goncalves LF, Yeo L, Mazor M, Hassan SS, Kusanovic JP . Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med 2010; 23: 646–657.
Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM . Alternative complement pathway activation fragment bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol 2008; 198: 385–389.
Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM . Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol 2011; 117: 75–83.
Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB . Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension 2013; 62: 1040–1045.
Guseh SH, Feinberg BB, Dawood HY, Yamamoto HS, Fichorova RN, Burwick RM . Urinary excretion of C5b-9 is associated with the anti-angiogenic state in severe preeclampsia. Am J Reprod Immunol 2015; 73: 437–444.
Burwick RM, Feinberg BB . Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 2013; 34: 201–203.
Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ . Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta 2012; 33: 233–243.
George EM, Granger JP . Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep 2011; 13: 269–275.
Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP . Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2008; 294: H541–H550.
Proctor LM, Arumugam TV, Shiels I, Reid RC, Fairlie DP, Taylor SM . Comparative anti-inflammatory activities of antagonists to C3a and C5a receptors in a rat model of intestinal ischaemia/reperfusion injury. Br J Pharmacol 2004; 142: 756–764.
Chen Y, Zhang H, Zhang Y, Lu N, Zhang L, Shi L . Exercise intensity-dependent reverse and adverse remodeling of voltage-gated Ca(2+) channels in mesenteric arteries from spontaneously hypertensive rats. Hypertens Res 2015; 38: 656–665.
Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA This pronouncement was written for the American College of Sports Medicine by exercise and hypertension. Med Sci Sports Exerc 2004; 36: 533–553.
Hagberg JM, Park JJ, Brown MD . The role of exercise training in the treatment of hypertension: an update. Sports Med 2000; 30: 193–206.
Campbell MK, Mottola MF . Recreational Exercise and occupational activity during pregnancy and birth weight: a Case-Control Study. Am J Obstet Gynecol 2001; 184: 403–408.
Dempsey JC, Butler CL, Williams MA . No need for a pregnant pause: physical activity may reduce the occurrence of gestational diabetes mellitus and preeclampsia. Exerc Sport Sci Rev 2005; 33: 141–149.
Marcoux S, Brisson J, Fabia J . The effect of leisure time physical activity on the risk of pre-eclampsia and gestational hypertension. J Epidemiol Community Health 1989; 43: 147–152.
Rudra CB, Sorensen TK, Luthy DA, Williams MA . A prospective analysis of recreational physical activity and preeclampsia risk. Med Sci Sports Exerc 2008; 40: 1581–1588.
Hinman SK, Smith KB, Quillen DM, Smith MS . Exercise in Pregnancy: A Clinical Review. Sports Health 2015; 7: 527–531.
Ramirez-Velez R, Aguilar de Plata AC, Escudero MM, Echeverry I, Ortega JG, Salazar B, Rey JJ, Hormiga C, López-Jaramillo P . Influence of regular aerobic exercise on endothelium-dependent vasodilation and cardiorespiratory fitness in pregnant women. J Obstet Gynaecol Res 2011; 37: 1601–1608.
Weissgerber TL, Wolfe LA, Davies GA . The role of regular physical activity in preeclampsia prevention. Med Sci Sports Exerc 2004; 36: 2024–2031.
Weissgerber TL, Davies GAL, Roberts JM . Modification of angiogenic factors by regular and acute exercise during pregnancy. J Appl Physiol 2010; 108: 1217–1223.
Yeo S, Steele NM, Chang MC, Leclaire SM, Ronis DL, Hayashi R . Effect of exercise on blood pressure in pregnant women with a high risk of gestational hypertensive disorders. J Reprod Med 2000; 45: 293–298.
Gavard JA, Artal R . Effect of exercise on pregnancy outcome. Clin Obstet Gynecol 2008; 51: 467–480.
Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA . Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension 2003; 41: 1273–1280.
Clapp JF III, Kim H, Burciu B, Lopez B . Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol 2000; 183: 1484–1488.
Clapp JF III, Kim H, Burciu B, Schmidt S, Petry K, Lopez B . Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol 2002; 186: 142–147.
Clapp JF III, Capeless EL . Neonatal morphometrics after endurance exercise during pregnancy. Am J Obstet Gynecol 1990; 163: 1805–1811.
Clapp JF . Long-term outcome after exercising throughout pregnancy: fitness and cardiovascular risk. Am J Obstet Gynecol 2008; 199: 489–489.
Clapp JF III . Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J Pediatr 1996; 129: 856–863.
Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K . Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension 2012; 60: 1545–1551.
