Abstract
Beckwith–Wiedemann Syndrome (BWS) is an overgrowth syndrome caused by a variety of molecular changes on chromosome 11p15.5. Children with BWS have a significant risk of developing Wilms tumours with the degree of risk being dependent on the underlying molecular mechanism. In particular, only a relatively small number of children with loss of methylation at the centromeric imprinting centre (IC2) were reported to have developed Wilms tumour. Discontinuation of tumour surveillance for children with BWS and loss of methylation at IC2 has been proposed in several recent publications. We report here three children with BWS reported to have loss of methylation at IC2 on clinical testing who developed Wilms tumour or precursor lesions. Using multiple molecular approaches and multiple tissues, we reclassified one of these cases to paternal uniparental disomy for chromosome 11p15.5. These cases highlight the current challenges in definitively assigning tumour risk based on molecular classification in BWS. The confirmed cases of loss of methylation at IC2 also suggest that the risk of Wilms tumour in this population is not as low as previously thought. Therefore, we recommend that for now, all children with a clinical or molecular diagnosis of BWS be screened for Wilms tumour by abdominal ultrasonography until the age of eight years regardless of the molecular classification.
Similar content being viewed by others
Introduction
Beckwith–Wiedemann Syndrome (BWS) (OMIM 130650) is an overgrowth disorder with an increased risk for tumour development in childhood. The clinical presentation is heterogeneous and can involve macrosomia, hemihyperplasia, macroglossia, abdominal wall defects, and other phenotypic features.1 BWS is pan-ethnic and has an estimated prevalence of 1/10 000–13 700 with a sex ratio of 1:1.2
BWS confers a risk for embryonal tumour development in the first eight years of life of ~7.5% (range of 4–21%).3 This risk includes a 3–5% risk of developing Wilms tumour (WT), a renal malignancy of embryonal origin.4 Tumour surveillance recommendations vary amongst centres; however, for WT, these have typically included abdominal ultrasound every 3 months to the age of 8 years.5
BWS is caused by a variety of genetic and/or epigenetic alterations that usually impact the regulation of imprinted genes on chromosome 11p15.5.6 Imprinting refers to the preferential or exclusive expression of the paternal or maternal allele of an imprinted gene. Imprinted gene expression is regulated by epigenetic mechanisms, including DNA methylation of CpG-rich imprinting centres (ICs). ICs regulate the expression of several imprinted genes that are clustered within domains. BWS is caused by dysregulation of one or two imprinted domains on chromosome 11p15.5, each regulated by an IC6 (Figure 1).
Map of chromosome 11p15 region: (a) Schematic representation of imprinting regulation in the chromosome 11p15 region. Orange represents maternally derived chromosomes while green represents paternally derived chromosomes. Typically, IC1 is methylated on the paternally derived chromosome resulting in IGF2 expression and silencing of H19 while on the maternally derived chromosome IC2 is methylated resulting in silencing of KCNQ1OT1 and expression of KCNQ1 and CDKN1C. (b) Map of KCNQ1OT1 (DMR (IC2) indicating the coverage of pyrosequencing, MS-MLPA, and the Infinium methylation array. IC2 overlaps the CpG island indicated by a green bar. Genome maps are adapted from the UCSC Genome Browser. (c) Map of H19 (DMR (IC1) indicating coverage of pyrosequencing, methylation-specific MLPA probes (MS-MLPA), copy-number specific MLPA probes (CN-MLPA), and the Infinium methylation array. CTCF target sites are indicated.
