Abstract
The aim of this study was to identify molecular pathways related to antidepressant response. We administered paroxetine to the DBA/2J mice for 28 days. Following the treatment, the mice were grouped into responders or non-responders depending on the time they spent immobile in the forced swim test. Hippocampal metabolomics and proteomics analyses revealed that chronic paroxetine treatment affects glutamate-related metabolite and protein levels differentially in the two groups. We found significant differences in the expression of N-methyl-d-aspartate receptor and neuronal nitric oxide synthase proteins between the two groups, without any significant alterations in the respective transcript levels. In addition, we found that chronic paroxetine treatment altered the levels of proteins associated with the ubiquitin–proteasome system (UPS). The soluble guanylate cyclase-β1, proteasome subunit α type-2 and ubiquitination levels were also affected in peripheral blood mononuclear cells from antidepressant responder and non-responder patients suffering from major depressive disorder. We submit that the glutamatergic system and UPS have a crucial role in the antidepressant treatment response in both mice and humans.
Similar content being viewed by others
Introduction
The high non-response rate to antidepressant treatment is a major problem in clinical practice. Over one-third of major depressive disorder (MDD) patients do not achieve full remission, experiencing symptom recurrence despite antidepressant treatment.1, 2, 3
Although the exact mechanism of antidepressant response remains unknown, numerous studies have tried to identify biological pathways as potential biomarkers for antidepressant treatment response. Serotonin transporter4 and serotonin autoreceptors5 have been found to be critical for the antidepressant response. In addition, alterations and abnormalities of the hypothalamus–pituitary–adrenal axis have been associated with antidepressant treatment outcome.6 The brain-derived neurotrophic factor gene Val66Met polymorphism has also been studied with regard to the antidepressant treatment response and was shown to result in antidepressant treatment resistance in rodents and humans.7, 8 A link between inflammatory cytokines and antidepressant response has been documented. Cerebrospinal fluid interleukin-1, interleukin-6 and tumour necrosis factor-α blood levels in MDD patients were significantly correlated with depression severity.9 High cytokine concentrations have been found in antidepressant treatment-resistant depression patients.10, 11, 12
Fast-acting antidepressant-like agents have also been investigated illuminating novel molecular pathways associated with antidepressant response. Scopolamine,13 an antagonist for muscarinic cholinergic receptors, and ketamine,14 an N-methyl-d-aspartate receptor (NMDAR) antagonist, have a rapid antidepressant-like effect in treatment-resistant depression patients. Low doses of ketamine were found to increase glutamate transmission that mediates brain-derived neurotrophic factor synthesis and synaptogenesis that are important for antidepressant-like action.15
Our previous studies have shown that energy metabolism pathways may be associated with antidepressant response. Acute ketamine treatment resulted in significant energy metabolism changes.16 This was also evidenced by hippocampal glycogen and energy metabolism alterations in chronic paroxetine-treated mice.17
In the current study, paroxetine-treated DBA/2 J mice that were previously shown to respond to chronic paroxetine treatment18 were grouped into responders or non-responders based on their forced swim test (FST) behaviour. Quantitative proteomics and metabolomics analyses were used to identify antidepressant response-associated pathways in brain samples. The relevance of the identified pathways was examined in peripheral blood mononuclear cells (PBMCs) taken from patients diagnosed with MDD.
Materials and methods
Details for mouse brain and blood collection, proteomics and metabolomics analyses, functional enrichment analysis, western blot analysis, quantitative reverse transcription PCR and immunoprecipitation are provided as a part of ‘Supplementary Information’.
Animal housing and husbandry
The experiments were carried out with male DBA/2 J mice (Charles River Laboratories, Chatillon-sur-Chalaronne, France). All the animals were between 8 and 10 weeks old and single housed for at least 1 week before the beginning of the experiments. The mice were kept under normal light and temperature conditions (12 light: 12 dark light cycle, lights on at 1900 h, temperature maintained at 23±2 °C and humidity at 55±5%) with standard bedding and nesting material, in polycarbonate cages (21 × 15 × 14 cm). Water and Altromin 1324 standard mouse chow (Altromin, Lage, Germany) were provided ad libitum. All the procedures were carried out in accordance with the European Communities Council Directive 2010/63/EU and approved by the committee for the Care and Use of Laboratory animals of the Government of Upper Bavaria, Germany.
Drug administration
The mice were treated with vehicle or 5 mg kg−1 paroxetine pills (Paroxetine hydrochloride; Carbone Scientific, London, UK) for 28 days, twice a day. The animals were randomly assigned to the vehicle- or paroxetine-treated experimental group. Vehicle or paroxetine was administered via customized palatable pellets (40 mg PQPellets, Phenoquest, Martinsried, Germany). To control for environmental effects, such as social stress between group-housed male mice, all the mice were single housed during vehicle and paroxetine treatment to ensure accurate dosing. The animals that did not take the pellets properly were excluded from further analysis.
Behavioural testing
Forced swim test
Each mouse was put into a glass beaker (height 24 cm, diameter 13 cm) that had been filled with 21±1 °C water up to a height of 15 cm. This meant that the animals were unable to touch the bottom or escape for 6 min testing period. Immobility time was measured for the entire 6 min test. The amount of time the mouse spent immobile was scored by an experienced observer who was blind to the experimental group.
Patient samples
The PBMCs obtained from 17 participants of the Munich Antidepressant Response Signature study were included for assessing protein expression levels (Supplementary Table 4). All the 17 depressed patients were treated for 4–6 weeks with different types of antidepressant drugs that included tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonergic and noradrenergic reuptake inhibitors, noradrenergic and selective serotonergic antidepressants, noradrenergic reuptake inhibitors and selective serotonin reuptake enhancers. Diagnosis was conducted according to DSM-IV criteria. Depression severity was evaluated using the 21-item Hamilton Depression Rating Scale. Responder patients were classified based on their clinical antidepressant treatment response corresponding to minimal 50% reduction in the Hamilton Depression Rating Scale score between baseline and after 6 weeks of treatment. The Munich Antidepressant Response Signature project was approved by the ethics committee of the Medical Faculty of the Ludwig Maximilians University Munich, Germany (submission number 318/00). Participants included in the study gave oral and written consents.
