Abstract
Maternal depressive symptoms influence neurodevelopment in the offspring. Such effects may appear to be gender-dependent. The present study examined contributions of prenatal and postnatal maternal depressive symptoms to the volume and microstructure of the amygdala in 4.5-year-old boys and girls. Prenatal maternal depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS) at 26 weeks of gestation. Postnatal maternal depression was assessed at 3 months using the EPDS and at 1, 2, 3 and 4.5 years using the Beck’s Depression Inventory-II. Structural magnetic resonance imaging and diffusion tensor imaging were performed with 4.5-year-old children to extract the volume and fractional anisotropy (FA) values of the amygdala. Our results showed that greater prenatal maternal depressive symptoms were associated with larger right amygdala volume in girls, but not in boys. Increased postnatal maternal depressive symptoms were associated with higher right amygdala FA in the overall sample and girls, but not in boys. These results support the role of variation in right amygdala structure in transmission of maternal depression to the offspring, particularly to girls. The differential effects of prenatal and postnatal maternal depressive symptoms on the volume and FA of the right amygdala suggest the importance of the timing of exposure to maternal depressive symptoms in brain development of girls. This further underscores the need for intervention targeting both prenatal and postnatal maternal depression to girls in preventing adverse child outcomes.
Similar content being viewed by others
Introduction
Perinatal maternal depression is associated with an increased risk for emotional,1, 2, 3 behavioral4 and cognitive problems,5 as well as multiple forms of psychopathology6, 7, 8 in the offspring. Compelling evidence also suggests the influence of perinatal maternal depressive symptoms on brain development,9, 10, 11, 12, 13, 14, 15 particularly on the amygdala, a brain structure critical for emotional processing,16, 17 stress reactivity18 and vulnerability to depression.19 Recent research showed a significant association between prenatal maternal depressive symptoms and the amygdala microstructure in neonates shortly after birth.11 Prenatal maternal depressive symptoms also modulate the development of amygdala functional organization in the first 6 months of life.12 Beyond prenatal maternal depression, increased postnatal maternal depressive symptoms associate with a larger amygdala volume in 10-year-old children.10 These are in line with findings on an increased amygdala volume in children under institutional rearing conditions.20, 21 Likewise, the first-degree relatives of patients with major depressive disorder were found to have a larger amygdala volume than healthy controls.22 These findings highlight the vulnerability of amygdala development in offspring to the exposure of an early adverse environment. Hence, the amygdala can be an interesting candidate brain structure for understanding the biological basis of the association between maternal emotional well-being and the mental health of the offspring. Nevertheless, the timing for the influence of maternal depression on amygdala development in early childhood remains unclear, which complicates models of risk as well as the design and timing of preventive interventions.
Prenatal and postnatal maternal depressive symptoms have an impact on the offspring possibly through distinct pathways. Depressed mothers during pregnancy exhibit a number of physiological changes that may influence intrauterine environment and hence implicate alterations in fetal development. In contrast, postnatal maternal depression influences the offspring most likely through forms of parenting14, 23, 24 that enhance fearfulness, social withdrawal25, 26, 27, 28 and predict an increased risk for later psychopathology in the offspring.29 One would expect that prenatal and postnatal maternal depressive symptoms independently influence brain development in offspring. Indeed, prenatal and postnatal maternal depression independently predicts an increased risk for depression in offspring.30 Interestingly, a very recent neuroimaging study suggested differential and independent influences of prenatal and postnatal maternal depressive symptoms on cortical morphology and white matter microstructure in children aged between 2.6 and 5.1 years old.31 Alternatively, environmental conditions at one stage in development influence the sensitivity to later conditions.32 Changes in the intrauterine environment due to prenatal maternal depression may increase the child’s susceptibility to postnatal maternal depression. Although this hypothesis is less investigated, Lusby et al.33 recently showed that higher levels of postnatal depressive symptoms were associated with greater relative right frontal electroencephalogram asymmetry specifically among infants whose mothers had higher levels of prenatal depressive symptoms, suggesting an interaction effect of prenatal and postnatal maternal depressive symptoms on neurodevelopment. Nevertheless, there is a lack of knowledge on how prenatal and postnatal maternal depressive symptoms interplay and impact brain development in offspring, particularly the amygdala.
An extensive meta-analysis34 shows that in community samples, the association between maternal depressive symptoms and internalizing problems is stronger in girls. Likewise, the association between maternal emotional well-being and amygdala-prefrontal connectivity is unique to girls.35 A higher maternal cortisol level in early gestation is associated with larger right amygdala volume in childhood, but only in girls.9 These findings underscore the importance of gender-dependent developmental pathways of neuronal substrates that underlie the risk for transgenerational transmission of depression from mother to child.