IC1, located at the telomeric end of chromosome 11p15.5, regulates the expression of two imprinted genes: the paternally expressed IGF2 (insulin-like growth factor 2), and the maternally expressed non-coding RNA H19. This IC is normally methylated on the paternal allele insulating the H19 promoter from a downstream enhancer, and allowing the enhancer access to the IGF2 promoter.6
IC2 is centromeric of IGF2-H19 on chromosome 11p15.5. It overlaps the promoter of a long non-coding, paternally expressed RNA transcript, KCNQ1OT1 that regulates in cis the expression of several maternally expressed imprinted genes, including CDKN1C. The CDKN1C gene functions as both a tumour suppressor gene and a negative regulator of foetal growth. IC2 is normally methylated on the maternal chromosome blocking the expression of the paternally expressed KCNQ1OT1 and permitting CDKN1C to be expressed.6
The molecular alterations detected in individuals with BWS, in descending order of frequency are: loss of DNA methylation at IC2 (IC2 LOM; 50–60%), paternal uniparental disomy of 11p15.5 (patUPD; 20–25%), gain of DNA methylation at IC1 (IC1 GOM; 5–7%) and loss of function mutations of the maternally derived CDKN1C gene (3–8%).7, 8 No causative molecular alteration is detected in 10–15% of children with a BWS phenotype.1
(Epi)genotype/phenotype correlations are well documented in BWS, including correlations of specific molecular aetiologies with tumour risk and tumour type.1, 4, 9, 10 The highest risk for tumour development, especially WT, occurs with patUPD of 11p15.5 or GOM at IC1. Tumour risk is significantly lower for LOM at IC2 or mutations in CDKN1C. Multiple investigators have concluded that LOM at IC2 does not confer an increased risk for WT. In fact, prior to the present report, only three cases of WT with LOM at IC2 were reported but without validation of the initial molecular findings.9, 10, 11
(Epi)genotype/phenotype correlations for BWS have prompted recommendations to revise tumour surveillance protocols for BWS based on the molecular classification. Scott et al.12 suggested that children with BWS and IC2 alterations do not require WT screening. Brioude et al.13 proposed that children with BWS and LOM at IC2 should have an ultrasound evaluation at clinical diagnosis and only continue with ultrasound evaluation if visceromegaly or ‘severe’ hemihyperplasia is present. In 2015, Mussa et al.4 questioned the rationale for WT surveillance for cases with LOM at IC2. Their guidelines from the Italian Scientific Committee on BWS noted that in the near future, clinical practice guidelines might cease recommending screening for these children.14 Most recently in 2016, Maas et al.11 stated that tumour screening is not indicated for children with BWS with LOM at IC2 based on an arbitrary 2% risk for specific tumours and the UK Wilms Tumour Surveillance Working Group designation of a 5% threshold for overall tumour development. However, the implementation of arbitrary risk thresholds for tumour surveillance may also impact long-term adverse health outcomes.
When proposing changes to tumour surveillance for BWS based on molecular subgroups, it is important to consider previously reported confounding issues including test sensitivity15 and somatic mosaicism.16 Currently, one of the most robust diagnostic tests for BWS used in clinical laboratories is methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA), which detects the 3 most common causative BWS-associated molecular alterations: IC1 GOM, IC2 LOM, and patUPD along with genomic copy number.14, 15 Other tests have also been used to test for targeted methylation changes consistent with a BWS diagnosis including pyrosequencing in research labs, and combined bisulfite restriction analysis and southern blotting in clinical labs. These tests measure the degree of methylation at specific CpG loci in IC1 and IC2. Simultaneous IC1 GOM and IC2 LOM implies the presence of patUPD; further molecular testing is required for confirmation.
We report here 3 cases of children with BWS and IC2 LOM at the time of initial clinical testing, who later developed WT or WT precursor lesions known as nephrogenic rests. Further investigations led to the molecular reclassification of one of these cases to patUPD of 11p15.5. Given these cases, we support continuation of current tumour surveillance recommendations for all children with BWS until more definitive data are available to support evidence-based modification of these recommendations.
Materials and methods
Clinical data and sample collection
Cases either presented at our centre or were referred to us from an external centre. Medical records were reviewed regarding clinical diagnosis of BWS, surveillance, cancer diagnosis, and treatment. All patients consented to be part of this study through an approved Hospital for Sick Children REB protocol. Blood was collected for analysis from all patients. Other tissues were obtained where available including skin, saliva, tumour, and non-neoplastic kidney from nephrectomy specimens.
Methylation-specific multiplex ligation-dependent probe amplification
MS-MLPA was performed at the Genome Diagnostic Laboratory at The Hospital for Sick Children. The MS-MLPA kit (MRC-Holland, Amsterdam, The Netherlands) was used with methylation-specific digestion at genomic sites representing IC1 and IC2 (Figure 1) as previously described.17 Microsatellite analysis was used to confirm cases of suspected patUPD.
