Abstract
The opioid antagonist naltrexone has been shown to attenuate the subjective effects of amphetamine. However, the mechanisms behind this modulatory effect are currently unknown. We hypothesized that naltrexone would diminish the striatal dopamine release induced by amphetamine, which is considered an important mechanism behind many of its stimulant properties. We used positron emission tomography and the dopamine D2-receptor radioligand [11C]raclopride in healthy subjects to study the dopaminergic effects of an amphetamine injection after pretreatment with naltrexone or placebo. In a rat model, we used microdialysis to study the modulatory effects of naltrexone on dopamine levels after acute and chronic amphetamine exposure. In healthy humans, naltrexone attenuated the subjective effects of amphetamine, confirming our previous results. Amphetamine produced a significant reduction in striatal radioligand binding, indicating increased levels of endogenous dopamine. However, there was no statistically significant effect of naltrexone on dopamine release. The same pattern was observed in rats, where an acute injection of amphetamine caused a significant rise in striatal dopamine levels, with no effect of naltrexone pretreatment. However, in a chronic model, naltrexone significantly attenuated the dopamine release caused by reinstatement of amphetamine. Collectively, these data suggest that the opioid system becomes engaged during the more chronic phase of drug use, evidenced by the modulatory effect of naltrexone on dopamine release following chronic amphetamine administration. The importance of opioid-dopamine interactions in the reinforcing and addictive effects of amphetamine is highlighted by the present findings and may help to facilitate medication development in the field of stimulant dependence.
Similar content being viewed by others
Introduction
Amphetamines have powerful effects on brain monoamine systems and give rise to an acute increase in extracellular dopamine (DA) levels in several parts of the brain.1 In humans, the use of positron emission tomography (PET) and DA D2-receptor radioligands to indirectly measure endogenous DA levels has consistently shown a DA increase in the striatum after administration of psychostimulants. Whereas some studies have shown a correlation between DA release and subjective euphoria,2, 3 others have indicated a relationship to drug wanting rather than liking.4, 5 In line with the latter observation, DA D2 antagonists do not consistently block amphetamine-induced euphoria.6 Consequently, other neurotransmitter systems than DA are thought to be involved in the actions of amphetamine.1
Several lines of evidence point to the importance of brain opioid systems in stimulant use disorders. An important finding that has been consistently replicated is that the non-selective opioid antagonist naltrexone (NTX) attenuates the subjective effects of amphetamine, both in healthy individuals and in patients with amphetamine dependence.7, 8, 9, 10 In clinical trials, NTX has also been found to reduce craving and prevent relapse to amphetamine dependence.11, 12, 13 However, the mechanism of action behind the clinical effects remains unclear and is relevant not only for the pharmacotherapy of amphetamine dependence, but also with regard to the specific contributions of brain DA and opioid systems in reward and motivation.5
The interaction between amphetamine and the opioid system has also been investigated in preclinical models. For instance, NTX attenuates reinstatement of amphetamine self-administration and the sensitized locomotor response to amphetamine, but has no effect on conditioned place preference in rats.14, 15, 16 On the basis of these results we hypothesized that NTX may attenuate the subjective effects of amphetamine via interaction with the dopamine system. To explore whether NTX may attenuate amphetamine-induced DA release we combined human and rodent laboratory models to investigate the effects of both acute and chronic amphetamine exposure.
Materials and methods
In order to study the effects of acute amphetamine administration on DA release in humans, we used PET and the dopamine D2-receptor radioligand [11C]raclopride. The sensitivity of this radioligand to stimulant-induced changes in brain DA concentration is well-established.17, 18 Since DA release is sensitive to expectations of amphetamine,19 we included a placebo arm in the study. For ethical reasons, only a limited number of doses of amphetamine may be given to human subjects in an experimental setting, and recruiting amphetamine dependent patients for repeated PET experiments would be very challenging. Therefore, we used a rat model to compare the acute and chronic effects of amphetamine, using in vivo microdialysis to analyze brain DA levels after both acute and chronic amphetamine administration.
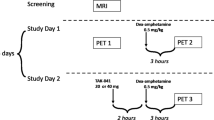
Human PET study
A cross-over randomized, placebo-controlled, double-blind design was used to test the hypothesis that pretreatment with NTX would attenuate the brain DA release induced by amphetamine.