Genest DS, Falcao S, Michel C, Kajla S, Germano MF, Lacasse AA, Vaillancourt C, Gutkowska J, Lavoie JL . Novel role of the renin-angiotensin system in preeclampsia superimposed on chronic hypertension and the effects of exercise in a mouse model. Hypertension 2013; 62: 1055–1061.
Falcao S, Bisotto S, Michel C, Lacasse AA, Vaillancourt C, Gutkowska J, Lavoie JL . Exercise training can attenuate preeclampsia-like features in an animal model. J Hypertens 2010; 28: 2446–2453.
Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC . Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 2012; 303: R520–R526.
Banek CT, Johnson BK, Gingery A, Bauer AJ, Gilbert JS . Exercise training attenuates placental ischemia induced hypertension and angiogenic imbalance in the rat. FASEB J 2011; 25: 836.
Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC . Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Compar Physiol 2012; 303: R520–R526.
Moraska A, Deak T, Spencer RL, Roth D, Fleshner M . Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1321–R1329.
Alexander BT, Llinas MT, Kruckeberg WC, Granger JP . L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension 2004; 43: 832–836.
Gilbert JS, Dukes M, LaMarca BB, Cockrell K, Babcock SA, Granger JP . Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am J Hypertens 2007; 20: 686–691.
Gilbert JS, LaMarca BB, Babcock SA, Cockrell K, Granger JP . Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increases in soluble Flt-1 expression. Hypertension 2007; 50: E108–E108.
Gilbert JS, Babcock SA, Arany M, Granger JP . Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble endoglin. Hypertension 2008; 52: E49–E49.
Clapp JF III, Rizk KH . Effect of recreational exercise on midtrimester placental growth. Am J Obstet Gynecol 1992; 167: 1518–1521.
Clapp JF III . The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur J Obstet Gynecol Reprod Biol 2003; 110 (Suppl 1): S80–S85.
Lotgering FK, Gilbert RD, Longo LD . Maternal and fetal responses to exercise during pregnancy. Physiol Rev 1985; 65: 1–36.
ACOG Committee on Obstetric Practice. Committee Opinion #267: exercise during pregnancy and the postpartum period. Obstet Gynecol 2002; 99: 171–173.
Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM . AMPK and PPARdelta agonists are exercise mimetics. Cell 2008; 134: 405–415.
Richter EA, Ruderman NB . AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J 2009; 418: 261–275.
Jorgensen SB, Richter EA, Wojtaszewski JF . Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol 2006; 574: 17–31.
Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP . AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol 2008; 586: 6021–6035.
Ouchi N, Shibata R, Walsh K . AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 2005; 96: 838–846.
Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D . Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 2000; 346 Pt 3: 659–669.
Moffat C, Harper ME . Metabolic functions of AMPK: aspects of structure and of natural mutations in the regulatory gamma subunits. IUBMB Life 2010; 62: 739–745.
Giugliano D, De Rosa N, Di Maro G, Marfella R, Acampora R, Buoninconti R, D'Onofrio F . Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care 1993; 16: 1387–1390.
Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ . Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994; 43: 647–654.
Glueck CJ, Bornovali S, Pranikoff J, Goldenberg N, Dharashivkar S, Wang P . Metformin, pre-eclampsia, and pregnancy outcomes in women with polycystic ovary syndrome. Diabet Med 2004; 21: 829–836.
Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, Senadheera S, Illanes SE, Kaitu'u-Lino TJ, Tong S . Metformin as a prevention and treatment for preeclampsia: effects on soluble Fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol 2016; 214: 356–356.
Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S . Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 2005; 54: 928–934.
Bosselaar M, Smits P, van Loon LJC, Tack CJ . Intravenous AICAR during hyperinsulinemia induces systemic hemodynamic changes but has no local metabolic effect. J Clin Pharmacol. 2011; 51: 1449–1458.
Buhl ES, Jessen N, Pold R, Ledet, Thomas F, Allan P, Steen B, Pedersen O, Schmitz O, Lund S . Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes 2002; 51: 2199–2206.
Ford RJ, Teschke SR, Reid EB, Durham KK, Kroetsch JT, Rush JW . AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens 2012; 30: 725–733.
Banek CT, Bauer AJ, Needham KM, Dreyer HC, Gilbert JS . AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol 2013; 304: H1159–H1165.
Acknowledgements
This work was supported in part by grants from the American Heart Association 10SDG2600040 and the National Institutes of Health HL114096 and a sub-contract from HD079547.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Gilbert, J. From apelin to exercise: emerging therapies for management of hypertension in pregnancy. Hypertens Res 40, 519–525 (2017). https://doi.org/10.1038/hr.2017.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.40
Keywords
This article is cited by
-
Physical exercise for a healthy pregnancy: the role of placentokines and exerkines
The Journal of Physiological Sciences (2023)