Pyrosequencing
All samples were analysed by pyrosequencing using assays for IC1 and IC2 developed in our laboratory. Sodium bisulfite conversion was done using the EpiTect Plus kit (Qiagen, Germantown, MD, USA). After conversion, the relevant loci were amplified by PCR. The PCR products were checked for purity by gel electrophoresis. Pyrosequencing utilised the Pyromark Q24 Pyrosequencer (Qiagen). Methylation at each IC was calculated as a mean of the methylation at each queried CpG site.18 Control samples were obtained from individuals at our hospital with normal neurodevelopment and growth. Gain or loss of methylation was defined as a mean methylation value 2 s.d.’s from the mean of the control population (Supplementary Table 1). Pyrosequencing data is publically available on the LOVD database (individual IDs 00103960, 00103962, and 00103963).
Methylation array
Blood samples from all 3 cases were analysed on the Infinium 450 K Methylation Array (Illumina, San Diego, CA, USA) after the same sodium bisulfite conversion process described above for pyrosequencing. Normalisation and background subtraction were performed using the standard protocol with the Genome Studio software suite (Illumina).19 We identified probes that represent CpG sites overlying known imprinted regions of chromosome 11p15.5 using a previously described method.18, 20 Gain or loss of methylation at each CpG site was defined as a beta value plus or −2.5 s.d.’s from the mean of the values in the control blood samples at the same site. Infinium data are available on the Gene Expression Omnibus GSE95488.
SNP array
SNP array technology was used to assess copy number variation (CNV) and loss of heterozygosity (LOH) in blood samples from all three patients. DNA from cases 1, 2 and 3 were analysed using the HumanOmni2.5 array (Illumina; available on the Gene Expression Omnibus GSE95488). In case 3, SNPs were also analysed on a CytoScan Dx Assay (Affymetrix, Santa Clara, CA, USA) in the Genome Diagnostics Laboratory at The Hospital for Sick Children. Probes on the chromosome 11p were inspected for beta allele frequencies deviating from 0, 0.5, or 1 (indicating LOH) and for log R ratios deviating from 0 (indicating CNVs). CNV Finder (Illumina) and BeadarraySNP available on bioconductor21 were applied genome wide to identify CNVs within and outside of the 11p region.
Results
Case 1
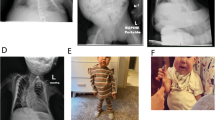
This female term infant presented with macrosomia, neonatal hypoglycaemia, a small umbilical hernia, and macroglossia (Figure 2a). Left-sided hemihyperplasia was subsequently noted. Abdominal ultrasound screening was commenced which was initially only remarkable for bilateral nephromegaly with normal serum creatinine levels.
A blood sample drawn shortly after birth was examined by MS-MLPA and LOM at IC2 was reported. The methylation level at IC1 was interpreted as ‘borderline’ GOM (see Table 1). Microsatellite STR analysis was carried out on DNA from the patient and her parents. The Genome Diagnostic laboratory reported that the test results did not support patUPD for chromosome 11p15.5.
At 15 months of age a routine screening ultrasound uncovered bilateral renal masses. Secondary imaging with computed tomography supported a likely diagnosis of WT and did not reveal any metastases. The patient received neoadjuvant chemotherapy consisting of vincristine, actinomycin, and doxorubicin with good radiologic response after 6 weeks. Given complete resolution of the right-sided lesion on imaging and intraoperative exam, a left partial nephrectomy was performed. Pathologic findings confirmed a diagnosis of favourable histology WT. Two nephrogenic rests were found in the resection specimen. The patient completed 15 additional weeks of chemotherapy with vincristine and actinomycin and did not require radiotherapy. She is currently in remission 6 years from initial diagnosis.
Follow-up MS-MLPA evaluation was undertaken in the Genome Diagnostics Laboratory on DNA extracted from the initial blood sample. This reanalysis confirmed LOM at IC2 but also found GOM at IC1 leading to a revised classification of paternal UPD at chromosome 11p15.5. At the time of surgery additional fresh samples were acquired for analysis by pyrosequencing in the research laboratory. These included skin for fibroblast culture, tumour, and non-neoplastic kidney samples. PatUPD was confirmed in both the non-neoplastic kidney and in the tumour sample. However, DNA methylation studies in the fibroblast and blood samples were consistent with LOM at IC2 (Table 2) supporting the presence of mosaicism. Interestingly, methylation at IC1 was increased in the kidney and tumour compared to blood and fibroblasts suggesting a dynamic process of methylation gain at this locus over the course of the development of neoplasia.
