Abstract
Innate immune signalling has an essential role in inflammation, and the dysregulation of signalling components of this pathway is increasingly being recognised as an important mediator in cancer initiation and progression. In some malignancies, dysregulation of inflammatory toll-like receptor (TLR) and interleukin-1 receptor (IL1R) signalling is typified by increased NF-κB activity, and it occurs through somatic mutations, chromosomal deletions, and/or transcriptional deregulation. Interleukin-1 receptor-associated kinase (IRAK) family members are mediators of TLR/IL1R superfamily signalling, and mounting evidence implicates these kinases as viable cancer targets. Although there have been previous efforts aimed at the development of IRAK kinase inhibitors, this is currently an area of renewed interest for cancer drug development.
Similar content being viewed by others
IRAK family kinases
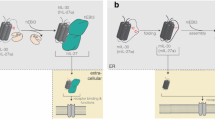
The interleukin-1 receptor-associated kinases (IRAKs) are key mediators of toll-like receptor (TLR) and interleukin-1 receptor (IL1R) signalling processes. TLR/IL1R-mediated signalling controls diverse cellular processes including inflammation, apoptosis, and cellular differentiation. TLR/IL1R signalling is achieved through differential recruitment of adaptor molecules such as MyD88, Mal/TIRAP, TRIF, and TRAM. These adaptors function in the subsequent recruitment and activation of IRAK family kinases. Four IRAK genes exist in the human genome (IRAK1, IRAK2, IRAK3, and IRAK4), and studies, particularly with transgenic mice, have revealed distinct, non-redundant biological roles. All IRAK proteins share a similar domain structure, including an N-terminal death domain important for dimerisation and MyD88 interaction, a proline/serine/threonine-rich (ProST) domain, and a kinase and/or pseudokinase domain. However, only IRAK1, IRAK2, and IRAK3 contain a C-terminal domain, which is required for TRAF6 activation (Figure 1). Further biochemical characterisation has revealed differential posttranslational modification, cellular localisation, and regulation of IRAK family members. Although IRAKs are categorised as serine/threonine protein kinases, only IRAK1 and IRAK4 exhibit kinase activity. Human epidemiological studies, as well as transgenic mouse models, have linked genetic variations in IRAK genes to a collection of diverse diseases including cancer.
Domain architecture, size, and expression pattern of human IRAK family protein kinases. IRAK proteins consist of an amino-terminal death domain, a ProST domain, a kinase or pseudokinase domain, and C-terminus domain important for TRAF6 interaction.
IRAK1
IRAK1, the first member of the IRAK family to be discovered, was identified through biochemical isolation of an IL1-dependent kinase activity which co-immunoprecipitated with the IL1R. Upon IL1R/TLR ligand binding, MyD88 is rapidly recruited to the receptor through its toll/interleukin-1 receptor (TIR) domain. IRAK1 interacts with MyD88 via its death-domain and undergoes subsequent activation. The activation and phosphorylation of IRAK1 is a multistep process, but initially requires a critical threonine at position 209 (T209), as mutation of this residue completely disrupts IRAK1 kinase activity (Kollewe et al, 2004). IRAK1 is subsequently phosphorylated at residues within its activation loop and ProST region. MyD88 only binds non-phosphorylated IRAK1, and upon phosphorylation IRAK1 is released from the receptor complex to bind the E3 ubiquitin ligase, TRAF6, to activate NF-κB.
IRAK1 is the substrate of additional covalent modifications, including ubiquitination and sumoylation, which impact function and localisation. After becoming phosphorylated, IRAK1 can undergo K48-linked ubiquitination and subsequent rapid degradation via the 26S proteasome (Yamin and Miller, 1997). Additionally, IRAK1 can be modified through the addition of K63-linked polyubiquitin chains. This is thought to be an activating mark, as mutation of K63-linked ubiquitin sites on IRAK1 prevents NEMO binding and NF-κB activation (Conze et al, 2008). The proportion of higher molecular weight modified forms of IRAK1 increases upon LPS stimulation and is thought to result from ubiquitination, in addition to hyperphosphorylation of the ProST domain (Figure 1). IRAK1 localises to both the cytoplasm and nucleus; however, the modified, higher-molecular weight form is predominantly found in the nuclear fraction, and sumoylation of IRAK1 is necessary for nuclear entry (Su et al, 2007). Thus, posttranslational modifications of IRAK1 are necessary for regulating diverse functions, including nuclear trafficking, degradation, and kinase activation.