Subjects
Seven healthy males aged 20–45 years were recruited via flyers posted at Karolinska Institutet, Stockholm, Sweden. The sample size was based on previous studies demonstrating significant effects of amphetamine on [11C]raclopride binding in healthy controls,2, 4, 19 as well as our own work on NTX and amphetamine.7, 8 Exclusion criteria included (1) DSM-IV diagnosis of major Axis-1 psychiatric disorder including any history of substance use disorder (including nicotine), (2) use of a psychoactive substance within the past 30 days, (3) history of serious medical conditions, (4) consumption of more than the equivalent of 60 g of pure alcohol per week, (5) positive result on alcohol breath analyzer at the test sessions, (6) traces of opiates, cannabis, amphetamines or benzodiazepines in the urine at screening or during test days. All participants provided written informed consent and were paid an equivalent of €500 for their participation. The study was approved by the Stockholm Regional Ethical Review Board, the Radiation Safety Committee at Karolinska Institutet and the Swedish Medical Products Agency and conducted in accordance with Good Clinical Practice (ICH GCP, 1996) and the Declaration of Helsinki.
Experimental procedure
Prior to the PET measurements, all subjects underwent a structural MR scan (1.5 T) to exclude intracranial pathology and obtain anatomical references for definition of regions of interests (ROIs). In total, each subject underwent three PET examinations with [11C]raclopride, ~1 week apart: at baseline; after placebo+amphetamine administration; and after NTX+amphetamine administration (denoted here as baseline, placebo+amphetamine, and NTX+amphetamine, respectively). The order of the two latter examinations was randomized.
On test days, subjects arrived at the laboratory at 0800 hours and received a standardized breakfast. Subjective and physiological measures were evaluated throughout the experimental procedure. At 0900 hours, subjects received either a capsule of NTX (50 mg) or placebo. One hour post ingestion of study medication, subjects underwent a PET examination with [11C]raclopride, using the ECAT HR 47 (CTI/Siemens, Knoxville, TN, USA) PET system run in 3D mode. Prior to each emission scan, a transmission scan was performed for attenuation correction. The subjects received an intravenous dose of amphetamine 0.3 mg kg−1, immediately followed by a saline solution of [11C]raclopride (223–268 MBq, specific radioactivity 193–1131 GBq μmol−1) injected as a bolus. The cannula was then flushed with 10 ml saline. Immediately following [11C]raclopride administration, PET emission data were obtained for 51 min.20 To minimize movement artifacts, an individual plastic helmet was made for all participants and used together with a head fixation system. The reconstructed data were displayed as 47 horizontal sections with a center-to-center distance of 3.125 mm.
Regions of interest
ROIs were manually delineated on individual structural MR images, based on previously published guidelines21, 22 in which the striatum is divided into limbic, associative and sensorimotor subregions based on their differential connectivity.23 The same ROIs were used for the three experiments and all ROIs were combined to create a ROI for the whole striatum. The MR images were reoriented to the AC–PC plane and then used for co-registration to PET images using SPM2. The average values of right and left ROIs were used to increase the signal-to-noise ratio for the quantification. A ROI for cerebellum was drawn below the appearance of the petrosal bone in five slices corresponding to a thickness of 10 mm. ROIs were applied to the PET images using the co-registration parameters to extract regional time activity curves. [11C]raclopride binding potential (BPND) was calculated using the simplified reference tissue model24 with cerebellum as a reference region.
Subjective and cardiovascular measures
A visual analog rating scale was administered to describe drug effects. The visual analog rating scale comprised four scales: ‘feel the drug ’; ‘like the effect’; ‘feel aroused’; and ‘want more’, providing a composite measure of subjective effects. The subjects rated their experiences starting at the time of NTX/placebo administration and continuing at designated time points. To measure physiological effects of amphetamine, heart rate and blood pressure were recorded manually at the same time points as the subjective measures.