Investigation for LOH and for CNVs via SNP array was undertaken on the blood sample drawn after diagnosis of the renal tumour and revealed copy number neutral LOH spanning all of 11p15 and the telomeric end of 11p14 (rs11604127_rs56192161; Figure 3). This finding was in contrast to the STR analysis undertaken on the initial blood sample. As well, LOM at IC2 (cg00000924_cg25306939) and GOM at IC1 in blood were also found on the methylation array supporting the classification of paternal UPD 11p15.5. Therefore, the molecular aetiology for BWS in this patient was revised from the initial report of LOM at IC2 to mosaic patUPD.
SNP Array data from the 3 cases in this series at 11p15.5 and 11p15.4. For each case the first set of data represents the B allele frequency (BAF, green) and the second set of data represents the log R ratio (LRR, blue). In the bottom panel, a red arrow marks IC1 (chr11:2019627-2024297) while a purple arrow marks IC2 (chr11:2720411-2722087).
Case 2
This male infant was conceived via in vitro fertilisation and intracytoplasmic sperm injection. Prenatal ultrasound identified macrosomia and an abdominal wall defect. The baby was born at term and was noted to have macrosomia and an omphalocele as well as hypoglycaemia. Follow-up examination also revealed left-sided hemihyperplasia, a nevus simplex, a hemangioma, and a muscular ventricular septal defect (Figure 2b).
At 2 years of age, screening by abdominal ultrasonography detected bilateral small renal lesions. It was unclear on initial imaging whether these represented WT or nephrogenic rests and follow-up was planned with serial imaging. There was no biopsy and no cancer-directed therapy. Three clinicians with expertise in childhood renal tumours including Dr Bruce Beckwith interpreted the findings to be consistent with hyperplastic nephrogenic rests (WT precursors). The child was subsequently followed closely with diagnostic imaging and the lesions resolved without therapeutic intervention.
A saliva sample from this patient was obtained at 5 years of age and was clinically tested with MS-MLPA. This sample was found to have LOM at IC2 without GOM at IC1. A blood sample obtained at 9 years of age and tested by MS-MLPA and pyrosequencing indicated LOM at IC2 with normal methylation at IC1. SNP array testing on this blood sample showed no evidence of LOH or CNV. Methylation array data in blood also indicated LOM at IC2 but not GOM at IC1. LOM was also detected at other loci on the methylation array. In this case, multiple clinically available tests (MS-MLPA, SNP array) and research tests (pyrosequencing) on two tissues supported a diagnosis of IC2 LOM without another clear molecular aetiology for developing hyperplastic nephrogenic rests.
Case 3
This male infant was found to have an omphalocele on antenatal ultrasonography and molecular testing on amniocyte DNA indicated a diagnosis of BWS with LOM at IC2. In the neonatal period, other features of BWS were apparent including macrosomia, macroglossia, and bilateral nephromegaly (Figure 2c). He also had gastroesophageal reflux, significant constipation, and right undescended testicle repaired with orchidopexy. MS-MLPA was performed on a cord blood sample that revealed LOM at IC2 and normal methylation at IC1.
Screening with abdominal ultrasonography commenced at birth. At 21 months of age an echogenic lesion of uncertain clinical significance was found in the left lower renal pole. Follow-up imaging at 24 months of age showed no changes but 6 weeks later the lesion had increased in size. Given a presumptive diagnosis of WT, neoadjuvant chemotherapy consisting of vincristine and actinomycin was initiated for 6 weeks which resulted in a partial response. He then underwent left partial nephrectomy and a pathologic confirmation of stage 1 favourable histology WT was made. There was one perilobar nephrogenic rest found in the resection specimen (Figure 4). He completed 15 additional weeks of chemotherapy and is now in remission 18 months from tumour diagnosis though bilateral renal cysts have recently been noted on imaging.