Hierarchical clustering analysis
Hierarchical clustering analysis is a method to build and split different cluster hierarchies. It has been applied to identify subgroups of cells and animals based on marker protein expression or behavioural parameters.19, 20 Hierarchical clustering analysis was carried out with SPSS (SPSS version 21, IBM SPSS, Chicago, IL, USA) to separate vehicle- or paroxetine-treated mice into subgroups. Based on FST immobility time, three subgroups that include long-time floating, intermediate-time floating and short-time floating groups were stratified for each treatment condition (vehicle or paroxetine).
Statistical analysis
Statistical analyses of the FST behavioural data and covariates were performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). The t-test or one-way analysis of variance were used to assess statistical significance between groups. For the identification of significantly altered metabolites, metabolite peak intensities were median and auto-scaled normalized. Metabolites with missing values, 30 for the hippocampus in all replicates, were excluded from data analysis. Metabolite level differences between paroxetine-treated long-time floating (PLF) and paroxetine-treated short-time floating (PSF) groups were calculated using t-test (P<0.05), followed by false discovery rate correction (q<0.1). Proteomics data were corrected for multiple testing by Benjamini–Hochberg.
Western blot data were also analysed with GraphPad Prism 5. One-way analysis of variance with Tukey’s test was used to evaluate the protein level difference among the groups. Spearman correlation (r) was used to assess the correlation of proteins with FST immobile time of mice. Pearson’s correlation (r) was used to evaluate the correlation of proteins with clinical antidepressant response in depressed patients. Data were expressed as the mean±s.e.m. Statistical data were considered significant at P<0.05. To check normal distribution of data, D’Agostino’s normality test was used. Sample size was determined based on our previous results.17, 21
Results
Identification of protein–metabolite network related to heterogeneous antidepressant response in mice
The mice were treated with paroxetine (5 mg kg−1, twice a day) for 28 days. Three animal subgroups were identified according to FST immobile time (F3,141=132.1, P<0.0001; Figure 1a). To investigate the systemic effect of chronic paroxetine treatment on hippocampal molecular pathways, proteomic analyses were performed of the two extreme groups, PLF and PSF groups, which resulted in significant protein expression differences both in membrane- and cytoplasm-associated fractions (Figure 1b). GluN1 and GluN2B proteins were found to be shared by four enriched functional pathways related to amyotrophic lateral sclerosis, Alzheimer’s disease, Huntington’s disease and long-term potentiation. Based on this finding, we further investigated protein–protein interaction network using our proteomics data. The analysis showed extensive interactions among pathways related to glutamatergic transmission (Figure 1c). Hippocampal metabolite profiling data also showed altered levels of relevant glutamatergic receptor modulators and metabolites that are part of glutamate metabolism pathway (Figure 1d). After false discovery rate correction, several metabolites were at significantly higher levels in PSF compared with the PLF mice (Figure 1e).
Identification of hippocampal protein–metabolite networks associated with the heterogeneous antidepressant treatment response in mice. (a) Paroxetine-treated mice were categorized into paroxetine-treated long-time floating (PLF), paroxetine-treated intermediate-time floating (PIF) and paroxetine-treated short-time floating (PSF) groups based on FST immobile time, n(VEH/PLF/PIF/PSF)=47/9/14/72. (b) Representative proteomics profiles and enriched pathways between the PLF and PSF groups. Proteomics profile differences between the two groups enriched several functional pathways. The shared protein signatures among the pathways were obtained using Venn diagram analysis. In the heat map, colours denote log2 ratio. Numbers in the enriched pathways indicates the number of proteins identified, n=5 per group. (c) Protein interaction network analysis based on proteomics data. In the interaction pathway map, colours denote fold difference between the two groups. Proteins with >20% fold change and adjusted P-value <0.05 were considered significant, n=5 per group. Designations for proteins in the heat map and protein interaction networks are based on Uniprot database. In the network, line thickness indicates the confidence of protein–protein interaction data. Small node represents protein with unknown three-dimensional (3D) structure. Large node represents protein with known or predicted 3D structure. (d) Volcano plot comparing PLF and PSF metabolomes. Metabolites with |log2(fold change)|>0.3 and –log10(P-value)>1.3 were considered significant, corresponding to >20% fold change and 0.05<P, n=5 per group. (e) Glutamate pathway-related metabolite differences between PLF and PSF mice, n=5 per group. Data are expressed as the mean±s.e.m. ***P<0.001 vs VEH, ##P<0.01 vs PLF, ###P<0.001 vs PLF and PIF (one-way analysis of variance (ANOVA) with Tukey’s test, Figure 1a), *q<0.1, **q<0.05, ***q<0.001 (two-tailed t-test followed by false discovery rate (FDR) correction, Figure 1e). FST, forced swim test; VEH, vehicle.
Western blot analyses were performed to assess glutamatergic pathway protein level differences between the PLF and PSF mice (Figure 2 and Supplementary Figure 1). The GluN1, phospho-GluN1 (P-GluN1), phospho-GluN2A (P-GluN2A) and P-GluN2B levels were significantly different between the PLF and PSF groups (GluN1: F3,16=18.27, P<0.0001; P-GluN1: F3,16=3.43, P<0.05; P-GluN2A: F3,16=7.12, P<0.01; P-GluN2B: F3,16=17.30, P<0.0001; Figure 2a). GluN1 and phospho-GluN2B (P-GluN2B) proteins were significantly upregulated in PLF mice, whereas PSF mice did not show NMDAR subunit and phosphorylation level changes when compared with control group. Ca2+/calmodulin-dependent protein kinase 2 (CaMK2), phospho-CaMK2 (P-CaMK2) and glycogen synthase kinase-3β (GSK-3β) protein levels were significantly upregulated in PLF mice, whereas PSF mice exhibited a similar level of NMDAR signalling proteins except phospho-extracellular signal-regulated kinase (P-ERK), which were upregulated compared with the other groups. P-ERK, CaMK2 and GSK-3β levels showed significant differences between the PLF and PSF mice (P-ERK: F2,12=12.44, P<0.01; CaMK2: F2,12=6.62, P<0.05; GSK-3β: F2,12=19.75, P<0.001).