In this study, we examined the extent to which prenatal and postnatal maternal depressive symptoms interactively or independently contribute to the development of the amygdala structure in 4.5-year-old children. We employed both structural magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI)36 to assess the volume and microstructure of the amygdala. Prenatal and postnatal maternal depressive symptoms were assessed at multiple time points. Given considerable evidence for sexually dimorphic associations between maternal mental health and neurodevelopmental outcomes,37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 we further explored the above questions in the full, female and male samples, respectively, to elucidate potential gender differences of the amygdala volume and microstructure in response to maternal depressive symptoms in early life. If both prenatal and postnatal maternal depressive symptoms interactively influence amygdala development, we hypothesized that this interaction would influence both the size and structural organization of the amygdala. If prenatal and postnatal maternal depressive symptoms independently contribute to amygdala development, the effects of maternal depressive symptoms on the size and structural organization may presumably be dependent on the underlying developmental processes of the brain, such as neurogenesis, synaptogenesis and so on. The pronounced relationship between maternal depressive symptoms and the amygdala would be greater in girls than in boys.
Materials and methods
Participants
Three hundred and forty-two mother–child dyads who participated in the prospective GUSTO (Growing Up in Singapore Towards healthy Outcomes) birth cohort study were recruited for neuroimaging study when the children were 4.5 years of age. The GUSTO cohort recruited pregnant Singapore citizens, or permanent residents of Chinese, Malay or Indian ethnic backgrounds from two major birthing hospitals in Singapore at the first antenatal visit (see Soh et al.48 for further details). The GUSTO study was approved by the National Healthcare Group Domain Specific Review Board and the Sing Health Centralized Institutional Review Board. Written informed consent was obtained from mothers.
Maternal education, ethnicity, age and monthly household income were extracted from survey questionnaires conducted as part of a scheduled appointment during the 26th week of pregnancy. Birth outcomes, including gestational age, birth weight, APGAR (Appearance, Pulse, Grimace, Activity, and Respiration) score and gender, were obtained from the hospital record.
This study only included children with gestational age ⩾34 weeks, birth weight ⩾2 kg and a 5-min APGAR score ⩾8 to avoid potential effects of birth complications on the brain development and with maternal reports on depression scales.
Maternal depression scales
The Edinburgh Postnatal Depression Scale (EPDS) questionnaire was administered to mothers at 26 weeks of pregnancy and 3 months after delivery to assess depressive symptomatology. The EPDS49 is a widely used 10-item self-report scale designed as a screening instrument for postnatal depression and valid for use in prenatal and early postnatal depression. Each item of the EPDS is scored on a four-point scale (0–3), and items 3 and 5–10 are reverse-scored.
The Beck’s Depression Inventory-II (BDI-II) was administered to mothers at 1, 2, 3 and 4.5 years postpartum. The BDI-II is a widely used 21-item questionnaire that assesses the existence and severity of symptoms of depression and predicts the severity of clinical depressive symptoms.50 Each item of the BDI-II is scored on a four-point scale (0–3). Higher total scores indicate more severe depressive symptoms.
Prorating imputation was performed when 8 or 9 questions were answered on the EPDS or 19 or 20 questions were answered for the BDI-II. All EPDS and BDI measures were standardized. As the scores of postnatal maternal depressive symptoms were highly correlated with each other (r>0.5, P<0.001), the averaged score across the postnatal points was computed to quantify the severity of postnatal maternal depressive symptoms up to 4.5 years postpartum.
MRI acquisition and quality check
Children underwent MRI scans at age of 4.5 years (±1month) using a 3 T Siemens Skyra scanner (Siemens, Munich, Germany) with a 32-channel head coil at KK Women’s and Children’s Hospital. Children were recruited during a 4-year home visit. Children went through an MRI home training program prior to the MRI visit and on-site MRI training (see details in the Supplementary Material). The image protocols were: (i) high-resolution isotropic T1-weighted magnetization prepared rapid gradient recalled echo (192 slices, 1 mm thickness, in-plane resolution 1 mm, sagittal acquisition, field of view 192 × 192 mm, matrix=192 × 192, repetition time=2000 ms, echo time=2.08 ms, inversion time=877 ms, flip angle=9°, scanning time=3.5 min); (ii) isotropic axial diffusion-weighted imaging protocol (single-shot echo-planar sequence, 69 slices of 2.0 mm thickness, with no inter-slice gaps, matrix 96 × 96, field of view 192 × 192 mm, repetition time=8200 ms, echo time=85 ms, flip angle=90°, 30 diffusion-weighted images with b=1000 s mm−2, 5 baseline images without diffusion weighting, GRAPPA=3, scanning time=5.5 min).