Pathology findings from partial nephrectomy. (a–d) Treated WT. (a) Macroscopic appearance of the tumour measuring only 0.5 cm in diameter. (b) The central portion of the specimen is mainly necrotic with isolated differentiated epithelial elements. (c) The more peripheral portion consists of blastema (lower left corner) with differentiated tubules in a fibrotic stroma. (d) Intra-renal vein (vessel wall marked by arrow) containing a tumour thrombus composed of fibrous stroma and rare differentiated tubules. (e and f) perilobar nephrogenic rest showing a well circumscribed rest surrounded by normal renal parenchyma. The rest is composed of nests of tubules separated by varying amounts of fibrous stroma. (original magnifications: a × 1, b, c × 200, d × 100, e × 10, and f × 100).
At the time of nephrectomy, tissue samples were obtained for testing including blood, skin from the left and right sides of the abdominal incision site, and non-neoplastic kidney. Pyrosequencing results on all of these samples were consistent with LOM at IC2 with normal methylation at IC1. A sample of the tumour for molecular analysis was not available due to necrosis of the specimen.
The blood sample obtained at the time of diagnosis with WT was also examined using the methylation array which confirmed the finding of LOM at IC2 and normal methylation at IC1. This sample also showed evidence of LOM at multiple other imprinted regions (Table 3).
Given the unusual finding of WT in a child with BWS caused by LOM at IC2, further clinical testing was pursued. Sanger sequencing of the WT1 gene and MLPA did not reveal any mutations, duplications, or deletions. A clinical SNP array was consistent with a 46XY karyotype without CNVs or LOH at any region including 11p15. Thus this child’s WT developed in the context of LOM at IC2 without another clear molecular aetiology for WT.
Discussion
We present three cases of patients with BWS and WT or nephrogenic rests who, on initial clinical testing, had a molecular classification of LOM at IC2. In one of these cases (case 1) the molecular classification was subsequently revised to patUPD of 11p15.5. In two other cases, however, the molecular diagnosis of IC2 LOM was confirmed by testing of multiple tissues using both epigenetic and genetic test modalities. These cases demonstrate that individuals with BWS and LOM at IC2 on molecular testing are, in fact, at risk for developing WT although the magnitude of this risk is yet to be defined.
Disparate molecular findings have been previously reported in individuals with BWS on repeat testing over time and across different tissue samples. Alders et al. presented patients with BWS and macroglossia who had either no molecular diagnosis or LOM at IC2 in blood. Examining DNA extracted from tongue tissue, they revised one patient’s diagnosis to patUPD and two others to isolated GOM at IC1.16 Our study of multiple tissues in case 1 showed that different tissues can demonstrate different methylation values at IC1 and IC2. These findings suggest that molecular test results in constitutional tissues such as blood, saliva, and fibroblasts may not accurately reflect the molecular status of cells in the organs at risk for tumour development. In addition, there appears to be a potential selective growth advantage of blood cells with patUPD of 11p15.22 That is, some patients may, as they age, have an increase in the proportion of cells in blood with patUPD. Therefore, both the timing of tissue sampling and the actual tissue sampled may influence the testing outcome.
The fact that one case of BWS and LOM IC2 was reclassified to patUPD at 11p15.5 also points to challenges inherent in current clinical testing modalities suggesting that they are not always definitive. A reduced sensitivity of MS-MLPA to detect gain of methylation at IC1 versus loss at IC2 can impact the detection of 11p15 UPD in the presence of somatic mosaicism.15 This can lead to MS-MLPA findings that are borderline or below the level of detection of 11p15 UPD in blood.23 As can be seen in Figure 1, only selected regions in the ICs are represented on the MS-MLPA assay. Therefore, methylation changes may be underestimated or overestimated. Alternate novel testing approaches could be considered as an adjunct to improve sensitivity. Russo et al15 have proposed the utilisation of a number of ‘complementary’ testing approaches to enhance molecular diagnostic classification for BWS. They also comment on the challenges of interpreting borderline cases and the clinical variability of BWS. Future adequately powered studies of BWS with more sensitive testing modalities across multiple tissues will be required to better define the frequency of this type of misclassification.