Chronic paroxetine treatment resulted in differential expression of glutamatergic pathway proteins between PLF and PSF groups. (a) NMDAR subunits and signalling protein and phosphorylation levels among the groups. The NMDAR subunit proteins were blotted using membrane-associated fraction. The NMDAR signalling proteins were blotted using cytoplasm-associated fraction, n=5 per group. (b) Glutamate metabolism and synapse/vesicle trafficking pathway protein level differences among the groups, n=5 per group. The proteins were blotted using cytoplasm-associated fraction. Data are expressed as the mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 vs VIF, #P<0.05, ##P<0.01, ###P<0.001 vs PLF (one-way analysis of variance (ANOVA) with Tukey’s test). Coomassie brilliant blue staining was used as a loading control. The VIF mice were selected as a control group. NMDAR, N-methyl-d-aspartate receptor; PLF, paroxetine-treated long-time floating; PSF, paroxetine-treated short-time floating; SYNJ1, synaptojanin 1; VIF, vehicle-treated intermediate-time floating.
We also investigated proteins in the pathways of glutamate metabolism and synapse/vesicle trafficking (Figure 2b). Although chronic paroxetine treatment increased glutamine synthetase protein expression both in PLF and PSF mice, only glutamate dehydrogenase 1 (GDH1) level was significantly distinct between the two groups (F2,12=5.42, P<0.05). Synapsin and SYNJ1 (synaptojanin 1) protein expressions were downregulated by chronic paroxetine treatment without significant differences between the PLF and PSF mice.
To determine whether chronic paroxetine treatment differentially regulated nitric oxide (NO) system in relation to glutamatergic pathway changes, we extended our study to investigate NO-related proteins and metabolites (Figure 3 and Supplementary Figure 2a). NO system-related proteins including postsynaptic density protein-95 (PSD-95), neuronal nitric oxide synthase (nNOS), carboxy-terminal PDZ ligand of nNOS (CAPON) and soluble guanylate cyclase-β1 (sGC-β1) showed significant level differences between PLF and PSF groups (PSD-95: F2,12=5.90, P<0.05; nNOS: F2,12=15.57, P<0.001; CAPON: F2,12=7.55, P<0.01; sGC-β1: F2,12=9.78, P<0.01; Figure 3a). Altered levels of citrulline whose conversion from arginine is catalysed by nNOS protein were detected (Figure 3b). Taken together, through integration of proteomic and metabolomic data, we were able to identify a systemic protein–metabolite network that is differentially affected between the PLF and PSF groups (Figure 3c).
Differential effect of chronic paroxetine treatment on PSD-95/nNOS complex. (a) PSD-95, nNOS, CAPON and sGC-β1 protein level differences among groups, n=5 per group. The proteins were blotted using cytoplasm-associated fraction. (b) Arginine and citrulline levels in PLF and PSF mice, n=5 per group. (c) Affected protein–metabolite network following chronic paroxetine treatment. Upward-pointing red arrow indicates higher biosignature level in PSF compared to PLF mice. Downward-pointing blue arrow indicates lower biosignature level in PSF compared with PLF mice. Data are expressed as the mean±s.e.m. **P<0.01 vs PLF (two-tailed t-test), ***P<0.001 vs VIF, #P<0.05 vs PLF (one-way analysis of variance (ANOVA) with Tukey’s test, Figure 3a). *q<0.1 (two-tailed t-test followed by false discovery rate (FDR) correction, Figure 3b). Coomassie Brilliant Blue staining was used as a loading control. VIF mice were selected as a control group. CAPON, carboxy-terminal PDZ ligand of nNOS; mGluR2/3, metabotropic glutamate receptor 2 and 3; NMDAR, N-methyl-d-aspartate receptor; nNOS, neuronal nitric oxide synthase; PLF, paroxetine-treated long-time floating; PSD-95, postsynaptic density protein-95; PSF, paroxetine-treated short-time floating; sGC-β1, soluble guanylate cyclase-β1; VIF, vehicle-treated intermediate-time floating.
Chronic paroxetine treatment induces differential ubiquitin–proteasome system profiles
To examine whether differential protein expression levels between PLF and PSF mice were caused by transcriptional alterations, we performed quantitative reverse transcription PCR analysis for NMDAR subunits PSD-95 and nNOS (Figure 4a). As we did not see any transcript level differences between the groups, we next examined the possible involvement of ubiquitination-induced proteasomal degradation in the observed protein expression differences. Although no ubiquitinated GluN1, GluN2A and PSD-95 difference was detected between the PLF and PSF mice (Figure 4b), the PSF mice showed greater total proteasome subunit α type-2 (PSMA2) and ubiquitination levels compared with PLF mice (PSMA2: F2,12=44.89, P<0.0001; ubiquitination: F2,12=13.69, P<0.001; Figure 4c and Supplementary Figure 2b). Although ubiquitination level in the PLF mice showed significant reduction, its level in PSF was similar to that in control mice.
Differential effect of chronic paroxetine treatment on UPS. (a) Quantitative reverse transcription PCR data of NMDAR subunits, PSD-95 and nNOS showed no transcription differences between PLF and PSF groups, n=6 per group. (b) Immunoprecipitation (IP) study of ubiquitinated NMDAR subunits and PSD-95. IP with ubiquitin antibody was followed by western blot (WB) analysis of the proteins. No ubiquitinated protein level differences was observed between the PLF and PSF groups, n=3 per group. The proteins were blotted using total lysate extract. (c) PSMA2 and ubiquitination level differences between the PLF and PSF groups, n=5 per group. The proteins were blotted using cytoplasm-associated fraction. *P<0.05, **P<0.01, ***P<0.001 vs VIF, ##P<0.01, ###P<0.001 vs PLF (one-way analysis of variance (ANOVA) with Tukey’s test). Coomassie brilliant blue staining is shown as loading control. VIF mice were selected as a control group. NMDAR, N-methyl-d-aspartate receptor; nNOS, neuronal nitric oxide synthase; PLF, paroxetine-treated long-time floating; PSD-95, postsynaptic density protein-95; PSF, paroxetine-treated short-time floating; PSMA2, proteasome subunit α type-2; UPS, ubiquitin–proteasome system; VIF, vehicle-treated intermediate-time floating.
Taken together, we found significant glutamatergic and ubiquitin–proteasome system (UPS) pathway protein differences between the PLF and PSF groups, further supported by a significant correlation of protein levels with FST immobility time (Supplementary Table 3).