The image quality was verified immediately after the acquisition through visual inspection when children were still in the scanner. A scan was repeated when ring artifact on T1-weighted images and signal loss on DTI were large (see an example in Supplementary Figure S1). The image was removed from the study if no acceptable image was acquired after three repetitions.
Structural MRI and DTI analysis
FreeSurfer was used to label each voxel in the T1-weighted image as gray matter, or white matter, or cerebrospinal fluid, or subcortical structures (for example, hippocampus, amygdala, thalamus, caudate, putamen and globus pallidus).51 FreeSurfer employed a Markov random field model that requests for a prior probability obtained from a training data set with T1-weighted images and their manual structural labels. In this study, we reconstructed the prior probability in the Markov random field model based on the manual segmentation of 30 Asian children and embedded it in FreeSurfer (replacing RB_all_2008-03-26.gca under freesurfer/average). FreeSurfer was then performed to each T1-weighted image in this study. Post-processing quality check was conducted following by the instruction on https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingData. The segmentation accuracy was assessed using a volume overlap ratio (VOR) between the automated and manual segmentation.51 The VOR values for the amygdala is 0.90±0.05, suggesting the high accuracy of the automated segmentation when compared with the manual labeling.
Within individual subjects, diffusion-weighted images were corrected for motion and eddy current distortions using affine transformation to the image without diffusion weighting.52 Using multivariate least-square fitting, six elements of the diffusion tensor were then determined, from which fractional anisotropy (FA) was calculated. The amygdala mask in the T1-weighted image was then superimposed to the FA images through affine transformation obtained between the image without diffusion weighting and T1-weighted image. Mean FA values were computed for the amygdala and used in the following statistical analysis.
Statistical analysis
Multiple regression analyses were used to examine the interactive, independent and main effects of prenatal and postnatal maternal depression on the amygdala volume and FA. These regression analyses were repeated for three groups of subjects: (1) the overall sample, (2) girls and (3) boys.
Interaction model (interactive effects)
The interaction of prenatal and postnatal maternal depression was formed as the product of the two standardized predictors. A hierarchical order of entry was used to enter predictors. Covariates were entered in the first block followed by prenatal maternal depression and postnatal maternal depression in the second block and the interaction term in the third block.
Reduced model (independent effects)
In cases where the interaction term was not significant, a reduced regression model without the inclusion of the interaction term was used to consider independent effects of prenatal and postnatal maternal depression on the amygdala volume and FA.
Separate models (main effects)
The main effects of prenatal or postnatal maternal depression on bilateral amygdala volume and FA were also examined in two separate models. In the first model, covariates were entered in the first block followed by prenatal maternal depression in the second block. In the second model, covariates were entered in the first block followed by postnatal maternal depression in the second block.
Confounding variables
This study considered variables that are either related to the amygdala volume or risk factors for maternal depression. Hence, the age at MRI, maternal ethnicity, maternal education and total brain volume were included as common covariates in the regression analysis to control for potential influences on the amygdala volume. The same covariates except for total brain volume were included in the regression analysis on the amygdala FA. In addition, gender was also used as covariate when analyzing the overall sample. Covariates that had a categorical level of measurement (that is, maternal ethnicity) were dummy coded before they were entered into the regression model to ensure their suitability for regression. As household income was highly correlated with maternal education (r=0.521; P<0.001) and maternal age was highly correlated with household income (r=0.281; P<0.001), these were not further included as covariates to avoid potential collinearity problems.
Results
Demographics
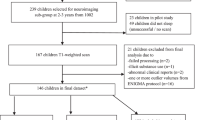
Of the 342 subjects who underwent MRI, 77 subjects had unusable T1 or DTI data due to image quality, 4 did not meet the inclusion criteria and 26 mothers of infants did not complete depression questionnaires (that is, EPDS or BDI). Hence, the total sample size in this study included 235 subjects. Among them, 203 (95 boys and 108 girls) had good T1 data and 188 (88 boys and 100 girls) had good DTI data. Table 1 lists the demographic information of the full, boy and girl samples that were used in this study. We also list the demographic information of 77 subjects who were not used in this study due to image quality in Supplementary Table S1.
The mothers of boys and girls did not differ in prenatal (t233=1.68, P=0.094), and postnatal maternal depressive symptoms (t233=1.12, P=0.264), maternal education (t231=−0.650, P=0.516) and maternal ethnicity ( =1.942, P=0.379).