We report here two additional cases of WT in children with BWS and LOM at IC2. Cases 2 and 3 in our series are the first cases for which LOM at IC2 was confirmed by extensive molecular testing undertaken in multiple tissues. Although it is possible that case 3 developed a sporadic WT that was not associated with his diagnosis of BWS, a number of the clinical features of the case argue against this possibility. In particular, perilobar nephrogenic rests were noted in the resection specimen which are known to be associated with WT arising in children with BWS.24 Similarly, the nephrogenic rests that characterised case 2 are in keeping with BWS. The presence of nephrogenic rests in the resection specimens of these two cases also underscores the risk of developing future metachronous malignancies. These 2 new cases of BWS and WT/nephrogenic rests in children with LOM at IC2 echo previous reports of a small but real risk of developing WT in children with LOM at IC2. Previous reports include three other children with BWS and LOM at IC2 in peripheral blood cells who developed WT10, 11 and an additional two cases of WT with LOM at IC2 in normal kidney tissue as well as tumour tissue.25
There are several limitations to the present study. This is a case series and therefore, the incidence of WT in the population of children with BWS and LOM at IC2 cannot be defined. Second, our analysis of multiple tissues using multiple techniques is more comprehensive and therefore not directly comparable to other reports of children with LOM at IC2 in the literature.
The development of tumour surveillance guidelines for paediatrics tumour predisposition syndromes such as BWS is complex and requires consideration of a number of different factors including tumour risk and test sensitivity/specificity. As noted previously, to define criteria for tumour surveillance, Maas et al.11 utilised threshold of 5% for overall tumour incidence proposed by the UK Wilms Tumour Surveillance Working Group and an additional 2% threshold for specific tumours. However, in order to set such a threshold using an evidence-based approach, one must consider the true incidence of tumour development in BWS with IC2 LOM, the risks associated with late diagnosis, and the sensitivity and specificity of clinical testing modalities such as the most commonly used MS-MLPA platform.
In the fourth National WT Study, children with BWS who had undergone screening had smaller tumours at diagnosis than were found in earlier studies.26 The benefit of diagnosis at an early stage is indisputable. Currently early stage WT can be effectively treated without radiation and with minimal use of anthracyclines.27 This reduction of therapy significantly reduces the risk of late effects of treatment including cardiomyopathy and secondary malignancy.28 Furthermore, some centres are now performing nephron-sparing surgery in selected children with early stage WT. Early reports of the outcomes of these procedures indicate that they can be done safely in this population without increasing the risk of tumour recurrence.29 Importantly, there is also emerging evidence that the risk of long-term renal dysfunction is reduced with this surgical approach30 as it preserves more normal renal tissue. These data suggest that the ability to perform nephron-sparing surgery is of clear benefit to children with BWS who will continue to be at risk for tumour development and may require further renal resection. In the 3 cases reported here, tumour surveillance was initiated at birth and malignancy was detected at an early stage. Notably, in cases 1 and 3 early detection allowed treatment with only two chemotherapeutic agents following nephron-sparing surgery. In 2014, Samuel et al.31 suggested that treatment related morbidity and increased quality of life needed to emerge as tumour surveillance goals equally important to survival. Indeed, the rationale for a screening programme in children with BWS has been to detect WT at an early stage to minimise potential treatment related-sequelae and to enhance long-term health and well being. With respect to quality of life, it is particularly important to consider that children with BWS and LOM at IC2 are also at greater risk than the general population to develop renal dysfunction/renal failure even at a young age due to developmental renal issues that can result in cystic and calcified lesions.32 Therefore, the frequency and types of long-term adverse outcomes need to be carefully considered before advocating for limiting surveillance for individuals with BWS and LOM at IC2.
We recognise, of course, the benefits of reducing screening in children with BWS. Such a reduction can decrease medical costs and can potentially reduce the anxiety that parents face every 3 months awaiting screening results. However, for parents and children, the benefits of screening and the potential for early treatment and reduction of risk for renal failure may outweigh the psychological and logistic burdens of screening. In terms of medical costs, screening all BWS patients has been shown before to be cost effective.5 Such screening protocols could potentially be found to be even more cost effective if the long-term issues of renal dysfunction/renal failure were considered. Although potential cost benefits could be realised by screening a subset of children with BWS, in our opinion, clinical testing techniques and risk estimates for BWS molecular subgroups are not yet adequately developed to achieve this goal.