Glutamatergic and UPS pathway protein regulation in vehicle-treated subgroups
We further investigated glutamatergic and UPS pathway protein expressions in the hippocampus of vehicle-treated subgroups to ascertain whether these protein levels were differentially regulated by FST in the absence of paroxetine treatment (Supplementary Figure 3). We found significant NMDAR subunit expression and phosphorylation level differences between vehicle-treated long-time floating (VLF) and vehicle-treated short-time floating (VSF) groups (P-GluN2A: F2,12=79.64, P<0.0001; GluN2B: F2,12=20.45, P<0.0001; P-GluN2B: F2,12=19.93, P<0.001; Supplementary Figure 3b). Vehicle-treated short-time immobile mice exhibited lower NMDAR subunits and phosphorylation levels than their counterparts, VLF mice. Other glutamatergic and UPS pathway proteins, with the exception of PSD-95, showed no expression level difference between the vehicle-treated subgroups (PSD-95: F2,12=30.24, P<0.0001; Supplementary Figure 3c). We therefore conclude that NMDAR and PSD-95 expression level differences between VLF and VSF mice are independent of paroxetine treatment.
Glutamatergic and ubiquitin–proteasome system pathway protein regulation in the prefrontal cortex
Glutamatergic and UPS pathways were also assessed in the prefrontal cortex (Supplementary Figure 4a). We found that prefrontal GluN2A and GluN2B protein levels were significantly lower in PSF compared with PLF mice, whereas none of the prefrontal NMDAR subunit phosphorylation showed a difference. Metabolomics profiles were also investigated in the prefrontal cortex (Supplementary Figure 4b). No significant enrichment was observed for metabolic pathways, and glutamatergic pathway-related metabolites were barely different between the PLF and PSF groups, except for carbamoyl phosphate. Other pathway proteins including nNOS, CAPON and PSMA2 were also found to be differentially regulated between the two groups (Supplementary Figure 4c). Although a significant increase of prefrontal nNOS levels was found in PSF mice, nNOS metabolites including arginine and citrulline were not different between the PLF and PSF groups indicating no change in prefrontal NO pathway activity (Supplementary Figure 4d).
sGC-β1, PSMA2 and ubiquitin protein expression levels are associated with clinical antidepressant treatment response in PBMCs from MDD patients
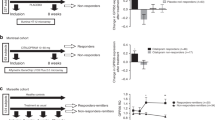
To investigate the relevance of the identified biosignatures, sGC-β1, PSMA2 and ubiquitination levels were analysed in MDD patients’ PBMCs (Figure 5, Supplementary Figure 5). All three proteins were differentially expressed between the antidepressant responder and non-responder patient groups, especially 6 weeks after admission (T6; Figure 5a). Although sGC-β1 protein levels were significantly reduced in both groups at T6, PSMA2 protein levels were significantly reduced only in the responder patients’ PBMCs at T6. Ubiquitination levels were not altered by chronic antidepressant treatment in either group. However, they were lower in responder compared with non-responder patients. All three proteins level at T6 significantly correlated with clinical antidepressant response (Figure 5b). Only PSMA2 protein level change between baseline (T0) and T6 samples was significantly correlated with the clinical antidepressant treatment response (Figure 5c).
sGC-β1, PSMA2 and ubiquitination levels in human PBMCs from antidepressant responder and non-responder patients. (a) sGC-β1, PSMA2 and ubiquitination level differences between antidepressant non-responder (NR) and responder patients (R) at baseline (T0) and after 6 weeks treatment (T6), n=17. (b) Correlation of protein levels at T6 with clinical antidepressant treatment response, n=17. (c) Correlation of protein level changes (between T0 and T6) with clinical antidepressant treatment response, n=17. *P<0.05, **P<0.01 vs NR (two-tailed t-test). ##P<0.01, ###P<0.001, ####P<0.0001 vs T0 (two-tailed paired t-test). Data are expressed as the mean±s.e.m. The proteins were analysed using total lysate extract. Pearson correlation coefficients (r) with P-values are indicated in the correlation graphs. PBMC, peripheral blood mononuclear cell; PSMA2, proteasome subunit α type-2; sGC-β1, soluble guanylate cyclase-β1.
Discussion
In the present study, we investigated molecular pathway differences between mice that responded and mice that did not respond to chronic paroxetine treatment. PLF mice were classified as non-responders based on their long-time immobility in FST, whereas PSF mice exhibited a significantly shorter FST immobility time and were classified as responders. As the FST is a behavioural test commonly used to evaluate antidepressant-like effects in mice,21, 22 we decided to categorize animals based on their FST immobility time. In pilot studies, we investigated whether baseline (pre-treatment) FST impacts on post-treatment FST. Baseline FST outcome did not correlate with post-treatment FST phenotype, suggesting that the heterogeneous FST immobility distribution in paroxetine-treated mice results from chronic paroxetine treatment and is not an inherent behavioural trait of the animal (data not shown). The variability DBA/2 J mice had in the time they spent immobile in the FST at the end of treatment provided a model for investigating the heterogeneous antidepressant treatment response.
To further analyse behavioural differences between the PLF and PSF groups, we have performed female-urine-sniffing test, which has been used to evaluate anhedonic-like behaviour of animals.23 Sniffing time differences between the groups showed strong tendency towards statistical significance (P=0.052, data not shown).
Our results suggest that proteins and metabolites associated with the glutamatergic pathway are affected by chronic antidepressant treatment in a distinctive way in responders and non-responders. The glutamatergic pathway has previously been associated with MDD pathobiology and antidepressant response. In depressed patients, significantly elevated serum, plasma and cerebrospinal fluid glutamate levels were found.24, 25, 26, 27, 28 A single-nucleotide polymorphism in metabotropic 7 glutamate receptor was shown to be involved in the onset of the clinical antidepressant effect.29 Glutamate release decreases with chronic fluoxetine, desipramine, reboxetine, venlafaxine or agomelatine treatment.30, 31 Numerous studies have shown that chronic antidepressant treatment regulates glutamatergic receptor expression in rodent hippocampus.32, 33, 34
Other proteins investigated in the current study, CaMK2,35 GDH1,36, 37 GSK-3β,38, 39 P-ERK,40, 41, 42 SYNJ1,43, 44, 45 have been associated with MDD pathology and/or antidepressant treatment response.