=1.942, P=0.379).
Relations between maternal depressive symptoms and the amygdala volume
The full sample (Table 2) showed no interaction of prenatal and postnatal maternal depressive symptoms on the left (β=0.005, P=0.944, df=192) or right (β=0.098, P=0.141, df=191) amygdala volumes. Similarly, there was no independent effect of prenatal maternal depressive symptoms on left (β=0.095, P=0.150, df=193) and right (β=0.097, P=0.137, df=192) amygdala volumes after adjusting for postnatal maternal depressive symptoms. There were no effects of postnatal maternal depressive symptoms on left (β=0.030, P=0.648, df=193) or right (β=−0.003, P=0.958, df=192) amygdala volumes after adjusting for prenatal maternal depressive symptoms.
The main effects remained largely similar when prenatal and postnatal maternal depressive symptoms were considered in separate models. There was a trend for a significant effect for prenatal maternal depressive symptoms in relation with the left amygdala volume (β=0.109, P=0.061, df=194) and right amygdala volume (β=0.096, P=0.097, df=193). Postnatal maternal depressive symptoms did not significantly predict left (β=0.074, P=0.203, df=194) or right (β=0.042, P=0.466, df=193) amygdala volume.
The effects of prenatal and postnatal maternal depressive symptoms on the amygdala volumes in the boy sample were the same as those for the full sample (Table 2). Interestingly, in girls, greater prenatal maternal depressive symptoms predicted a larger right amygdala volume with (β=0.219, P=0.043, df=99; Table 2; Figure 1a) and without (β=0.195, P=0.042, df=100) adjusting for postnatal maternal depressive symptoms. There were no independent effects of postnatal maternal depressive symptoms on the left (β=0.009, P=0.930, df=99) or right (β=−0.050, P=0.622, df=99) amygdala volumes.
Relations between maternal depressive symptoms and the amygdala microstructure
The full sample (Table 3) did not show a significant interaction of prenatal and postnatal maternal depressive symptoms on the left amygdala (β=−0.025, P=0.779, df=178) or right amygdala (β=−0.046, P=0.597, df=177) FA. There were no independent effects of prenatal maternal depressive symptoms on the left (β=0.026, P=0.768, df=179) or right (β=−0.020, P=0.811, df=178) amygdala FA after adjusting for postnatal maternal depressive symptoms. Likewise, there was no independent effect of postnatal maternal depression on left (β=0.105, P=0.218, df=179) amygdala FA. However, greater postnatal maternal depressive symptoms strongly predicted greater right (β=0.233, P=0.005, df=178) amygdala FA in the overall sample (Figure 1b).
The aforementioned relations remained essentially the same when prenatal and postnatal maternal depressive symptoms were entered into separate regression models. Prenatal maternal depressive symptoms did not predict the left amygdala (β=0.079, P=0.297, df=180) FA or right amygdala (β=0.097, P=0.195, df=179) FA. Postnatal maternal depressive symptoms did not significantly predict the left (β=0.117, P=0.112, df=180), but strongly significantly predicted the right (β=0.223, P=0.002, df=179) amygdala FA.
The effects of prenatal and postnatal maternal depressive symptoms on the amygdala microstructure in girls (Table 3; Figure 1c) were the same as those in the full sample. Again, in girls, greater postnatal maternal depressive symptoms predicted a higher right amygdala FA value with (β=0.348, P=0.001, df=92; Figure 1c) and without adjusting for prenatal maternal depressive symptoms (β=0.325, P=0.001, df=93). In contrast, the boy sample did not show any significant effects of prenatal and postnatal maternal depressive symptoms on the amygdala microstructure (Table 3).
Discussion
The present study investigated the relationship of prenatal and postnatal maternal depressive symptoms with amygdala volume and microstructure in children using a community sample. This study did not show any evidence on interaction of prenatal and postnatal maternal depressive symptoms on amygdala structure. However, this study revealed that greater prenatal maternal depressive symptoms predicted a larger right amygdala volume in girls, whereas postnatal maternal depressive symptoms associated with right amygdala microstructure in the overall sample and in girls, but not in boys. These findings suggested independent, differential influences of prenatal and postnatal maternal depressive symptoms on the structural development of the amygdala, with evidence for gender-specific effects.
