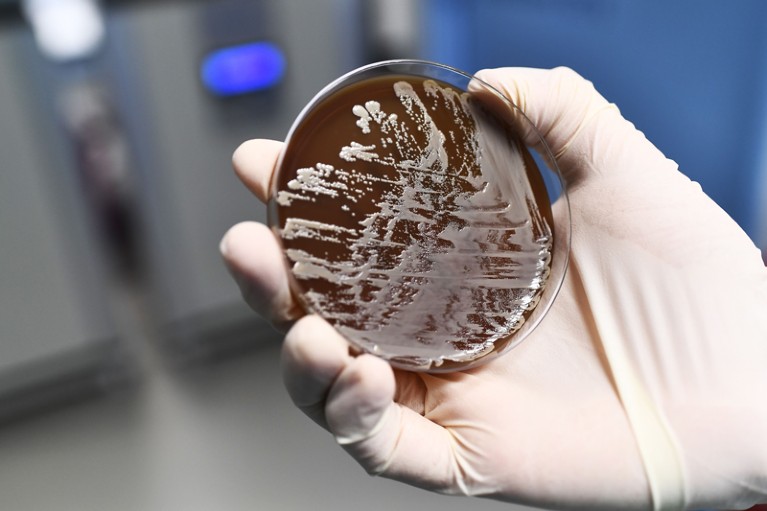
As the threat from antibiotic resistance grows, experts have suggested some ways forward.Credit: Anne-Christine Poujoulat/AFP/Getty
Accounts differ on exactly how long Alexander Fleming was away from his London laboratory on holiday before he discovered the impact of penicillin. It might have been two weeks; it might have been four. But we do know it was long enough for the famous stray Penicillium mould to develop and wipe out colonies of bacteria on his discarded petri plates. Some things — the growth of microbial life among them — simply can’t be rushed.
Today, that creates a problem. Getting an infectious agent identified and the best antibiotic prescribed within a typical eight-hour working day is close to impossible. It generally takes several days, and sometimes more. And the longer it takes to start treatment, the more time the infection has to take hold.
It is understandable, then, that physicians would rather not wait. One way to speed things up is to take a best guess at the diagnosis and throw a broad-spectrum antibiotic at it (one that works against a number of bacteria). That approach can save lives, but it brings its own problems. The World Health Organization predicts that, without urgent action, the spread of antibiotic-resistant bacteria will lead to a resurgence in deaths from minor injuries and previously benign infections.
A good place to focus urgent action is the delay between a person becoming ill and receiving effective treatment. Shortening that time would reduce unnecessary prescribing, minimize the spread of resistance and, most importantly, give people the best chance of recovering.
Speeding up that process will require major advances in what microbiologists call antimicrobial susceptibility testing. Conventionally, this testing can be broken down into two steps. First, laboratories culture and identify the infectious agent. And second, they show which antibiotic is most likely to be effective.
In theory, the technology exists to hasten both of those stages. Advances in genomics mean that rapid DNA sequencing can identify bacteria within hours. It can also quickly and accurately detect antibiotic resistance and susceptibility for tuberculosis (The CRyPTIC Consortium and the 100,000 Genomes Project N. Engl. J. Med. 379, 1403–1415; 2018).
Developed further, this and other technologies could deliver results within an hour of a sample being taken from a patient. That would be a game-changer — but it has not yet happened. Why?
Talk to the people involved — physicians, researchers, testing labs, regulators and commercial firms among them — and all will offer their own reasons. One result of such discussion is published this week— a consensus statement that seeks to find common ground on defining the obstacles and recommending ways to overcome them (A. van Belkum et al. Nature Rev. Microbiol. https://doi.org/10.1038/s41579-018-0098-9; 2018).
The statement is signed by specialists who represent organizations from the French diagnostics company bioMérieux to the European Commission’s Joint Programming Initiative on Antimicrobial Resistance, which coordinates national research programmes. It’s a landmark effort and a triumph of cooperation and communication for the community. Now the hard work really begins: addressing the issues in the roadmap.
One challenge is regulation. Regions and countries tend to have their own requirements and processes to approve the marketing of new diagnostics and to validate them after market release, so developers have a gargantuan task to meet all the different demands. Here, communication, harmonization and standardization are needed: policymakers need to sit together and agree on a common set of rules.
Another issue is how institutions collect and compile information about resistant strains and the usefulness of antibiotics. If the information were made available in real time and more samples analysed, the statement says, then a “smart antibiogram” could be developed to guide treatment. That could bring down the time to treatment — and time saved is lives saved.
Perhaps the biggest obstacle is cost. Current diagnostic tests might be slow, but they are cheap. Modern diagnostics tend to be more expensive to develop and use. That could change: rising antibiotic resistance could shake up clinical practice and the health-care market so fundamentally that most of today’s treatments and diagnostics become obsolete. In that case, what we now regard as too much of an investment will seem comparatively cheap. The world must not wait for such dire circumstances. Policymakers repeatedly say that action is needed on antibiotic resistance. The community has responded with a way forward.

 Four stories of antibacterial breakthroughs
Four stories of antibacterial breakthroughs
 Antibacterial drug discovery in the resistance era
Antibacterial drug discovery in the resistance era