IRAK1-deficent mice have been used to interrogate the role of IRAK1 in IL1/TLR-mediated activation of NF-κB and MAPK signalling pathways. IRAK1−/− macrophages display decreased LPS-induced IKKβ activation and NF-κB DNA binding. Additionally, primary mouse embryonic fibroblasts isolated from IRAK1−/− mice displayed reduced IL1-induced p38 and JNK activation. These studies have revealed a critical role for IRAK1 in nuclear STAT3 activation and subsequent IL-10 gene expression. This is of clinical relevance because elevation of IL-10 levels is a common phenomenon among atherosclerosis patients, and interestingly, this coincides with IRAK1 nuclear localisation (Huang et al, 2004).
Depending on the cellular context, a kinase-dead IRAK1 mutant can rescue the loss of NF-κB activation observed in IRAK1-deficient cells. Both wild-type and kinase-dead IRAK1 are capable of activating NF-κB transcriptional activity (Maschera et al, 1999). Recently, a catalytically inactive IRAK1 D359A mutant mouse was reported (Pauls et al, 2013). Bone-marrow-derived monocytes from this mouse did not exhibit impairment in the activation of the canonical IKK complex, MAPK activation, or the production of IL6, IL10, and TNF-α mRNA. However, plasmacytoid dendritic cells from IRAK1 D359A mice exhibit delayed TLR7- and TLR9-induced IFN-α and IFN-β mRNA production. Thus, the catalytic requirement of IRAK1 appears to be context and cell-type specific.
IRAK2
IRAK2 plays a critical role in proximal TLR signalling and in the activation of NF-κB. IRAK2 is a necessary component of a multimeric helical MyD88-IRAK4-IRAK2 signalling complex that is formed through death-domain interactions downstream of TLR/IL1R activation (Figure 2) (Lin et al, 2010). Unlike the other IRAK-family members, IRAK2 is capable of interacting with the TLR3 signalling adaptor Mal/TIRAP, and is recruited to TLR3 through death-domain interactions. Along with IRAK1, IRAK2 is also important in the formation of polyubiquitin chains associated with TRAF6 signalling. Interestingly, IRAK2-deficient mice are more resistant to LPS and CpG-induced septic shock than IRAK1-deficient animals. Although IRAK1 and IRAK2 function redundantly in initial TLR signalling responses, IRAK2 plays a critical role in late-phase TLR signalling, namely in cytokine production (Kawagoe et al, 2008). Mouse knock-in studies have established that the IRAK2-TRAF6 interaction is rate-limiting for the late phase cytokine production in bone-marrow-derived monocytes and plasmacytoid dendritic cells, and that this interaction is critical to sustaining NF-κB signalling during prolonged activation of MyD88 signalling (Pauls et al, 2013).
IRAK family signalling network. Activated TLR/IL1R results in the assembly of the Myddosome, a multiprotein complex composed of MyD88, IRAK4, and IRAK2, which activates the serine/threonine kinase, IRAK1, through IRAK4-dependent phosphorylation. IRAK1 associates with an E3 ubiquitin ligase, TRAF6, which mediates the activation of the IKK complex, resulting in transcription of NF-κB target genes. The IRAK1/TRAF6 complex can also activate JNK and p38 signalling through assembly of a catalytically active TAB2-TAB3-TAK1 complex. In monocytes and macrophages, IRAK3 negatively regulates IRAK signalling through suppression of IRAK4 and IRAK1 activation. TRAF6 also regulates other proteins (as indicated by ‘?’) that may contribute to immune signalling and malignancies.
IRAK3 (IRAK-M)
Human IRAK3 gene expression is restricted to monocytes and macrophages. Although initial studies reported that IRAK3 could activate NF-κB, more recent literature has demonstrated the powerful negative regulatory role IRAK3 plays within the context of TLR signalling (Figure 2). IRAK3−/− macrophages exhibit elevated levels of inflammatory cytokines upon TLR ligand challenge, and IRAK3−/− mice show a hyper-inflammatory response to bacterial infection (Kobayashi et al, 2002). Additionally, endotoxin tolerance is significantly reduced in IRAK3−/− cells; thus, IRAK3 regulates TLR signalling and innate immune homeostasis. At the molecular level, IRAK3 exerts negative regulatory effects through preventing (i) the dissociation of IRAK1 and IRAK4 from MyD88, and (ii) the formation of the IRAK1-TRAF6 signalling complex (Kobayashi et al, 2002). Recently, IRAK3 was identified as a regulator of haematopoiesis in a functional zebrafish screen, and thus could potentially play a role in HSC self-renewal and differentiation (Eckfeldt et al, 2005).