Our study underscored the importance of the right amygdala in relation to maternal depressive symptoms. Our findings are consistent with previous reports showing differential effects of maternal mood on the right versus the left amygdala.9, 11 Although the biological basis for such asymmetric development effects is unknown, our findings are consistent with the hypothesis that maternal emotional well-being selectively affects neural structures implicated in the processing of negatively valenced emotional information and in the accompanying of stress responses.53, 54, 55, 56, 57 This is particularly applied to the right amygdala because its activation associates with a negative attentional bias and is considered as an endophenotype for both anxiety and depression.58 During stress induction, the right amygdala responds at equally high levels for threat-related and positively valenced stimuli.59 Childhood maltreatment associates with both a selective increase in right amygdala volume60 and a negative attentional bias.61 Moreover, greater prenatal maternal depressive symptoms associate with the right amygdala microstructure in neonates shortly after birth11 and greater postnatal maternal depressive symptoms predict a larger right amygdala volume in 10-year-old children.10 These findings implicated that the right amygdala could be a neural origin for transgenerational transmission of risk for mood disorders from mother to child.
Though limited, there is evidence suggesting that children born to depressed mothers may be more susceptible to postnatal maternal depression.1 Greater levels of postnatal depressive symptoms associate with greater abnormal right frontal electroencephalogram asymmetry particularly among infants whose mothers had greater levels of depressive symptoms during pregnancy.33 This is contradictory to what was observed in this study on the right amygdala. This could be partially due to differences in the level of the severity of maternal depressive symptoms. This study was based on the community sample, whereas existing literature focuses on clinical samples of depressed mother–child dyads.33 On the other hand, these findings could suggest that interactive or independent effects of maternal depressive symptoms are brain region specific, which could be mediated via common or distinct mechanisms.
Prenatal and postnatal maternal depressive symptoms independently contributed to the amygdala structural development in 4.5-year-old girls, which is consistent with the findings on the cortisol level among 4.5-year-old children.62 Greater maternal depressive symptoms during pregnancy predicted larger right amygdala volume in 4.5-year-old girls. A greater severity of postnatal maternal depressive symptoms associated with higher right amygdala FA at 4.5 years of age, again with effects apparent in girls. On the other hand, Lupien et al.10 found that larger bilateral amygdala volumes in 10-year-old children continually exposed to postnatal maternal depression since birth compared to those without exposure of postnatal maternal depression. Thus, the influence of maternal depressive symptoms on the specific measure of the amygdala differs as a function of the age of the offspring. Indeed, using the same birth cohort, we previously found that greater prenatal maternal depressive symptoms associated with lower FA values of the right amygdala, but with no association with the amygdala volume in neonates shortly after birth.11 Even though biological mechanisms underlying these findings are unclear, these may not surprising given the fact that various ontogenetic processes of development occur in the brain at different periods of time,63, 64 such that structural imaging studies are tracking a ‘moving target’. Our findings are also consistent with the results of comparable studies of corticolimbic development showing that the nature of the environmental influence on neural structure is dependent upon developmental timing.65 For instance, the preschool age is a sensitive period for the influence of maternal support on the trajectory of hippocampal development.66 High maternal support was also found to be associated with lower activation of the amygdala to fearful faces in adolescence.67 Likewise, our studies provided new evidence that the influence of maternal depressive symptoms on the amygdala depends on developmental timing in early life.11 The development of the amygdala begins at an early embryonic stage68 and continues well into postnatal life.69, 70 Axon myelination is thought to peak during the early postnatal period,71 whereas the development of axonal connections is known to be dependent on the postnatal environment. The postnatal environment includes the influence of parental care, which associates with developmental trajectories in corticolimbic structures.66 The development of individual differences in the amygdala structural and functional connectivity is influenced by the quality of the postnatal rearing environment,10, 20, 21, 72 with evidence for accelerated maturation in response to environmental adversity.73
Our findings were apparent in 4.5-year-old girls not boys, suggesting that the maternal influences on the amygdala development may be dependent on gender, whereby the same environmental condition leads to differential effects on boys and girls. The association between maternal depressive symptoms and internalizing problems is stronger in girls.34 In line with these findings, prenatal stress also leads to sexually dimorphic developmental consequences later in life.38, 39, 40, 41, 74 There exist gender-specific responses of the fetal-placental unit to stress, where female foetuses are more susceptible to changes in stress levels.75, 76 There is similar evidence for a positive association between prenatal maternal cortisol levels and right amygdala volume in girls, but not boys at 7 years of age.9 Hence, gender-dependent effects on neurodevelopmental outcomes are not unique to the influence of maternal mood but they cut across various forms of maternal adversity, including maternal depression, maternal stress, maternal cortisol and so on. However, little is known on what mechanisms could explain such gender-dependent effects on neurodevelopmental outcomes. Although substantial evidence supports the idea that gender differences in response to stress may be partly due to sex hormones,77, 78 we reported that sexually dimorphic developmental consequences (for example, amygdala) due to prenatal maternal depression are not shown shortly after birth11 but become pronounced at age of 4.5 years using the same sample as that in this study. This raises the question on when sex hormones starts to play a role in differentiating developmental consequences due to maternal environment between boys and girls, which needs future investigation.