The NO system has been implicated in the treatment,22, 46, 47, 48, 49, 50, 51, 52, 53, 54 as well as the pathobiology of depression.49, 50, 55, 56, 57 We, therefore, further investigated PSD-95, nNOS, CAPON and sGC-β1. PSD-95 and nNOS proteins have been shown to associate using PDZ domain-based interaction. They are part of a tertiary complex with NMDAR and produce NO.58, 59, 60 CAPON sequesters nNOS and negatively regulates its association with NMDAR/PSD-95 complex.61 As CAPON overexpression has been shown to disrupt PSD-95/nNOS complex,62 high CAPON levels in PSF mice may prevent catalytic activation of nNOS, which may lead to low levels of citrulline, a product metabolite of nNOS. sGC-β1 is a subunit of soluble guanylate cyclase (sGC) that mediates NO signalling pathways.63, 64 sGC-β1 level difference was found significant in post-treatment PBMCs samples from depressed patients implicating the role of NO system in clinical antidepressant response.
Among the investigated proteins, only PSD-95 protein levels were lower in both short-time floating animals (VSF and PSF) compared with their counterparts, long-time floating animals (VLF and PLF). Thus, PSD-95 expression may be affected by FST, and not by chronic paroxetine treatment.
Although NMDAR subunit and phosphorylation level differences were also found significant between VLF and VSF mice, the protein expression pattern was opposite to that found in the PLF and PSF groups suggesting that NMDAR expression and activity was distinctly affected in the absence and presence of chronic paroxetine treatment.
Our results were further corroborated by metabolite profiling data. Although several metabolites including alanine, asparagine, glutamate, glutamine and glutathione did not reach statistical significance after false discovery rate correction, they showed differential levels between the PLF and PSF groups (P<0.05), which supports a glutamatergic pathway activity difference between the two groups. Alanine and citrate are known to regulate NMDAR activity.65, 66 Glutathione was found to be an NMDAR agonist.67, 68 Sarcosine is also an NMDAR co-agonist.69, 70 The role of serine as a potent co-agonist for NMDAR has been well demonstrated.71, 72 In addition, taurine is suggested to interact directly with NMDAR and regulate its function.73 Low serum asparagine, serine and taurine levels characterized non-responder patients after undergoing 5 weeks of antidepressant treatment.74 In addition, glutathione, sarcosine and taurine administrations have antidepressant-like effects suggesting their elevated levels might be relevant to the favourable paroxetine response.68, 70, 75 Xanthurenic acid was shown to be an agonist for metabotropic glutamate receptor 2 and 3 (mGluR2/3).76 These metabolite level differences indicate higher hippocampal glutamatergic activity in PSF compared with PLF mice. As GDH1 catabolizes glutamate, low levels of GDH1 protein might be the cause of the observed elevated glutamate levels in PSF mice. Alternatively, high glutamate levels might induce a compensatory feedback regulation of GDH1 protein expression to prevent pathway overactivation. Altered NMDAR subunit levels are consistent with the observed glutamate levels in the PLF and PSF mice. More glutamate and other glutamatergic receptor modulators could result in reduced NMDAR expression as previously reported in studies with l-trans-pyrrolidine-2,4-dicarboxylate, a high-affinity glutamate reuptake inhibitor.77, 78
Our proteomics data derived from the membrane-associated fraction also showed that levels for vesicular glutamate transporter 1, a presynaptic protein that regulates glutamate release, were significantly higher in the PSF than in the PLF mice. This suggests that PSF mice may have a greater glutamate release compared with the PLF mice.
When we investigated NMDAR subunits and PSD-95 protein ubiquitination differences between the PLF and PSF groups, we found that chronic antidepressant treatment induced different regulation of UPS proteins between drug responders and non-responders, both in animals and humans. This is supported by evidence from other studies, which suggests that UPS may be linked to MDD treatment. In this regard, it has been shown that single-nucleotide polymorphisms in proteasome subunit α7 (PSMA7), proteasome 26 S non-ATPase subunit 9 (PSMD9) and proteasome 26 S non-ATPase subunit 13 (PSMD13) are associated with the clinical antidepressant response.79, 80 We therefore submit that UPS proteins such as PSMA2 and ubiquitination are of interest for evaluating and predicting clinical antidepressant treatment response.
PSMA2 is an essential subunit that facilitates 20 S core proteasome particle formation that further interacts with 19 S regulatory particle to form 26 S proteasome.81, 82 Ubiquitination, covalent conjugation of ubiquitin, acts as a signal to guide proteasome to proteins destined for degradation.83 The fact that hippocampal PSMA2 and ubiquitination expression patterns are inconsistent between mouse and human may be due to different tissue sources (hippocampus vs PBMCs). Owing to the limited amount of mouse blood, we were unable to isolate sufficient PBMC material to proof that this is indeed the case. Other proteins related to psychiatric disorders and the antidepressant response including p11 and brain-derived neurotrophic factor have also been shown to be inversely correlated between brain and periphery.84, 85 In addition, ubiquitination levels were higher in antidepressant non-responder patients. As stressful events are known to alter the UPS pathway,86, 87 that might be a reflection of that.
Interestingly, UPS mediates NMDAR degradation.88, 89 Tai et al.90 reported that NMDA treatment of cultured hippocampal neurons decreases UPS activity, implicating an interplay between glutamatergic and proteasome pathways. This is in agreement with our data, which also point to an involvement of both pathways in the different response of the PLF and PSF mouse groups. Although we failed to find a relationship between ubiquitination and distinct NMDAR subunit protein levels between PLF and PSF mice, other protein degradation mechanisms might cause the observed differential expression. These include ubiquitination-like modifications such as human leuokocyte antigen-F associated transcript 10,91, 92 neural precursor cell expressed developmentally downregulated protein 8 (ref. 93) and small ubiquitin-related modifier,94 all shown to be involved in proteasomal degradation. In addition, autophagic protein degradation may be relevant for differential protein expression in paroxetine-treated mice.95, 96
Although individual housing has been shown to regulate stress response,97 Hilakivi et al.98 have shown that long-term (10–20 days) single housing did not result in a different stress response compared with group-housing in DBA/2 mice. Although the effect of single housing cannot be excluded, FST immobility distribution of the PLF and PSF groups can be the result of chronic paroxetine treatment based on distinct pathway protein expression patterns observed between the vehicle- and paroxetine-treated subgroups.