IRAK4
IRAK4 is the closest homologue to the Drosophila Pelle protein. As the only IRAK member in the fly, Pelle is a signalling mediator of the Toll-Dorsal pathway during embryonic development. Following the engagement of TLR agonists or IL1, IRAK4 is recruited to the protein adaptor MyD88 through death-domain interactions (Suzuki et al, 2002). IRAK4, IRAK2, and MyD88 can form a large oligomeric left-handed helical signalling complex, termed the Myddosome (Figure 2) (Lin et al, 2010). The assembly of this higher-order complex leads to the IRAK4-mediated recruitment and phosphorylation of IRAK1. Interestingly, overexpression of IRAK4 mutants containing truncations within the N-terminal kinase domain can suppress IL1-inducible recruitment of wild-type IRAK4 to the IL1R complex, and prevent association with IRAK1, whereas enabling sequestration of MyD88 (Medvedev et al, 2005). In contrast to IRAK1-deficient mice, IRAK4−/− animals display a severe impairment in inflammatory cytokine expression and NF-κB activation upon challenge with TLR ligands or IL1, and are completely resistant to LPS-mediated septic shock (Suzuki et al, 2002). Additionally, IL1-induced JNK and p38 activation is completely defective in cells lacking IRAK4.
Studies examining kinase-dead IRAK4 knock-in mice demonstrated the requirement of kinase activity for certain IRAK4-dependent activities. Similar to IRAK4-deficient animals, IRAK4 kinase-dead mice are resistant to TLR-induced septic shock (Koziczak-Holbro et al, 2008). Perhaps surprisingly, macrophages from IRAK4 kinase-dead mice were capable of activating NF-κB through IL1, TLR2, TLR4, and TLR7, suggesting kinase-dispensable activities of IRAK4. Interestingly, although IL1/TLR-induced NF-κB activation was not greatly impaired in IRAK4 kinase-dead knock-in mice, there was severe impairment of IL1/TLR-induced cytokine production and JNK activation (Koziczak-Holbro et al, 2008). Further studies examining IRAK4-deficient human cells reconstituted with kinase-dead IRAK4 have revealed redundancies in IRAK4 kinase activity. In human fibroblasts, kinase-dead IRAK4 was capable of restoring IL1-induced NF-κB, JNK activation, and IL8 gene expression to a similar degree as IRAK4 (Qin et al, 2004). Thus, there may be context-specific redundancies between IRAK kinase activities.
Human IRAK4 deficiency has been described as an autosomal recessive disorder (Day et al, 2004). As a result of IRAK4 deficiency, patients suffer from recurrent infections caused by Gram-positive pyogenic bacteria such as Streptococcus pneumoniae. Blood cells from these patients fail to generate pro-inflammatory cytokines upon stimulation with IL1β, IL18, and TLR agonists. Thus, the immunological phenotype of IRAK4−/− mice is consistent with that of IRAK4-deficient patients.
Dysregulated IRAK signalling in cancer
The link between inflammation and cancer dates back to 1863, when Rudolf Virchow first observed leukocyte-infiltrates in tumour tissues. It is now widely accepted that inflammation contributes to cancer pathogenesis. Moreover, it is evident that an inflammatory microenvironment is an important characteristic of human tumours. Not surprisingly, many environmental cancer risk factors are associated with chronic inflammation. For example, induction of inflammation by bacterial and viral infections increases cancer risk (Ferreri et al, 2009). Similarly, tobacco smoke and obesity are tumour-promoting factors by triggering chronic inflammatory signalling (Park et al, 2010; Takahashi et al, 2010). IL1β, a pro-inflammatory cytokine and an activator of IRAK signalling, plays a direct role in tumour cell growth, angiogenesis, invasion, drug resistance, and metastasis (Vidal-Vanaclocha et al, 2000; Carmi et al, 2013). Similarly, depending on the tumour cell context, TLRs participate in a myriad of protumour responses (Table 1). Thus, as necessary mediators of IL1R and TLR-inflammatory signalling, the IRAK-family kinases represent rational cancer drug targets.