To the best of our knowledge, this study is the first to combine multimodal magnetic resonance images in probing amygdala volume and microstructure within a relatively large sample of young children with the objective of understanding the timing of the influences of maternal depressive symptoms on amygdala development. Moreover, several unique features of the study include the analysis of the interactive or independent effects of prenatal and postnatal maternal depressive symptoms on amygdala volume and FA as well as the assessment of gender effects in the sample. Nevertheless, in consideration of both scientific importance and subject burden, our study only assessed prenatal maternal depression at one time point, namely, 26 weeks of gestation, given the fact that the second and third trimesters during pregnancy are critical periods of neural migration and synaptogenesis in the fetal brain. Additional measurements would have allowed for a better understanding of the specificity of timing during pregnancy. However, the existing data suggest that individual differences in depressive symptoms are in general stable across pregnancy.79, 80 In addition, our assessment of maternal depression was based on a common screening tool designed to elicit a subjective report of emotional well-being, but did not constitute a clinical assessment. The reported results are thus best considered as being associated with self-reported depressive symptoms and not with clinical depression, per se. Furthermore, potential factors, such as parenting, should be considered to understand possible pathways mediating the association of maternal depression and the amygdala structure in children. Finally, imaging children at this age is very challenging. Our study discarded 22.5% images acquired because of signal loss that was inspected qualitatively rather than quantitatively due to the lack of ground truth. In general, training children outside the scanner can significantly improve image acquisition.81 Our study had carefully designed the training program to get children familiar and cooperative with the scanning procedure and instruction, and to improve image quality (Supplementary Material).
Our study sought to investigate the effect of prenatal and postnatal maternal depressive symptoms on the volume and microstructure of the amygdala in 4.5-year-old children and the presence of sexually dimorphic development. Our findings indicate the independent and differential contributions of prenatal and postnatal maternal depressive symptoms to amygdala volume and microstructure in girls, but not in boys. Our study thus emphasizes the importance of the timing of exposure to maternal depressive symptoms, which further facilitates the understanding of the plasticity and vulnerability of the brain to maternal depression in early life, as well as the importance of gender.
References
Goodman SH, Gotlib IH . Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev 1999; 106: 458–490.
Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA . Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy 2004; 6: 319.
Chong SC, Broekman BF, Qiu A, Aris IM, Chan YH, Rifkin-Graboi A et al. Anxiety and depression during pregnancy and temperament in early infancy: findings from a multi-ethnic, asian, prospective birth cohort study. Infant Ment Health J 2016; 37: 584–598.
Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM . Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Dev Psychol 2000; 36: 759–766.
Sohr-Preston SL, Scaramella LV . Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin Child Fam Psychol Rev 2006; 9: 65–83.
Klein DN . A family study of major depressive disorder in a community sample of adolescents. JAMA 2001; 285: 2063.
Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H . Offspring of depressed parents: 20 years later. Am J Psychiatry 2006; 163: 1001–1008.
Hammen C, Brennan PA . Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch Gen Psychiatry 2003; 60: 253–258.
Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA . Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA 2012; 109: E1312–E1319.
Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108: 14324–14329.
Rifkin-Graboi A, Bai J, Chen H, Hameed WBr, Sim LW, Tint MT et al. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 2013; 74: 837.
Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 2015; 5: e508.
Soe NN, Wen DJ, Poh JS, Li Y, Broekman BF, Chen H et al. Pre- and post-natal maternal depressive symptoms in relation with infant frontal function, connectivity, and behaviors. PLoS ONE 2016; 11: e0152991.
Wen DJ, Soe NN, Wee SL, Sanmugam S, Kwek K, Chong Y-S et al. Infant frontal EEG asymmetry in relation with postnatal maternal depression and parenting behavior. Transl Psychiatry 2017; 7: e1057.
Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD et al. Maternal anxiety and infants' hippocampal development: timing matters. Transl Psychiatry 2013; 3: e306.
Phelps EA, LeDoux JE . Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005; 48: 175–187.
Weiskrantz L . Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol 1956; 49: 381–391.
Bogdan R, Hariri AR . Neural embedding of stress reactivity. Nat Neurosci 2012; 15: 1605–1607.