Statistical analysis
Statistical evaluation of BPND data for each ROI was conducted using two-way repeated-measures analysis of variance (ANOVA) with Greenhouse–Geisser correction. Three comparisons of binding potential values were estimated by the ANOVA: (1) baseline vs amphetamine; (2) baseline vs NTX+amphetamine; (3) placebo+amphetamine vs NTX+amphetamine. Condition by region interactions in the ANOVA, were investigated further with post hoc t-tests. All statistical tests were two-tailed and the threshold for significance was set at P<0.05. The secondary outcome of subjective measures was defined as the mean score of the four visual analog rating scale items for the various time points during each test day, comparing the NTX+amphetamine and placebo+amphetamine conditions. A group composite score was calculated as an aggregate of the mean scores for each time point. This score was compared between the two conditions with repeated-measures ANOVA.
Microdialysis
We used in vivo microdialysis to investigate the effects of NTX on amphetamine-induced DA release in freely moving rats. First, two different acute amphetamine doses were tested. In a second experiment we investigated the effects of amphetamine reinstatement, that is, a challenge dose of amphetamine after a period of chronic treatment followed by abstinence.
Animals
Male Wistar rats 250–380 g, corresponding to 9–12 weeks at arrival, (BK Universal, Sollentuna, Sweden or Taconic, Ejeby, Denmark) were housed four per cage in a temperature (±21 °C) and humidity (±40–50%) controlled environment on a 12 h light/dark cycle (lights on 0700 hours). Food and water were available ad libitum. All experiments were conducted during the light phase of the cycle. Animals were handled in accordance with the guidelines of the Swedish National Board of Laboratory Animals and the study was approved by the Stockholm Regional Ethical Review Board (acute experiment) or Gothenburg (chronic experiment), Sweden.
Drugs
Dexamphetamine sulfate (Apoteket, Stockholm, Sweden) and NTX (Sigma Chemicals, Stockholm, Sweden) were dissolved in physiological saline (sodium chloride 0.9% (w/v)). All drugs were administered intraperitoneally (i.p.), and injected at a volume of 1 or 2 ml kg−1 of body weight.
Surgical procedure
Rats in the acute experiment were anaesthetized with a mix of fentanyl citrate (0.39 mg kg−1) and fluanisone (12.5 mg kg−1, Hypnorm, Janssen-Cilag) and midazolam (6.25 mg kg−1, Dormicum, Roche) diluted in distilled water (1:1:2; 5 ml kg−1 i.p.) or in the chronic experiment, by isoflurane (Apoteket) and mounted in a stereotaxic frame. Dialysis probes were implanted in the Nucleus Accumbens (N.Acc) with stereotaxic coordinates anteriorposterior: +1.6 mm: mediolateral −1.4 mm: dorsoventral −8.2 mm relative to bregma and the dural surface, in accordance with an anatomical atlas.25 After surgery, animals were individually housed and allowed 2 days of recovery before initiation of the experiment.
Microdialysis procedures
The microdialysis experiments were conducted approximately 48 h after surgery. Dialysis occurred through a semi-permeable membrane (Filtral AN69, Hospal Industrie, Meyzieu, France) with an active surface length of 2-2.25 mm. The dialysis probe was perfused with a physiological solution (Ca Cl2 (1.3 mm), NaCl (147 mm), KCl (3.0 mm), MgCl2 (1.0 mm), Na2HPO4 (1.0 mm), NaH2PO4 (0.2 mm)) at a rate of 2 or 2.5 μl min−1 set by a microperfusion pump. Dialysate was collected over 15 min intervals (37.5 μl) in the two acute dialysis studies and over 20 min intervals (40 μl) in the chronic dialysis study, after which the samples were injected into a high-performance liquid chromatography system. On-line quantification of DA in the dialysate was accomplished by electrochemical detection (ESA, Chelmsford, MA, USA or Dionex P580, Västra Frölunda, Sweden). After baseline measurements in the acute dialysis studies, rats were treated with either NTX (3 mg kg−1 i.p) or saline (1 mg kg−1 i.p) 30 min before given an amphetamine (0.5 or 2 mg kg−1 i.p) or saline injection (1 mg kg−1 i.p). In the chronic dialysis study, rats were conditioned to amphetamine using a protocol, which induces robust locomotor sensitization to amphetamine.16 Briefly, rats received daily injections of either saline or amphetamine (2 mg kg−1) for 10 consecutive days after which the animals were left untreated for another ten days. Surgery was performed 8 days into the drug-free period. In the following microdialysis experiment, the rats received an injection with NTX or vehicle, followed 40 min later by a saline injection for the previously saline-treated rats and amphetamine (0.5 mg kg−1 i.p.) for the previously amphetamine treated rats. Dialysate was collected for 180 min after the last drug administration. Rats were randomly assigned to different treatment groups with a minimum of three experimental groups represented on each experimental day in order to avoid systematic errors. The technician performing the DA analysis was blind to treatment group.