Lymphoid Malignancies
Cancer-specific dependencies on IRAK signalling became evident following the discovery of oncogenically active MyD88 mutations in activated B-cell-like diffuse large B-cell lymphoma (Table 1) (ABC DLBCL) (Ngo et al, 2011). Notably, in a large set of tumour biopsies, sequence analysis of the MyD88 coding region revealed that 29% of ABC DLBCL tumours harboured the L265P single amino acid substitution within the MyD88 TIR domain. This mutation is absent in other DLBCL subtypes, including germinal centre B-cell-like DLBCL and Burkitt’s lymphoma. The L265P MyD88 mutant promotes cell survival through spontaneous assembly of a protein-signalling complex containing IRAK1 and IRAK4, leading to IRAK4 kinase activation, IRAK1 phosphorylation, and activated JAK-STAT and NF-κB signalling. Strikingly, in ABC DLBCL cell lines harbouring L265P MyD88 mutations, RNAi-mediated knockdown of MyD88, IRAK4, or IRAK1 eliminated NF-κB activation and induced rapid apoptosis. Thus, in this context, sustained MyD88-IRAK signalling is necessary for ABC DLBCL pathogenesis and tumour cell survival. In shRNA rescue experiments, IRAK4 kinase activity was necessary to prevent RNAi-induced apoptosis; conversely, kinase-dead IRAK1 was capable of rescuing RNAi-induced apoptosis. Thus, in ABC DLBCL, IRAK1, and IRAK4 have divergent kinase activities, and interestingly, IRAK1 appears to possess non-catalytic pro-survival activity. Ultimately, this study supports the development of IRAK4-selective kinase inhibitors for the treatment of tumours harbouring oncogenic MyD88 mutations.
In a related lymphocytic haematological malignancy, Waldenström’s Macroglobulinaemia (WM), suppression of IRAK signalling appears to be a promising therapeutic approach (Table 1). The common somatic L265P mutation of MyD88 is even more prevalent in WM, occurring in 91% of patients. Treon and colleagues (Treon et al, 2012) first reported IRAK1/4 kinase inhibitor-mediated apoptosis of primary MyD88 L265P-expressing cells derived from WM patient marrow (Yang et al, 2013). This study was the first to uncover Bruton’s tyrosine kinase (BTK) as an important binding partner of MyD88 L265P, and showed that the L265P mutant activates BTK in WM. Because BTK and IRAK signalling converge on NF-κB, the authors hypothesised that combined BTK and IRAK inhibition would provide a synergistic apoptotic effect. Indeed, potent synergistic WM cell killing was observed when combining the prototype BTK inhibitor, ibrutinib, with a small-molecule inhibitor of IRAK1/4. However, unlike in ABC DLBCL, the relative contribution from either IRAK1 or IRAK4 to WM cell survival is still unclear and remains an important question for future studies. Thus far, data collected from ongoing phase II trials with ibrutinib point towards very promising activity in WM (Akinleye et al, 2013). Combining ibrutinib with an IRAK kinase inhibitor would therefore be a rational approach and may provide a synergistic efficacy profile for WM patients.
Myeloid malignancies
Activation and overexpression of IRAK1 in myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) has been recently reported (Table 1) (Rhyasen et al, 2013). IRAK1 is a validated target of miR-146a, a microRNA that contributes to the pathogenesis of MDS patients harbouring a common cytogenetic abnormality, del(5q) (Starczynowski et al, 2010). However, IRAK1 activation appears to be a more general feature of MDS and AML and is readily observed in non-del(5q) patients, suggesting alternate mechanisms of IRAK1 dysregulation. One possibility explaining IRAK1 activation in non-del(5q) MDS patients is through the reported overexpression of TLR1/2/6 (Wei et al, 2013). Marrow cells from MDS patients harbouring mutations within TLR2 exhibit markedly increased pIRAK1 levels (Wei et al, 2013). Our research examined a small-molecule inhibitor of IRAK1/4 (IRAK-Inh) in MDS. The pharmacological effects of IRAK-Inh included a dose-dependent effect on cell growth, apoptosis, and progenitor cell function. Additional validation was carried out through RNAi-mediated knockdown of IRAK1 in MDS/AML cells, similarly resulting in apoptosis, and impaired MDS/AML-progenitor cell function. Interestingly small-molecule inhibition of IRAK1/4 was ineffective against primary AML cells, as well as the promyelocytic leukaemia HL60 cell line. A potential explanation was offered through integrated gene-expression analysis, which uncovered compensatory upregulation of BCL2 mRNA in IRAK-Inh-treated AML cells. A combined BCL2/IRAK inhibitory strategy was utilised to examine AML cell dependency on BCL2 activity within the context of inhibited IRAK1. The combination of the BH3 mimetic, ABT263, and IRAK-Inh elicited powerful collaborative cytotoxicity against all MDS and IRAK-Inh-refractory AML cells tested, both in primary cell culture and tumour xenograft models. Furthermore, in vitro studies of this drug combination against normal CD34+ cells exhibited a differential response when compared against their malignant counterparts, thus suggesting a reasonable therapeutic window. It remains to be seen whether this drug combination will prove effective in other tumour types, but these findings implicate IRAK1 as a druggable target in MDS and AML.