Price JL, Drevets WC . Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35: 192–216.
Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 2010; 13: 46.
Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry 2009; 50: 943–951.
Romanczuk-Seiferth N, Pöhland L, Mohnke S, Garbusow M, Erk S, Haddad L et al. Larger amygdala volume in first-degree relatives of patients with major depression. NeuroImage Clin 2014; 5: 62–68.
Fleming AS, Ruble DN, Flett GL, Shaul DL . Postpartum adjustment in first-time mothers: relations between mood, maternal attitudes, and mother-infant interactions. Dev Psychol 1988; 24: 71–81.
Rifkin-Graboi A, Kong L, Sim LW, Sanmugam S, Broekman BF, Chen H et al. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl Psychiatry 2015; 5: e668.
Degnan KA, Almas AN, Fox NA . Temperament and the environment in the etiology of childhood anxiety. J Child Psychol Psychiatry 2010; 51: 497–517.
Bruder-Costello B, Warner V, Talati A, Nomura Y, Bruder G, Weissman M . Temperament among offspring at high and low risk for depression. Psychiatry Res 2007; 153: 145–151.
Moffitt TE, Caspi A, Newman DL, Silva PA . Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. Arch Gen Psychiatry 1996; 53: 1033–1039.
Murray L, Halligan SL, Goodyer I, Herbert J . Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: a preliminary study. J Affect Disord 2010; 122: 218–223.
Biederman J, Faraone SV, Hirshfeld-Becker DR, Friedman D, Robin JA, Rosenbaum JF . Patterns of psychopathology and dysfunction in high-risk children of parents with panic disorder and major depression. Am J Psychiatry 2001; 158: 49–57.
Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 Years. JAMA Psychiatry 2013; 70: 1312–1319.
Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, Dewey D . Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol Psychiatry 2016; 80: 859–868.
Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH . For better and for worse: differential susceptibility to environmental influences. Curr Dir Psychol Sci 2007; 16: 300–304.
Lusby CM, Goodman SH, Bell MA, Newport DJ . Electroencephalogram patterns in infants of depressed mothers. Dev Psychobiol 2014; 56: 459–473.
Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D . Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011; 14: 1–27.
Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci 2012; 15: 1736–1741.
Qiu A, Mori S, Miller MI . Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol 2015; 66: 853–876.
Quarini C, Pearson RM, Stein A, Ramchandani PG, Lewis G, Evans J . Are female children more vulnerable to the long-term effects of maternal depression during pregnancy? J Affect Dis 2016; 189: 329–335.
Sandman CA, Glynn LM, Davis EP . Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res 2013; 75: 327–335.
Grey KR, Davis EP, Sandman CA, Glynn LM . Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology 2013; 38: 1178–1185.
Buss C, Davis EP, Hobel CJ, Sandman CA . Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress 2011; 14: 665–676.
David IWP, Jones A . Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol 2006; 572: 45–50.
Paul RH . The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat 2008; 2008: 193–201.
Murray L, Cooper PJ . Postpartum Depression and Child Development. Guilford Press: New York, USA, 1997.
Murray L . The impact of postnatal depression on infant development. J Child Psychol Psychiatry 1992; 33: 543–561.
Sharp D, Hay DF, Pawlby S, Schmücker G, Allen H, Kumar R . The impact of postnatal depression on boys' intellectual development. J Child Psychol Psychiatry 1995; 36: 1315–1336.
Kurstjens S, Wolke D . Effects of maternal depression on cognitive development of children over the first 7 years of life. J Child Psychol Psychiatry 2001; 42: 623–636.
Murray L, Fiori-Cowley A, Hooper R, Cooper P . The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev 1996; 67: 2512–2526.
Soh SE, Lee SSM, Hoon SW, Tan MY, Goh A, Lee BW et al. The methodology of the GUSTO cohort study: a novel approach in studying pediatric allergy. Asia Pac Allergy 2012; 2: 144–148.
Cox JL, Holden JM, Sagovsky R . Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987; 150: 782–786.
Beck AT, Ward CH, Mendelson MM, Mock JJ, Erbaugh JJ . An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355.
Huang H, Ceritoglu C, Li X, Qiu A, Miller MI, van Zijl PC et al. Correction of B0 susceptibility induced distortion in diffusion-weighted images using large-deformation diffeomorphic metric mapping. Magn Reson Imaging 2008; 26: 1294–1302.
Hamilton JP, Gotlib IH . Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry 2008; 63: 1155–1162.
Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport 1998; 9: 3301–3307.
Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A et al. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry 2008; 165: 872–880.
Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 2008; 63: 234–240.
Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL et al. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry 1997; 54: 233–241.
Beck AT . The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry 2008; 165: 969–977.
van Marle HJ, Hermans EJ, Qin S, Fernandez G . From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry 2009; 66: 649–655.
Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH . Sensitive periods of amygdala development: The role of maltreatment in preadolescence. Neuroimage 2014; 97: 236–244.
Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E et al. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry 2005; 162: 291–296.
Laurent HK, Leve LD, Neiderhiser JM, Natsuaki MN, Shaw DS, Harold GT et al. Effects of prenatal and postnatal parent depressive symptoms on adopted child HPA regulation: independent and moderated influences. Dev Psychol 2013; 49: 876–886.
Thompson RA, Nelson CA . Developmental science and the media: early brain development. Am Psychol 2001; 56: 5–15.
Stiles J, Jernigan TL . The basics of brain development. Neuropsychol Rev 2010; 20: 327–348.
Meaney MJ . Mother nurture and the social definition of neurodevelopment. Proc Natl Acad Sci USA 2016; 113: 6094–6096.
Luby JL, Belden A, Harms MP, Tillman R, Barch DM . Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci USA 2016; 113: 5742.
Romund L, Raufelder D, Flemming E, Lorenz RC, Pelz P, Gleich T et al. Maternal parenting behavior and emotion processing in adolescents—An fMRI study. Biol Psychol 2016; 120: 120–125.
Humphrey T . The development of the human amygdala during early embryonic life. J Comp Neurol 1968; 132: 135–165.
Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol 1996; 366: 223–230.
Holland D, Chang L, Ernst TM, Curran M, Buchthal SD, Alicata D et al. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol 2014; 71: 1266–1274.
Brody BA, Kinney HC, Kloman AS, Gilles FH . Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 1987; 46: 283–301.
Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM et al. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am J Psychiatry 2016; 173: 625–634.
Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM . Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 2015; 18: 1364.
Ellman LM, Schetter CD, Hobel CJ, Chicz‐DeMet A, Glynn LM, Sandman CA . Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol 2008; 50: 232–241.
Clifton VL . Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 2010; 31: S33–S39.
Clifton VL, Murphy VE . Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta 2004; 25: S45–S52.
Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N . Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 2010; 30: 431–438.
Handa RJ, Burgess LH, Kerr JE, O'Keefe JA . Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 1994; 28: 464–476.
Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C . Stability of mood states and biochemistry across pregnancy. Infant Behav Dev 2006; 29: 262–267.
Field T, Diego M, Hernandez-Reif M, Figueiredo B, Schanberg S, Kuhn C et al. Chronic prenatal depression and neonatal outcome. Int J Neurosci 2008; 118: 95–103.
Raschle NM, Lee M, Buechler R, Christodoulou JA, Chang M, Vakil M et al. Making MR imaging child's play - pediatric neuroimaging protocol, guidelines and procedure. J Vis Exp 2009; pii: 1309; doi:10.3791/1309.
Acknowledgements
The GUSTO study group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong-Fong Chong, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Keith M Godfrey, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D Holbrook, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Yung Seng Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A Rebello, Jenny L Richmond, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Mya Thway Tint, Hugo PS van Bever, Rob M van Dam, Inez Bik Yun Wong, PC Wong, Fabian Yap and George Seow Heong Yeo. This study is supported by National Medical Research Council (NMRC; NMRC/TCR/004-NUS/2008, NMRC/TCR/012-NUHS/2014, and NMRC/CBRG/0039/2013), the Hope for Depression Research Foundation and by The JPB Foundation through The JPB Research Network on Toxic Stress, A Project of the Center on the Developing Child at Harvard University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wen, D., Poh, J., Ni, S. et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry 7, e1103 (2017). https://doi.org/10.1038/tp.2017.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.74
This article is cited by
-
Brain structural and functional outcomes in the offspring of women experiencing psychological distress during pregnancy
Molecular Psychiatry (2024)
-
Maternal positive mental health during pregnancy impacts the hippocampus and functional brain networks in children
Nature Mental Health (2024)
-
Prevalence and factors associated with trajectories of antenatal depression: a prospective multi-center cohort study in Chengdu, China
BMC Pregnancy and Childbirth (2023)
-
Pathways link environmental and genetic factors with structural brain networks and psychopathology in youth
Neuropsychopharmacology (2023)
-
Lifelong effects of prenatal and early postnatal stress on the hippocampus, amygdala, and psychological states of Holocaust survivors
Scientific Reports (2023)