Statistical analysis
DA levels were expressed and statistically analyzed as percent of baseline levels. Baseline was defined as the average of the four-dialysate samples collected immediately before the first injection. The mean percent changes from baseline were then calculated for each 15/20 min sample for all rats in each group. Data were analyzed by one- or two-way ANOVA followed by Tukey’s multiple comparisons test using the GraphPad Prism software.(version 7.0b, GraphPad Software, San Diego, CA, USA) Data are presented as mean±s.e.m. where eight animals per group were estimated sufficient for a valid statistical outcome.
Results
Human PET study
Subjective and cardiovascular effects
Figure 1 shows the composite score of the subjective effects reported by the healthy subjects on the visual analog rating scale. As expected, there was a main effect for time point of measurement (F=419.6; P<0.001), showing that the amphetamine injection caused a subjective drug effect over time. Repeated measures ANOVA also revealed a main effect for treatment condition (F=482.1; P<0.001), such that the placebo+amphetamine condition produced a significantly stronger subjective drug effect than the NTX+amphetamine condition. In other words, NTX reduced the subjective effects of amphetamine. NTX did not produce any significant differences in heart rate or pulse (data not shown).
Subjective effects of amphetamine (Amph; 0.3 mg kg−1), after pretreatment with placebo or naltrexone (NTX; 50 mg), as measured by visual analog scales. Values represent the mean±S.E.M. An i.v. dose of amphetamine produced a significant increase in the reporting of subjective effects in healthy individuals (F=419.6; P<0.001). Repeated measures ANOVA revealed that pretreatment with NTX compared with placebo significantly attenuated the ratings of the subjective effects of amphetamine (F=482.1; P<0.001).
Effects on dopamine release
Two-way repeated-measures ANOVA revealed a main effect of condition (F=9.76, P=0.015), a main effect of brain region (F=67.76, P<0.001) and a condition-by-region interaction (F=4.21, P=0.024) on [11C]raclopride BPND. Post hoc paired t-tests demonstrated significantly decreased BPND in all striatal ROIs for both placebo+amphetamine and NTX+amphetamine as compared to baseline, indicating increased endogenous DA levels. However, there was no significant difference in BPND between placebo+amphetamine and NTX+amphetamine (Figure 2). The results were similar for all subregions of the striatum (Table 1).
PET [11C]raclopride BPND in the striatum (all subregions combined) at baseline and after an injection of amphetamine (0.3 mg kg−1), pre-treated with placebo or naltrexone (NTX). Values represent the mean and 95% confidence interval. There was no significant difference in the [11C]raclopride BPND between the experimental conditions placebo+amphetamine and NTX+amphetamine, for any of the subregions of the striatum. Amph, amphetamine; BPND, binding protein; PET, positron emission tomography.
Microdialysis
Basal levels of DA in the N.Acc did not differ significantly between the different treatment groups (P>0.05) in any of the in vivo microdialysis studies. Pooling of the data from all animals resulted in a mean DA level of 5.71±0.31 fmol min−1.
Two-way ANOVA of the data from the acute amphetamine (0.5 mg kg−1) experiment revealed significant time, treatment and interaction effects (Finteraction(42,252)=4.498, P<0.0001). NTX (3 mg kg−1) alone did not significantly influence DA output in the N.Acc. Amphetamine (0.5 mg kg−1) significantly increased DA output 15 min after administration (P<0.05) compared to saline, an effect that lasted for 45 min (Figure 3a). NTX (3 mg kg−1) pretreatment did not suppress amphetamine-induced DA release. When amphetamine was administered at a dose of 2.0 mg kg−1, two-way ANOVA revealed similar effects (Finteraction(42,248)=23.39, P<0.0001), and at this dose amphetamine caused a robust increase of DA output 15 min after administration compared to saline (P<0.001), an effect that lasted up to two hours (Figure 3b). NTX (3 mg kg−1) pretreatment did not affect the amphetamine-induced DA output at any time point.