PSF mice showed similar glutamatergic and UPS pathway protein levels as control mice, whereas PLF mice exhibited significantly different pathway protein levels compared with the other groups. This indicates a dysregulated pathway activity on chronic paroxetine treatment in the PLF mice that is associated with chronic paroxetine treatment non-response.
Heterogeneous FST immobility of vehicle-treated mice may be a reflection of a distinct individual response to FST, an acute stressful behavioural test. As protein expression levels were highly variable in vehicle-treated subgroups, only vehicle-treated intermediate-time floating group mice were selected and used for further analyses.
Our analysis also revealed that chronic paroxetine treatment did not induce significant differences in NMDAR subunit phosphorylation and glutamatergic metabolite levels in the prefrontal cortex. This suggests that there might be closer glutamatergic protein–metabolite interaction in the hippocampus than that in the prefrontal cortex. Although we found a significant nNOS protein level difference, the lack of PSD-95 and citrulline level differences indicates that prefrontal NO system is not differentially affected between the PLF and PSF groups. Significant PSMA2 level difference in the prefrontal cortex further supports PSMA2 protein as a biomarker candidate for the antidepressant response.
Although we were able to identify biomarker candidates that predict and evaluate the clinical antidepressant treatment response, a time-course analysis will be necessary to further corroborate these findings.
In the present study, we used wild-type stress naive DBA2/J mice to explore pharmacological heterogeneity of chronic paroxetine response. An animal model with a depression-like phenotype may provide additional information for the interaction of drug treatment and stress response.
Whether chronic paroxetine treatment response and the identified pathway activities remain in PSF mice after drug discontinuation will be investigated in a future study. An extension of the analysis to microdialysis should also add further information on the glutamate levels and release regulation.
The present study attempts to integrate quantitative proteomics and metabolomics data to improve our understanding of biological pathway changes relevant in the response to antidepressant treatment. What roles glutamatergic and ubiquitin systems have in the underlying mechanism of selective serotonin reuptake inhibitor response requires further studies with compounds able to modulate pathway activity. Pathway modulators may lead to novel drugs with antidepressant activities. In addition, the glutamatergic and ubiquitin systems activities could be used as a biosignature for the antidepressant treatment response.
References
Al-Harbi KS . Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 2012; 6: 369–388.
Thomas L, Kessler D, Campbell J, Morrison J, Peters TJ, Williams C et al. Prevalence of treatment-resistant depression in primary care: cross-sectional data. Br J Gen Pract 2013; 63: e852–e858.
Berlim MT, Turecki G . Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry 2007; 52: 46–54.
Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry 2009;195: 30–38.
Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci 2015; 18: 1606–1616.
Binder EB, Künzel HE, Nickel T, Kern N, Pfennig A, Majer M et al. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology 2008; 34: 99–109.
Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006;314: 140–143.
Kocabas NA, Antonijevic I, Faghel C, Forray C, Kasper S, Lecrubier Y et al. Brain-derived neurotrophic factor gene polymorphisms: influence on treatment response phenotypes of major depressive disorder. Int Clin Psychopharmacol 2011; 26: 1–10.
Martinez JM, Garakani A, Yehuda R, Gorman JM . Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety 2012; 29: 32–38.
Fitzgerald P, O'Brien SM, Scully P, Rijkers K, Scott LV, Dinan TG . Cutaneous glucocorticoid receptor sensitivity and pro-inflammatory cytokine levels in antidepressant-resistant depression. Psychol Med 2006; 36: 37–43.
Sluzewska A, Sobieska M, Rybakowski JK . Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology 1997; 35: 123–127.
Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H . Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22: 370–379.
Drevets WC, Zarate CA, Furey ML . Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry 2013; 73: 1156–1163.
Murrough JW, Iosifescu DV, Chang LC, Jurdi Al RK, Green CE, Perez AM et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–1142.
Duman RS, Li N . A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012; 367: 2475–2484.
Weckmann K, Labermaier C, Asara JM, Müller MB, Turck CW . Time-dependent metabolomic profiling of Ketamine drug action reveals hippocampal pathway alterations and biomarker candidates. Transl Psychiatry 2014; 4: e481.
Webhofer C, Gormanns P, Reckow S, Lebar M, Maccarrone G, Ludwig T et al. Proteomic and metabolomic profiling reveals time-dependent changes in hippocampal metabolism upon paroxetine treatment and biomarker candidates. J Psychiatr Res 2013; 47: 289–298.
Sillaber I, Panhuysen M, Henniger MSH, Ohl F, Kühne C, Pütz B et al. Profiling of behavioral changes and hippocampal gene expression in mice chronically treated with the SSRI paroxetine. Psychopharmacology (Berl) 2008; 200: 557–572.
Droy-Dupré L, Bossard C, Volteau C, Bezieau S, Laboisse CL, Mosnier J-F . Hierarchical clustering identifies a subgroup of colonic adenocarcinomas expressing crypt-like differentiation markers, associated with MSS status and better prognosis. Virchows Arch 2015; 466: 383–391.
Muehlmann AM, Bliznyuk N, Duerr I, Lewis MH . Repetitive motor behavior: further characterization of development and temporal dynamics. Dev Psychobiol 2015; 57: 201–211.
Webhofer C, Gormanns P, Tolstikov V, Zieglgänsberger W, Sillaber I, Holsboer F et al. Metabolite profiling of antidepressant drug action reveals novel drug targets beyond monoamine elevation. Transl Psychiatry 2011; 1: e58.
Doucet MV, Levine H, Dev KK, Harkin A . Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology 2013; 38: 1575–1584.
Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry 2010; 67: 864–871.
Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F . Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry 1993; 150: 1731–1733.
Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH . Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr (1970) 1982; 232: 299–304.
Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW . Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry 2000; 47: 586–593.
Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 1998; 37: 124–129.
Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Kawahara R . Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1155–1158.
Fabbri C, Drago A, Serretti A . Early antidepressant efficacy modulation by glutamatergic gene variants in the STAR*D. Eur Neuropsychopharmacol 2013; 23: 612–621.