A proximal IRAK signalling adaptor, IL1R-accessory protein (IL1RAP), is overexpressed on the surface of HSCs of AML and high-risk MDS patients and serves as an independent prognostic indicator in normal karyotype-AML (Barreyro et al, 2012). RNAi-mediated knockdown of IL1RAP decreases clonogenicity and increases AML cell death. More recently, selective killing of AML HSCs, and chronic myeloid leukaemia cells, has been achieved through antibody-based targeting of IL1RAP (Askmyr et al, 2013). Collectively, these studies implicate IL1RAP as a promising molecular target for MDS/AML and chronic myeloid leukaemia.
Tumour-infiltrating immune cells, particularly monocytes, exhibit characteristic immune tolerance after first exposure to cancer cells. This cancer-induced immune tolerance stems from the downregulation of pro-inflammatory genes. Interestingly, during this phenomenon, monocytes isolated from chronic myeloid leukaemia patients exhibit upregulated IRAK3 expression (del Fresno et al, 2005). Thus, IRAK3 becomes expressed in immune cells during cancer tolerance, acting to rapidly deactivate anti-tumour inflammatory responses through suppressing the activation of IRAK1/2/4 and thus dampening NF-κB activity. Increased monocytic IRAK3 expression is mediated by tumour-secreted hyaluronan through engagement of monocyte/macrophage-expressed CD44 and TLR4. Because IRAK3 expression is limited to monocytes and macrophages, small-molecule activators of IRAK3 may prove useful in activating host anti-tumour responses. Indeed, IRAK3-deficient mice are resistant to tumour cell growth and provide experimental proof-of-concept for this approach (Xie et al, 2007).
Solid tumours
Although there are fewer reports on IRAK-family kinases in the context of solid tumours, there is nascent evidence implicating IRAK1/4 signalling in melanoma (Srivastava et al, 2012). IRAK1 and IRAK4 are overexpressed and activated in melanoma cell lines, and as measured through immunohistochemistry, pIRAK4 is highly expressed in primary melanoma biopsies (Table 1). A small-molecule IRAK1/4 inhibitor was effective in sensitising melanoma cell lines to chemotherapy, and combined vinblastine plus IRAK1/4 inhibition has shown significant survival benefit, as compared with monotherapy, in a melanoma xenograft model. Thus, understanding the mechanism by which IRAK inhibition sensitises melanoma cells to chemotherapies may lead to the discovery of more effective targeted melanoma therapies.
Pre-clinical discovery and development of IRAK kinase inhibitors
Powers and colleagues (Powers et al, 2006) first reported the discovery and structure–activity relationships of small-molecule inhibitors of IRAK-family kinases (Supplementary Table 1). High-throughput screening efforts resulted in the identification of a novel series of N-acyl-2-aminobenzimidazoles that achieve sub-micromolar IRAK4 and IRAK1 half-maximal inhibitory concentrations (IC50). The same group reported crystal structures of IRAK4 kinase in complex with a similar N-acyl-2-aminobenzimidazole (Wang et al, 2006). The structures revealed a unique tyrosine gatekeeper residue, which creates an unusual ATP-binding site. Interestingly, when compared against the entire kinome, the tyrosine gatekeeper residue is exclusive to IRAK family of kinases. Because the gatekeeper residue is critical for kinase/small-molecule selectivity, the design of highly selective IRAK kinase inhibitors could be tractable through this unique molecular feature. Unlike IRAK4, the crystal structure of IRAK1 remains to be solved. The IRAK1 crystal structure will likely prove useful in the synthesis of improved IRAK1- and IRAK4-selective kinase inhibitors.
A series of additional efforts uncovered increasingly potent compound classes, including imidazo[1,2-a]pyridino-pyridines and benzimidazole-pyridines as low nanomolar IC50 IRAK4 and IRAK1 inhibitors (Supplementary Table 1) (Buckley et al, 2008a,2008b,2008c). However, there remains a paucity of published literature surrounding the effects of these compounds against disease models, including cancer.