Extracellular dopamine levels in the nucleus accumbens as measured by in vivo microdialysis in Wistar rats (n=5–6 per group). Rats received pretreatment with naltrexone (NTX; 3 mg kg−1) or vehicle at time point=0, followed by an injection of amphetamine (0.5 mg kg−1) or saline (a) and amphetamine 2.0 mg kg−1 or saline (b) at time point=30 min. Values represent the mean±s.e.m. Amphetamine (Amph; 0.5 mg kg−1) significantly increased dopamine (DA) output 15 min after administration (P<0.05) compared to baseline. NTX pretreatment did not suppress the amphetamine-induced DA release (a). Amphetamine at a dose of 2.0 mg kg−1 caused a robust increase of DA output 15 min after administration (P<0.01) compared to baseline. NTX pretreatment did not affect the amphetamine-induced DA output at any time point (b). Veh, vehicle.
In the chronic model, with daily administration of amphetamine (2 mg kg−1) for ten days and a subsequent drug-free period of ten days, two-way ANOVA revealed significant time, treatment and interaction effects (Finteraction (27,234)=8.365, P<0.0001). Post hoc analysis revealed that reinstatement with a challenge dose of amphetamine (0.5 mg kg−1) significantly increased DA output 20 min after administration compared to baseline (Figure 4). There was also a significant difference between NTX+vehicle treatment and NTX+amphetamine treatment (P=0.014) and between vehicle+amphetamine treatment and NTX+amphetamine treatment (P=0.030). Thus, NTX pretreatment attenuated the amphetamine-induced DA elevation by ~50% in this model with chronic amphetamine administration.
Extracellular dopamine levels in the nucleus accumbens as measured by in vivo microdialysis in Wistar rats (n=7–8 per group). All animals received 10 days of daily amphetamine 2 mg kg−1 (open symbols) or saline (closed symbols) administration followed by 10 days of abstinence before initiation of the dialysis experiment. On the day of the experiment the rats first received naltrexone (NTX) or vehicle, followed by an acute injection of either amphetamine (0.5 mg kg−1) or saline. Values represent the mean±s.e.m. Reinstatement with a challenge dose of amphetamine (Amph; 0.5 mg kg−1) significantly increased dopamine (DA) output 20 min after administration compared to baseline. A significant interaction (treatment x time, F21,182=10.121, P<0.001) was found between the groups and post hoc analysis revealed a significant difference (P=0.009), with NTX blunting the amphetamine-induced elevation by 50%. Veh, vehicle.
Discussion
In humans, pretreatment with NTX significantly attenuated the subjective effects of i.v. amphetamine, a finding that confirms earlier studies using oral amphetamine.7, 8 As expected, amphetamine led to a robust decrease in striatal [11C]raclopride binding, indicating an increase in extracellular DA levels. Contrary to our hypothesis, NTX did not attenuate the amphetamine-induced DA increase in the striatum.
For ethical reasons, repeated amphetamine injections to human research subjects should be avoided. Therefore, we used a rat model to further investigate the neurochemical effects of NTX pretreatment in amphetamine-exposed rats. In the acute model, the microdialysis data confirmed our PET results, that is, that NTX did not affect amphetamine-induced DA release in previously drug-naive animals. However, following chronic exposure to amphetamine, we found that NTX attenuated the amphetamine-induced DA response by approximately 50%. This finding is in agreement with our previous study on locomotor sensitization in which NTX had no effect on acute amphetamine-induced locomotion but attenuated the response in chronically treated animals.16 It also fits with observations in our previous experiments, where the effect of NTX on the subjective experience was of higher magnitude in dependent patients than in healthy individuals.7, 8
The findings from the acute experiments with both PET and in vivo microdialysis stand in contrast to the results of Schad et al., who found that pretreatment with naloxone attenuated the amphetamine-induced increase in extracellular DA in N. Acc. in rats.26 Potential explanations for this discrepancy include the use of a different rat strain, a cumulative, sub-cutaneous amphetamine-dosing schedule and pretreatment with naloxone rather than NTX. In addition, the limited sample size also makes the findings less certain and they have thus far not been replicated. To our knowledge, the present study is the first to utilize a translational methodology to examine the mechanism of an opioid antagonist in amphetamine use.