Bonanno G, Giambelli R, Raiteri L . Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci 2005; 25: 3270–3279.
Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS ONE 2010; 5: e8566.
Calabrese F, Guidotti G, Molteni R, Racagni G, Mancini M, Riva MA . Stress-induced changes of hippocampal NMDA receptors: modulation by duloxetine treatment. PLoS ONE 2012; 7: e37916.
Pittaluga A, Raiteri L, Longordo F, Luccini E, Barbiero VS, Racagni G et al. Antidepressant treatments and function of glutamate ionotropic receptors mediating amine release in hippocampus. Neuropharmacology 2007; 53: 27–36.
Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D et al. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry 2006; 59: 713–720.
Lotrich FE, Pollock BG . Candidate genes for antidepressant response to selective serotonin reuptake inhibitors. Neuropsychiatr Dis Treat 2005; 1: 17–35.
Bao X, Pal R, Hascup KN, Wang Y, Wang W-T, Xu W et al. Transgenic expression of Glud1 (glutamate dehydrogenase 1) in neurons: in vivo model of enhanced glutamate release, altered synaptic plasticity, and selective neuronal vulnerability. J Neurosci 2009; 29: 13929–13944.
Michaelis EK, Wang X, Pal R, Bao X, Hascup KN, Wang Y et al. Neuronal Glud1 (glutamate dehydrogenase 1) over-expressing mice: increased glutamate formation and synaptic release, loss of synaptic activity, and adaptive changes in genomic expression. Neurochem Int 2011; 59: 473–481.
Tsai S-J, Liou Y-J, Hong C-J, YW-Y Yu, Chen T-J . Glycogen synthase kinase-3beta gene is associated with antidepressant treatment response in Chinese major depressive disorder. Pharmacogenomics J 2008; 8: 384–390.
Joaquim HPG, Talib LL, Forlenza OV, Diniz BS, Gattaz WF . Long-term sertraline treatment increases expression and decreases phosphorylation of glycogen synthase kinase-3B in platelets of patients with late-life major depression. J Psychiatr Res 2012; 46: 1053–1058.
Qi X, Lin W, Li J, Li H, Wang W, Wang D et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis 2008; 31: 278–285.
Gourley S, Wu F, Kiraly D, Ploski J, Kedves A . Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 2008; 63: 353–359.
Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN . Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem 2001; 77: 916–928.
Mani M, Lee SY, Lucast L, Cremona O, Di Paolo G, De Camilli P et al. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron 2007; 56: 1004–1018.
Lüthi A, Di Paolo G, Cremona O, Daniell L, De Camilli P, McCormick DA . Synaptojanin 1 contributes to maintaining the stability of GABAergic transmission in primary cultures of cortical neurons. J Neurosci 2001; 21: 9101–9111.
Wong M-L, O'Kirwan F, Hannestad JP, Irizarry KJL, Elashoff D, Licinio J . St John's wort and imipramine-induced gene expression profiles identify cellular functions relevant to antidepressant action and novel pharmacogenetic candidates for the phenotype of antidepressant treatment response. Mol Psychiatry 2004; 9: 237–251.
Ghasemi M, Sadeghipour H, Mosleh A, Sadeghipour HR, Mani AR, Dehpour AR . Nitric oxide involvement in the antidepressant-like effects of acute lithium administration in the mouse forced swimming test. Eur Neuropsychopharmacol 2008; 18: 323–332.
Jesse C, Bortolatto C, Savegnago L . Involvement of l-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effect of tramadol in the rat forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1838–1843.
Dhir A, Kulkarni S . Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol 2007; 568: 177–185.
Wegener G, Volke V, Harvey B, Rosenberg R . Local, but not systemic, administration of serotonergic antidepressants decreases hippocampal nitric oxide synthase activity. Brain Res 2003; 959: 128–134.
Finkel MS, Laghrissi-Thode F, Pollock BG, Rong J . Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull 1996; 32: 653–658.
Hiroaki-Sato VA, Sales AJ, Biojone C, Joca SRL . Hippocampal nNOS inhibition induces an antidepressant-like effect: involvement of 5HT1A receptors. Behav Pharmacol 2014; 25: 187–196.
Joca SRL, Guimarães FS . Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl) 2006; 185: 298–305.
Tomaz VS, Cordeiro RC, Costa AMN, de Lucena DF, Nobre Júnior HV, de Sousa FCF et al. Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 2014; 268: 236–246.
Zhou Q-G, Hu Y, Hua Y, Hu M, Luo C-X, Han X et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 2007; 103: 1843–1854.
Zhou Q-G, Zhu L-J, Chen C, Wu H-Y, Luo C-X, Chang L et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci 2011; 31: 7579–7590.
Oliveira RMW, Guimarães FS, Deakin JFW . Expression of neuronal nitric oxide synthase in the hippocampal formation in affective disorders. Braz J Med Biol Res 2008; 41: 333–341.
Chrapko W, Jurasz P, Radomski MW, Archer SL, Newman SC, Baker G et al. Alteration of decreased plasma NO metabolites and platelet NO synthase activity by paroxetine in depressed patients. Neuropsychopharmacology 2006; 31: 1286–1293.
Doucet MV, Harkin A, Dev KK . The PSD-95/nNOS complex: new drugs for depression? Pharmacol Ther 2012; 133: 218–229.
Fedele E, Marchi M, Raiteri M . In vivo NO/cGMP signalling in the hippocampus. Neurochem Res 2001; 26: 1069–1078.
Bredt DS, Snyder SH . Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA 1989; 86: 9030–9033.
Sharma NM, Zheng H, Mehta PP, Li Y-F, Patel KP . Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardiovasc Res 2011; 92: 348–357.
Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH . CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 1998; 20: 115–124.
Hofmann F, Feil R, Kleppisch T, Schlossmann J . Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 2006; 86: 1–23.
Francis SH, Busch JL, Corbin JD, Sibley D . cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 2010; 62: 525–563.
Popescu G, Murthy S, Borschel W . Allosteric inhibitors of NMDA receptor functions. Pharmaceuticals 2010; 3: 3240–3257.