More recent selective IRAK4 inhibitors have utilised high-energy hydration sites to design three selective nanomolar IRAK4 ligands (Supplementary Table 1) (Robinson et al, 2010). The Ki of ND-346, ND-2110, and ND2158 for IRAK4 are 50, 7.5, and 1 nM, respectively (Chaudhary et al, 2012). Each candidate small molecule has demonstrated favourable pharmacokinetic/pharmacodynamic characteristics and efficacy in several murine autoimmune disease models (Chaudhary et al, 2012). The gain-of-function L265P mutation in MyD88 found in 29% of patients with ABC DLBCL served as rationale to pursue the combination of selective IRAK4 inhibitor (ND-2158) with leading BTK, SYK, and PI3Kδ inhibitors. Indeed, ND-2158 synergistically combines with either BTK, SYK, or PI3Kδ inhibitors to inhibit proliferation of the OCI-LY10 ABC DLBL cell line (Chaudhary et al, 2013). Ultimately, these approaches will likely bring a selective IRAK4 inhibitor into clinical development for lymphoma, wherein the clinical development strategy will revolve around combination trials with active FDA-approved agents.
With the recent approval of the covalent BTK inhibitor, ibrutinib, there has been a renewed interest in the development of irreversible kinase inhibitors. These small-molecule inhibitors form a covalent bond with a nucleophilic cysteine residue within the kinase ATP-binding pocket, thus irreversibly inactivating the target kinase. An effort undertaken by Gray and colleagues (Zhang et al, 2012), aimed at developing irreversible covalent inhibitors of JNK1/2/3, serendipitously led to the discovery of a covalent kinase inhibitor of IRAK1. KINOMEscan profiling revealed that the JNK-IN-7 phenylaminopyrimidine tool compound not only interacts with JNK1/2/3, but also IRAK1, exhibiting an enzymatic IC50 of ∼10 nM. Sequence alignment and subsequent examination of the IRAK4 crystal structure revealed cysteine-276 as the candidate reactive residue in IRAK1. Biochemical profiling demonstrated that JNK-7-IN was also capable of inhibiting Pellino 1 E3 ligase activity, suggesting that IRAK1 is a bona fide intracellular target. Thus, JNK-IN-7 may serve as a useful pharmacological probe to examine IRAK1-dependent cellular functions, and as a lead compound for the further development of increasingly potent covalent IRAK1 kinase inhibitors.
Outside the realm of rationally designed small-molecule inhibitors, there is perhaps only a single report of a natural product with activity against IRAK family kinases. Ginsenoside Rb1 and its metabolite, compound K, both derived from the root of Panax ginseng, a widely used herbal medicine, selectively inhibit IRAK1, but not IRAK2 or IRAK4 activation (Joh et al, 2011). Compound K has been extensively studied and has exhibited anti-inflammatory, anti-tumour, and anti-diabetic effects; however, prior to this study, the molecular mechanisms of this metabolite remained undefined. Orally administered ginsenoside Rb1 and compound K were capable of improving disease symptoms through IRAK1 inhibition, which resulted in reducing the expression of TNF-α, IL1β, IL6, and NO in a TNBS-induced colitis murine model. Both metabolites inhibited LPS-induced IRAK1 phosphorylation, IKKβ phosphorylation, NF-κB activation, and ERK and p38 activation. Biochemical profiling is necessary to determine whether either metabolite can directly inhibit the kinase activity of IRAK1. However, if direct enzymatic inhibition is confirmed, these natural metabolites may eventually provide a novel scaffold for the rational design of small-molecule IRAK1 kinase inhibitors.
References
Akinleye A, Furqan M, Adekunle O (2013) Ibrutinib and indolent B-cell lymphomas. Clin Lymphoma Myeloma Leuk 14: 253–260.
Askmyr M, Agerstam H, Hansen N, Gordon S, Arvanitakis A, Rissler M, Juliusson G, Richter J, Jaras M, Fioretos T (2013) Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood 121: 3709–3713.
Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A, Boultwood J, Paietta E, Ketterling RP, Cripe L, Fernandez HF, Greenberg PL, Tallman MS, Steidl C, Mitsiades CS, Verma A, Steidl U (2012) Overexpression of Il-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood 120: 1290–1298.
Buckley GM, Ceska TA, Fraser JL, Gowers L, Groom CR, Higueruelo AP, Jenkins K, Mack SR, Morgan T, Parry DM, Pitt WR, Rausch O, Richard MD, Sabin V (2008a) Irak-4 inhibitors. Part II: A structure-based assessment of imidazo[1,2-A]pyridine binding. Bioorg Med Chem Lett 18: 3291–3295.
Buckley GM, Fosbeary R, Fraser JL, Gowers L, Higueruelo AP, James LA, Jenkins K, Mack SR, Morgan T, Parry DM, Pitt WR, Rausch O, Richard MD, Sabin V (2008b) Irak-4 inhibitors. Part III: A series of imidazo[1,2-A]pyridines. Bioorg Med Chem Lett 18: 3656–3660.