An explanation for the effects of NTX on the subjective effects of amphetamine could be a direct effect of amphetamine on endogenous opioid release.27, 28, 29 We previously tested this hypothesis using PET and the μ opioid receptor ligand [11C]carfentanil. By using an i.v. amphetamine dose identical to the one in the present study in a cross-over, randomized experiment, we found no evidence of such an acute amphetamine-induced opioid release in healthy humans.30 Other studies have found reduced [11C]carfentanil binding in several brain regions three hours after an oral amphetamine dose, but it is unclear whether this is related to the subjective effects of the drug, since these effects follow within minutes after an intravenous injection.31, 32
The mechanisms whereby NTX attenuates the acute subjective effects of amphetamine in drug-naIve subjects are not fully understood. While NTX typically does not cause any subjective effects when administered on its own, its effects in models of chronic amphetamine exposure may be related to increased expression of endogenous opioids.33 Another possibility is that NTX might have other pharmacological effects besides being an opioid antagonist. Amphetamine has a complex mechanism of action that is still not fully understood and it affects several different neurotransmitters besides DA.1 It is possible that NTX interferes with such processes in ways that are still unknown. Since NTX works as a non-specific opioid antagonist, other opioid receptor subtypes besides the μ-receptor might also play a role, but this has not yet been systematically investigated.
An alternative possibility is that NTX does not alter the immediate actions of amphetamine at the brain stem or striatal levels, but instead affects higher-order cognitive and affective processing of the pharmacological stimulus. Expectation effects might be relevant in this context, since DA is involved in reward prediction and there also is plenty of evidence for the importance of opioid mechanisms in placebo effects.34 An individual expecting a pleasant effect from the injection of a study drug might actually experience and rate it as more pleasurable than someone without such expectations, and an opioid antagonist might attenuate this effect.35 In our PET study with [11C]carfentanil mentioned above, we found no evidence of expectancy-induced opioid release when the participants knew they would receive an amphetamine injection,30 but this might be different in individuals previously conditioned to amphetamine.
In a recent study of individuals with methamphetamine use disorder, pretreatment with NTX was found to reduce blood-oxygen-level dependent functional magnetic resonance imaging cue-reactivity and also alter the functional connectivity of certain mesolimbic and mesofrontal circuits.36 Since cue-induced stimulant craving has previously been shown to correlate with striatal DA release, this provides further evidence of the importance of DA-opioid interactions in stimulant addiction.37
One could speculate that different DA pathways are involved in different stages of amphetamine exposure.38, 39 In this study, we have studied the mesostriatal DA pathway, and possible downstream effects in areas like the ventral pallidum would not have been detected (Olive et al.40). Further studies are needed to investigate whether opioid antagonists modulate other DA projections after acute and chronic amphetamine exposure.
It may be informative to compare the findings described above with the literature on cocaine and the endogenous opioid system. Opioid antagonists have shown mixed results in rat models of cocaine-induced behaviors such as self-administration and reinstatement.26, 41 On the neurochemical level, there is evidence for upregulation of μ and δ opioid receptors following chronic cocaine exposure.42, 43 Consistent with this, human PET studies have shown increased prefrontal and striatal [11C]carfentanil binding in cocaine dependent patients compared to controls. These changes have also been shown to correlate with cocaine craving and risk of relapse.44, 45, 46, 47 Laboratory studies have shown that NTX does not affect the acute subjective effects of cocaine but does attenuate priming-induced cocaine craving.48 However, clinical trials of NTX for cocaine dependence have not produced any evidence for a relapse preventive effect.49 In other words, the preclinical rationale for NTX treatment of cocaine dependence seems quite convincing, but there is a lack of well-designed and adequately powered clinical trials to determine its possible clinical efficacy. For amphetamine, the evidence from clinical trials is stronger, but more research is needed to understand the mechanisms behind the therapeutic effect of NTX.
Earlier PET studies have found that injection of an opioid agonist (for example, heroin) has no significant effect on striatal DA release in humans.50, 51 Thus, whereas acute amphetamine administration produced no immediate endogenous opioid release30 and an opioid antagonist did not affect the DA release induced by acute amphetamine administration to drug-naive subjects, interactions between striatal DA and opioid systems may be related to chronic amphetamine exposure, as shown by the present microdialysis results. The physiological mechanisms behind this transition are still unclear. We hypothesize that enhanced interactions between brain DA and opioid systems correlate with stimulant addiction severity, but this remains to be investigated.