Westergaard N, Banke T, Wahl P, Sonnewald U, Schousboe A . Citrate modulates the regulation by Zn2+ of N-methyl-D-aspartate receptor-mediated channel current and neurotransmitter release. Proc Natl Acad Sci USA 1995; 92: 3367–3370.
Chueh SH . S-Nitrosoglutathione and glutathione act as NMDA receptor agonists in cultured hippocampal neurons1. Acta Pharmacol Sin 2006; 27: 853–860.
Rosa JM, Dafre AL, Rodrigues ALS . Antidepressant-like responses in the forced swimming test elicited by glutathione and redox modulation. Behav Brain Res 2013; 253: 165–172.
Zhang H, Hyrc K, Thio L . The glycine transport inhibitor sarcosine is an NMDA receptor co‐agonist that differs from glycine. J Physiol 2009; 587 (Pt 13): 3207–3220.
Huang C-C, Wei I-H, Huang C-L, Chen K-T, Tsai M-H, Tsai P et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry 2013; 74: 734–741.
Panatier A, Theodosis DT, Mothet J-P, Touquet B, Pollegioni L, Poulain DA et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 2006; 125: 775–784.
Mothet JP, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 2000; 97: 4926–4931.
Suárez LM, Solís JM . Taurine potentiates presynaptic NMDA receptors in hippocampal Schaffer collateral axons. Eur J Neurosci 2006; 24: 405–418.
Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpé S . Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand 1998; 97: 302–308.
Toyoda A, Iio W . Antidepressant-like effect of chronic taurine administration and its hippocampal signal transduction in rats. Adv Exp Med Biol 2013; 775: 29–43.
Fazio F, Lionetto L, Curto M, Iacovelli L, Cavallari M, Zappulla C et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci Rep 2015; 5: 17799.
Cebers G, Cebere A, Wägner A, Liljequist S . Prolonged inhibition of glutamate reuptake down-regulates NMDA receptor functions in cultured cerebellar granule cells. J Neurochem 1999; 72: 2181–2190.
Cebers G, Cebere A, Kovács AD, Högberg H, Moreira T, Liljequist S . Increased ambient glutamate concentration alters the expression of NMDA receptor subunits in cerebellar granule neurons. Neurochem Int 2001; 39: 151–160.
Gragnoli C . Proteasome modulator 9 gene SNPs, responsible for anti-depressant response, are in linkage with generalized anxiety disorder. J Cell Physiol 2014; 229: 1157–1159.
Wong M-L, Dong C, Maestre-Mesa J, Licinio J . Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry 2008; 13: 800–812.
Lecker SH, Goldberg AL, Mitch WE . Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 2006; 17: 1807–1819.
Park S, Li X, Kim HM, Singh CR, Tian G, Hoyt MA et al. Reconfiguration of the proteasome during chaperone-mediated assembly. Nature 2013; 497: 512–516.
Tai H-C, Schuman EM . Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 2008; 9: 826–838.
Svenningsson P, Berg L, Matthews D, Ionescu DF, Richards EM, Niciu MJ et al. Preliminary evidence that early reduction in p11 levels in natural killer cells and monocytes predicts the likelihood of antidepressant response to chronic citalopram. Mol Psychiatry 2014; 19: 962–964.
Elfving B, Plougmann PH, Müller HK, Mathé AA, Rosenberg R, Wegener G . Inverse correlation of brain and blood BDNF levels in a genetic rat model of depression. Int J Neuropsychopharmacol 2010; 13: 563–572.
Ryan MM, Lockstone HE, Huffaker SJ, Wayland MT, Webster MJ, Bahn S . Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol Psychiatry 2006; 11: 965–978.
Karssen AM, Her S, Li JZ, Patel PD, Meng F, Bunney WE et al. Stress-induced changes in primate prefrontal profiles of gene expression. Mol Psychiatry 2007; 12: 1089–1102.
Tsai N-P . Ubiquitin proteasome system-mediated degradation of synaptic proteins: an update from the postsynaptic side. Biochim Biophys Acta 2014; 1843: 2838–2842.
Kato A, Rouach N, Nicoll RA, Bredt DS . Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc Natl Acad Sci USA 2005; 102: 5600–5605.
Tai H-C, Besche H, Goldberg AL, Schuman EM . Characterization of the brain 26S proteasome and its interacting proteins. Front Mol Neurosci 2010; 3: pii: 12.
Hipp MS, Kalveram B, Raasi S, Groettrup M, Schmidtke G . FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol Cell Biol 2005; 25: 3483–3491.
Schmidtke G, Aichem A, Groettrup M . FAT10ylation as a signal for proteasomal degradation. Biochim Biophys Acta 2014; 1843: 97–102.
Enchev RI, Schulman BA, Peter M . Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol 2015; 16: 30–44.
Miteva M, Keusekotten K, Hofmann K, Praefcke GJK, Dohmen RJ . Sumoylation as a signal for polyubiquitylation and proteasomal degradation. Subcell Biochem 2010; 54: 195–214.
Gassen NC, Hartmann J, Schmidt MV, Rein T . FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy 2015; 11: 578–580.
Gassen NC, Hartmann J, Zschocke J, Stepan J, Hafner K, Zellner A et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med 2014; 11: e1001755.
Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 2003; 28: 540–558.
Hilakivi LA, Ota M, Lister RG . Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral 'despair'. Pharmacol Biochem Behav 1989; 33: 371–374.
Acknowledgements
This work was supported by the Max Planck Society. DIP is the recipient of a fellowship from the Kwanjeong Educational Foundation. We thank Professor Mathias Uhlen for providing PSMA2, SYNJ1, STXBP1 and SV2A antibodies from the HPA programme.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
IS is the CEO of Phenoquest AG. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Park, D., Dournes, C., Sillaber, I. et al. Delineation of molecular pathway activities of the chronic antidepressant treatment response suggests important roles for glutamatergic and ubiquitin–proteasome systems. Transl Psychiatry 7, e1078 (2017). https://doi.org/10.1038/tp.2017.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.39
This article is cited by
-
Alteration of transthyretin and thyroxine-binding globulin in major depressive disorder: multiple reaction monitoring-based proteomic analysis
Journal of Translational Medicine (2021)
-
Sialylated N-glycan profile during acute and chronic infections with Toxoplasma gondii in mice
Scientific Reports (2020)
-
Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response
Scientific Reports (2018)