The cases reported here support the maintenance of tumour surveillance including renal ultrasound screening for children with BWS caused by LOM at IC2. We advocate continuation of surveillance for three reasons: (1) the possibility of molecular misclassification of patUPD of chromosome 11p15 as LOM IC2 by the currently widely used chromosome 11p15 MS-MLPA testing; (2) the reality that children with LOM at IC2 are, in fact, at risk of developing WT even if that risk is lower than in other BWS molecular subgroups; and (3) the potential for long-term non-WT related renal complications.
In conclusion, we present 3 cases of children with BWS for whom clinical testing indicated LOM IC2. These children went on to develop WT or nephrogenic rests. Further testing led to reclassification to patUPD of 11p15.5 in one case. In the two other children, the molecular diagnosis of LOM at IC2 was retained following testing of multiple tissues and the use of multiple testing modalities. These findings support continued screening for WT in all children with a clinical diagnosis of BWS regardless of their underlying molecular alteration until such time that clinical testing can more accurately classify BWS cases and further data on prospectively ascertained cohorts are available to determine the true risk for WT in BWS and IC2 LOM. Ultimately, a decision about which molecular subgroups of BWS patients should be screened should include not only the absolute tumour risk but also the long-term risks for adverse outcomes. Research to improve the reliability of molecular testing, to further clarify the aetiology for tumour development in BWS, and to quantify the long-term risks for renal failure for children with BWS will ultimately allow for evidence-based tumour surveillance. In this era of whole-genome sequencing, these risks will ultimately be best quantified by a multi-omic approach to risk assessment
References
Weksberg R, Shuman C, Beckwith JB : Beckwith-Wiedemann syndrome. Eur J Human Genet 2009; 18: 8–14.
Mussa A, Russo S, De Crescenzo A et al: Prevalence of Beckwith-Wiedemann syndrome in North West of Italy. Am J Med Genet 2013; 161A: 2481–2486.
Tan TY, Amor DJ : Tumour surveillance in Beckwith-Wiedemann syndrome and hemihyperplasia: a critical review of the evidence and suggested guidelines for local practice. J Paediatr Child Health 2006; 42: 486–490.
Mussa A, Russo S, De Crescenzo A et al: (Epi)genotype–phenotype correlations in Beckwith–Wiedemann syndrome. Eur J Hum Genet 2016; 24: 183–190.
McNeil DE, Brown M, Ching A, DeBaun MR : Screening for Wilms tumor and hepatoblastoma in children with Beckwith-Wiedemann syndromes: a cost-effective model. Med Pediatr Oncol 2001; 37: 349–356.
Choufani S, Shuman C, Weksberg R : Molecular findings in Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 2013; 163C: 131–140.
Bliek J, Maas SM, Ruijter JM et al: Increased tumour risk for BWS patients correlates with aberrant H19 and not KCNQ1OT1 methylation: occurrence of KCNQ1OT1 hypomethylation in familial cases of BWS. Hum Mol Genet 2001; 10: 467–476.
Weksberg R, Nishikawa J, Caluseriu O et al: Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum Mol Genet 2001; 10: 2989–3000.
Mussa A, Molinatto C, Baldassarre G et al: Cancer risk in Beckwith-Wiedemann syndrome: a systematic review and meta-analysis outlining a novel (epi)genotype specific histotype targeted screening protocol. J Pediatr 2016; 176: 142–149.e1.
Ibrahim A, Kirby G, Hardy C et al: Methylation analysis and diagnostics of Beckwith-Wiedemann syndrome in 1,000 subjects. Clin Epigenetics 2014; 6: 11.
Maas SM, Vansenne F, Kadouch DJM et al: Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet 2016; 170: 2248–2260.
Scott RH, Walker L, Olsen OE et al: Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child 2006; 91: 995–999.
Brioude F, Lacoste A, Netchine I et al: Beckwith-Wiedemann syndrome: growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Horm Res Paediatr 2013; 80: 457–465.
Mussa A, Di Candia S, Russo S et al: Recommendations of the Scientific Committee of the Italian Beckwith-Wiedemann Syndrome Association on the diagnosis, management and follow-up of the syndrome. Eur J Med Genet 2016; 59: 52–64.
Russo S, Calzari L, Mussa A et al: A multi-method approach to the molecular diagnosis of overt and borderline 11p15.5 defects underlying Silver–Russell and Beckwith–Wiedemann syndromes. Clin Epigenetics 2016; 8: 1–15.