Buckley GM, Gowers L, Higueruelo AP, Jenkins K, Mack SR, Morgan T, Parry DM, Pitt WR, Rausch O, Richard MD, Sabin V, Fraser JL (2008c) Irak-4 inhibitors. Part 1: a series of amides. Bioorg Med Chem Lett 18: 3211–3214.
Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, Voronov E (2013) The role of Il-1beta in the early tumor cell-induced angiogenic response. J Immunol 190: 3500–3509.
Chaudhary D, Robinson S, Masse CE, Wessel MD, Watts S, Greenwood J, Shelley M, Brewer M, Harriman G, Frye LL, Wester RT, Kapeller R, Romero D (2012) Identification of highly potent and selective interleukin-1 receptor- associated kinase 4 inhibitors for the treatment of rheumatic diseases. [abstract]. Arthritis Rheum 64 (Suppl 10): 1062.
Chaudhary D, Romero Dl WN, Greenwood Robinson S Jr, Shelley M, Morin M, Kapeller R, Westlin WF (2013) Synergistic blockade of activated B-cell-like DLBCL proliferation with a selective inhibitor of IRAK4 in combination with inhibition of the B-cell receptor signaling network [abstract]. Blood 122.
Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD (2008) Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated Nf-KappaB activation. Mol Cell Biol 28: 3538–3547.
Day N, Tangsinmankong N, Ochs H, Rucker R, Picard C, Casanova JL, Haraguchi S, Good R (2004) Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J Pediatr 144: 524–526.
del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, Escoll P, Baos R, Caveda L, Garcia F, Arnalich F, Lopez-Collazo E (2005) Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol 174: 3032–3040.
Eckfeldt CE, Mendenhall EM, Flynn CM, Wang TF, Pickart MA, Grindle SM, Ekker SC, Verfaillie CM (2005) Functional analysis of human hematopoietic stem cell gene expression using zebrafish. Plos Biol 3: E254.
Ferreri AJ, Ernberg I, Copie-Bergman C (2009) Infectious agents and lymphoma development: molecular and clinical aspects. J Intern Med 265: 421–438.
Huang Y, Li T, Sane DC, Li L (2004) Irak1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J Biol Chem 279: 51697–51703.
Joh EH, Lee IA, Jung IH, Kim DH (2011) Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation—the key step of inflammation. Biochem Pharmacol 82: 278–286.
Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S (2008) Sequential control of toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol 9: 684–691.
Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA (2002) IRAK-M is a negative regulator of toll-like receptor signaling. Cell 110: 191–202.
Kollewe C, Mackensen AC, Neumann D, Knop J, Cao P, Li S, Wesche H, Martin MU (2004) Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J Biol Chem 279: 5227–5236.
Koziczak-Holbro M, Gluck A, Tschopp C, Mathison JC, Gram H (2008) IRAK-4 kinase activity-dependent and -independent regulation of lipopolysaccharide-inducible genes. Eur J Immunol 38: 788–796.
Lin SC, Lo YC, Wu H (2010) Helical assembly in the Myd88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465: 885–890.
Maschera B, Ray K, Burns K, Volpe F (1999) Overexpression of an enzymically inactive interleukin-1-receptor-associated kinase activates nuclear factor-kappaB. Biochem J 339 (Pt 2): 227–231.
Medvedev AE, Thomas K, Awomoyi A, Kuhns DB, Gallin JI, Li X, Vogel SN (2005) Cutting edge: expression of IL-1 receptor-associated kinase-4 (IRAK-4) proteins with mutations identified in a patient with recurrent bacterial infections alters normal IRAK-4 interaction with components of the IL-1 receptor complex. J Immunol 174: 6587–6591.
Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL, Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Staudt LM (2011) Oncogenically active Myd88 mutations in human lymphoma. Nature 470: 115–119.
Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140: 197–208.
Pauls E, Nanda SK, Smith H, Toth R, Arthur JS, Cohen P (2013) Two phases of inflammatory mediator production defined by the study of IRAK2 and IRAK1 knock-in mice. J Immunol 191: 2717–2730.
Powers JP, Li S, Jaen JC, Liu J, Walker NP, Wang Z, Wesche H (2006) Discovery and initial sar of inhibitors of interleukin-1 receptor-associated kinase-4. Bioorg Med Chem Lett 16: 2842–2845.
Qin J, Jiang Z, Qian Y, Casanova JL, Li X (2004) IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J Biol Chem 279: 26748–26753.
Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, Mathews L, Ferrer M, Southall N, Guha R, Keller J, Thomas C, Beverly LJ, Cortelezzi A, Oliva EN, Cuzzola M, Maciejewski JP, Mulloy JC, Starczynowski DT (2013) Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell 24: 90–104.
Robinson DD, Sherman W, Farid R (2010) Understanding kinase selectivity through energetic analysis of binding site waters. ChemMedChem 5: 618–627.
Srivastava R, Geng D, Liu Y, Zheng L, Li Z, Joseph MA, Mckenna C, Bansal N, Ochoa A, Davila E (2012) Augmentation of therapeutic responses in melanoma by inhibition of IRAK-1,-4. Cancer Res 72: 6209–6216.
Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, Hirst M, Hogge D, Marra M, Wells RA, Buckstein R, Lam W, Humphries RK, Karsan A (2010) Identification of mir-145 and mir-146a as mediators of the 5q- syndrome phenotype. Nat Med 16: 49–58.
Su J, Richter K, Zhang C, Gu Q, Li L (2007) Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol 44: 900–905.
Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC (2002) Severe impairment of interleukin-1 and toll-like receptor signalling in mice lacking IRAK-4. Nature 416: 750–756.
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M (2010) Tobacco smoke promotes lung tumorigenesis by triggering Ikkbeta- and JNK1-dependent inflammation. Cancer Cell 17: 89–97.
Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL, Laramie JM, Skifter DA, Lincoln SE, Hunter ZR (2012) Myd88 L265p somatic mutation in Waldenstrom's Macroglobulinemia. N Engl J Med 367: 826–833.
Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, Carrascal T, Walsh P, Reznikov LL, Kim SH, Novick D, Rubinstein M, Dinarello CA (2000) IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci USA 97: 734–739.
Wang Z, Liu J, Sudom A, Ayres M, Li S, Wesche H, Powers JP, Walker NP (2006) Crystal structures of IRAK-4 kinase in complex with inhibitors: a serine/threonine kinase with tyrosine as a gatekeeper. Structure 14: 1835–1844.
Wei Y, Dimicoli S, Bueso-Ramos C, Chen R, Yang H, Neuberg D, Pierce S, Jia Y, Zheng H, Wang H, Wang X, Nguyen M, Wang SA, Ebert B, Bejar R, Levine R, Abdel-Wahab O, Kleppe M, Ganan-Gomez I, Kantarjian H, Garcia-Manero G (2013) Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 27: 1832–1840.
Xie Q, Gan L, Wang J, Wilson I, Li L (2007) Loss of the innate immunity negative regulator IRAK-M leads to enhanced host immune defense against tumor growth. Mol Immunol 44: 3453–3461.
Yamin TT, Miller DK (1997) The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem 272: 21540–21547.
Yang G, Zhou Y, Liu X, Xu L, Cao Y, Manning RJ, Patterson CJ, Buhrlage SJ, Gray N, Tai YT, Anderson KC, Hunter ZR, Treon SP (2013) A mutation in Myd88 (L265p) supports the survival of lymphoplasmacytic cells by activation of bruton tyrosine kinase in Waldenstrom Macroglobulinemia. Blood 122: 1222–1232.
Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, Laughlin JD, Park H, Lograsso PV, Patricelli M, Nomanbhoy TK, Sorger PK, Alessi DR, Gray NS (2012) Discovery of potent and selective covalent inhibitors of JNK. Chem Biol 19: 140–154.
Acknowledgements
DTS is supported by Cincinnati Children’s Hospital Research Foundation, American Society of Hematology (ASH), National Institute of Health (RO1HL111103), Gabrielle’s Angel Foundation, and Department of Defense grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
PowerPoint slides
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rhyasen, G., Starczynowski, D. IRAK signalling in cancer. Br J Cancer 112, 232–237 (2015). https://doi.org/10.1038/bjc.2014.513
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.513
Keywords
This article is cited by
-
Role of innate immunological/inflammatory pathways in myelodysplastic syndromes and AML: a narrative review
Experimental Hematology & Oncology (2023)
-
MicroRNAs as the critical regulators of tyrosine kinase inhibitors resistance in lung tumor cells
Cell Communication and Signaling (2022)
-
IRAK1-regulated IFN-γ signaling induces MDSC to facilitate immune evasion in FGFR1-driven hematological malignancies
Molecular Cancer (2021)
-
Protein phosphatases in TLR signaling
Cell Communication and Signaling (2021)
-
Mechanism of the fungal-like particles in the inhibition of adipogenesis in 3T3-L1 adipocytes
Scientific Reports (2021)