In summary, the reduction of amphetamine’s acute subjective effects by NTX in drug-naive humans was not related to changes in DA transmission. Similarly, NTX did not have any effect on extracellular DA in rodents following an acute dose of amphetamine. In contrast, NTX significantly attenuated DA release caused by a challenge dose of amphetamine in rodents chronically exposed to amphetamine. The results suggest that the mechanisms whereby NTX modulates the effects of amphetamine are different in acute compared to chronic use. The current findings have the potential of advancing the knowledge of the mechanism of action of NTX as a pharmacological treatment for stimulant dependence for which at present, there exists no approved treatment.
References
Sulzer D, Sonders MS, Poulsen NW, Galli A . Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 2005; 75: 406–433.
Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 2001; 49: 81–96.
Swanson JM, Volkow ND . Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev 2003; 27: 615–621.
Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A . Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 2002; 27: 1027–1035.
Smith KS, Berridge KC, Aldridge JW . Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A 2011; 108: E255–E264.
Brauer LH, de Wit H . Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry 1996; 39: 26–32.
Jayaram-Lindström N, Wennberg P, Hurd YL, Franck J . Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol 2004; 24: 665–669.
Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J . Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology 2008; 33: 1856–1863.
Marks KR, Lile JA, Stoops WW, Rush CR . Separate and combined impact of acute naltrexone and alprazolam on subjective and physiological effects of oral d-amphetamine in stimulant users. Psychopharmacology (Berl) 2014; 231: 2741–2750.
Ray LA, Bujarski S, Courtney KE, Moallem NR, Lunny K, Roche D et al. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: a Double-Blind, Placebo-Controlled Laboratory Study. Neuropsychopharmacology 2015; 40: 2347–2356.
Jayaram-Lindström N, Hammarberg A, Beck O, Franck J . Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry 2008; 165: 1442–1448.
Grant JE, Odlaug BL, Kim SW . A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol 2010; 20: 823–828.
Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Föhr J et al. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry 2012; 169: 531–536.
Häggkvist J, Lindholm S, Franck J . The effect of naltrexone on amphetamine-induced conditioned place preference and locomotor behaviour in the rat. Addict Biol 2009; 14: 260–269.
Häggkvist J, Lindholm S, Franck J . The opioid receptor antagonist naltrexone attenuates reinstatement of amphetamine drug-seeking in the rat. Behav Brain Res 2009 30; 197: 219–224.
Häggkvist J, Björkholm C, Steensland P, Lindholm S, Franck J, Schilström B . Naltrexone attenuates amphetamine-induced locomotor sensitization in the rat. Addict Biol 2011; 16: 20–29.
Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 1994; 16: 255–262.
Laruelle M . Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20: 423–451.
Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci 2007 11; 27: 3998–4003.
Cervenka S, Bäckman L, Cselényi Z, Halldin C, Farde L . Associations between dopamine D2-receptor binding and cognitive performance indicate functional compartmentalization of the human striatum. NeuroImage 2008; 40: 1287–1295.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D-R, Huang Y et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23: 285–300.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–1057.
Joel D, Weiner I . The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 2000; 96: 451–474.
Lammertsma AA, Hume SP . Simplified reference tissue model for PET receptor studies. NeuroImage 1996; 4 (3 Pt 1): 153–158.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. 4th edn, San Diego, CA, USA: Academic Press, 1998.
Schad CA, Justice JB, Holtzman SG . Naloxone reduces the neurochemical and behavioral effects of amphetamine but not those of cocaine. Eur J Pharmacol 1995; 275: 9–16.
Olive MF, Koenig HN, Nannini MA, Hodge CW . Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 2001; 21: RC184.
Xia Y, He L, Whistler JL, Hjelmstad GO . Acute amphetamine exposure selectively desensitizes kappa-opioid receptors in the nucleus accumbens. Neuropsychopharmacology 2008; 33: 892–900.
Schad CA, Justice JB, Holtzman SG . Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther 2002; 300: 932–938.