Alders M, Maas SM, Kadouch DJM et al: Methylation analysis in tongue tissue of BWS patients identifies the (EPI)genetic cause in 3 patients with normal methylation levels in blood. Eur J Med Genet 2014; 57: 293–297.
Eggermann T, Schönherr N, Eggermann K et al: Use of multiplex ligation-dependent probe amplification increases the detection rate for 11p15 epigenetic alterations in Silver-Russell syndrome. Clin Genet 2008; 73: 79–84.
Choufani S, Shapiro JS, Susiarjo M et al: A novel approach identifies new differentially methylated regions (DMRs) associated with imprinted genes. Genome Res 2011; 21: 465–476.
Pidsley RY, Wong CC, Volta M et al: A data-driven approach to preprocessing Illumina 450 K methylation array data. BMC Genomics 2013; 14: 293.
Inbar-Feigenberg M, Choufani S, Cytrynbaum C et al: Mosaicism for genome-wide paternal uniparental disomy with features of multiple imprinting disorders: diagnostic and management issues. Am J Med Genet 2013; 161A: 13–20.
Oosting J BeadarraySNP: Normalization and Reporting of Illumina SNP bead arrays: R Package Version 1.40.0. Bioconductor, 2014.
Vinatier I, Martin X, Costa J-M et al: A late onset sickle cell disease reveals a mosaic segmental uniparental isodisomy of chromosome 11p15. Blood Cells Mol Dis 2015; 54: 53–55.
Slatter RE, Elliott M, Welham K et al: Mosaic uniparental disomy in Beckwith-Wiedemann syndrome. J Med Genet 1994; 31: 749–753.
Beckwith JB, Kiviat NB, Bonadio JF : Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol 1990; 10: 1–36.
Niemitz EL, Feinberg AP, Brandenburg SA et al: Children with idiopathic hemihypertrophy and beckwith-wiedemann syndrome have different constitutional epigenotypes associated with wilms tumor. Am J Hum Genet 2005; 77: 887–891.
Porteus MH, Narkool P, Neuberg D et al: Characteristics and outcome of children with Beckwith-Wiedemann syndrome and Wilms' tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 2000; 18: 2026–2031.
Green DM, Breslow NE, D'Angio GJ et al: Outcome of patients with stage II/favorable histology wilms tumor with and without local tumor spill: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 2013; 61: 134–139.
Armenian SH, Robison LL : Childhood cancer survivorship: an update on evolving paradigms for understanding pathogenesis and screening for therapy-related late effects. Curr Opin Pediatr 2013; 25: 16–22.
Romão Rodrigo LP, Pippi Salle JL, Shuman C et al: Nephron sparing surgery for unilateral wilms tumor in children with predisposing syndromes: single center experience over 10 Years. J Urol 188: 1493–1499.
Romão Rodrigo LP, Lorenzo AJ : Renal function in patients with Wilms tumor. Urol Oncol 34: 33–41.
Samuel N, Villani A, Fernandez CV, Malkin D : Management of familial cancer: sequencing, surveillance and society. Nat Rev Clin Oncol 2014; 11: 723–731.
Goldman M, Smith A, Shuman C et al: Renal abnormalities in beckwith-wiedemann syndrome are associated with 11p15.5 uniparental disomy. J Am Soc Nephrol 2002; 13: 2077–2084.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors disclose no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Brzezinski, J., Shuman, C., Choufani, S. et al. Wilms tumour in Beckwith–Wiedemann Syndrome and loss of methylation at imprinting centre 2: revisiting tumour surveillance guidelines. Eur J Hum Genet 25, 1031–1039 (2017). https://doi.org/10.1038/ejhg.2017.102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2017.102
This article is cited by
-
Hallmark discoveries in the biology of Wilms tumour
Nature Reviews Urology (2024)
-
Imprinting disorders
Nature Reviews Disease Primers (2023)
-
Genetic and epigenetic features of bilateral Wilms tumor predisposition in patients from the Children’s Oncology Group AREN18B5-Q
Nature Communications (2023)
-
Tumorprädispositionssyndrome und Nephroblastom
Die Radiologie (2022)
-
Clinically-relevant postzygotic mosaicism in parents and children with developmental disorders in trio exome sequencing data
Nature Communications (2019)