Guterstam J, Jayaram-Lindström N, Cervenka S, Frost JJ, Farde L, Halldin C et al. Effects of amphetamine on the human brain opioid system—a positron emission tomography study. Int J Neuropsychopharmacol 2013; 16: 763–769.
Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry 2012; 72: 371–377.
Mick I, Myers J, Stokes PRA, Erritzoe D, Colasanti A, Bowden-Jones H et al. Amphetamine induced endogenous opioid release in the human brain detected with [11C]carfentanil PET: replication in an independent cohort. Int J Neuropsychopharmacol 2014; 17: 2069–2074.
Turchan J, Maj M, Przewłocka B, Przewłocki R . Effect of cocaine and amphetamine on biosynthesis of proenkephalin and prodynorphin in some regions of the rat limbic system. Pol J Pharmacol 2002; 54: 367–372.
Peciña M, Zubieta J-K . Molecular mechanisms of placebo responses in humans. Mol Psychiatry 2015; 20: 416–423.
Mitchell SH, Laurent CL, de Wit H . Interaction of expectancy and the pharmacological effects of d-amphetamine: subjective effects and self-administration. Psychopharmacology (Berl) 1996; 125: 371–378.
Courtney KE, Ghahremani DG, Ray LA . The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans. Neuropsychopharmacology 2016; 41: 2872–2881.
Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006; 26: 6583–6588.
Wiskerke J, Schetters D, van Es IE, van Mourik Y, den Hollander BRO, Schoffelmeer ANM et al. μ-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. J Neurosci 2011; 31: 262–272.
Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 2006; 63: 1386–1395.
Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT . Presynaptic versus postsynaptic localization of mu and delta opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci 1997; 17: 7471–7479.
Gerrits MAFM, Kuzmin AV, van Ree JM . Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. Eur Neuropsychopharmacol 2005; 15: 297–303.
Unterwald EM, Horne-King J, Kreek MJ . Chronic cocaine alters brain mu opioid receptors. Brain Res 1992; 584: 314–318.
Unterwald EM, Rubenfeld JM, Kreek MJ . Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport 1994; 5: 1613–1616.
Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ . Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med 1996; 2: 1225–1229.
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M et al. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry 2005; 57: 1573–1582.
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML et al. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008; 200: 475–486.
Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B et al. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biol Psychiatry 2010; 68: 697–703.
Comer SD, Mogali S, Saccone PA, Askalsky P, Martinez D, Walker EA et al. Effects of acute oral naltrexone on the subjective and physiological effects of oral D-amphetamine and smoked cocaine in cocaine abusers. Neuropsychopharmacology 2013; 38: 2427–2438.
Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, Moeller FG . High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addict 2009; 18: 356–362.
Daglish MRC, Williams TM, Wilson SJ, Taylor LG, Eap CB, Augsburger M et al. Brain dopamine response in human opioid addiction. Br J Psychiatry 2008; 193: 65–72.
Watson BJ, Taylor LG, Reid AG, Wilson SJ, Stokes PR, Brooks DJ et al. Investigating expectation and reward in human opioid addiction with [(11) C]raclopride PET. Addict Biol 2014; 19: 1032–1040.
Acknowledgements
We thank Dr Per Stenkrona and the staff at the PET centre, Karolinska Institutet for the assistance with the PET experiment and Rosita Stomberg for technical assistance in the microdialysis experiment. This work was funded by grants from the Swedish Research Council 2012-2607 (JF) and 2014-3887 (ME), the Swedish Research Council for Health, Working life and Welfare 2013-1849 (JF) and the Swedish Brain Foundation (JF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jayaram-Lindström, N., Guterstam, J., Häggkvist, J. et al. Naltrexone modulates dopamine release following chronic, but not acute amphetamine administration: a translational study. Transl Psychiatry 7, e1104 (2017). https://doi.org/10.1038/tp.2017.79
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.79
This article is cited by
-
Blunted endogenous opioid release following an oral dexamphetamine challenge in abstinent alcohol-dependent individuals
Molecular Psychiatry (2020)
-
Serotonin concentration enhancers at clinically relevant doses reduce [11C]AZ10419369 binding to the 5-HT1B receptors in the nonhuman primate brain
Translational Psychiatry (2018)
-
Verification of a genetic locus for methamphetamine intake and the impact of morphine
Mammalian Genome (2018)