Abstract
Although a number of studies have analyzed the relation between the DRD2/ANKK1 gene Taq1A polymorphism and smoking cessation, the results remain controversial. The primary objective of the present study was to determine whether this variant indeed has any effect on smoking cessation. The A1-dominant model that considers A1/* (*=A1 or A2) and A2/A2 as two genotypes and compares their frequencies in current and former smokers was applied. A total of 22 studies with 11 075 subjects were included in the meta-analyses. Considering the potential influence of between-study heterogeneity, we conducted stratified meta-analyses with the Comprehensive Meta-Analysis statistical software (version 2.0). Results based on either cross-sectional or longitudinal studies consistently showed a statistically significant association between Taq1A A1/* genotypes and smoking cessation. Further, a more significant association of the variant with smoking cessation was detected when both types of studies were combined. However, there was marginal evidence of heterogeneity among studies (I2=33.9%; P=0.06). By excluding other ethnicities and subjects with cancer, the meta-analysis on the basis of 9487 Caucasians demonstrated that Taq1A A1/* genotypes indeed were significantly associated with smoking cessation under both the fixed- and random-effects models (pooled OR 1.22; 95% CI 1.11–1.34; P=3.9 × 10−5 for both models). No evidence of between-study heterogeneity or publication bias was observed. Thus, we conclude that the polymorphism of Taq1A has an important role in the process of abstaining from smoking, and smokers carrying A2/A2 genotype have a higher likelihood of smoking cessation than those who carry A1/A1 or A1/A2.
Similar content being viewed by others
Introduction
Cigarette smoking is the most common preventable cause of many diseases that contribute to about 6 million deaths worldwide each year.1 Twin and family studies indicate that smoking behaviors are influenced by both genetic and environmental factors.2, 3, 4 There exists a considerable interest in identifying genetic factors encouraging smoking cessation, of which the heritability is estimated to be ~50%.5, 6
The dopaminergic reward system has a crucial role in the etiology of smoking initiation and the subsequent development of nicotine dependence. A great number of studies have focused on determining whether variants in genes with a dopaminergic function could contribute to the heritable variation of smoking behaviors. This reward system consists of three parts: dopamine receptors, dopamine transporters and enzyme targets, which jointly modulate the concentrations of dopamine in synaptic clefts. Because of increasing synaptic dopamine concentrations to a higher level than those stimulated by natural rewards such as sexual intercourse and food, nicotine from cigarette smoke is considered a partial moderating factor of smoking maintenance, and variants in those genes linked with increasing the concentrations of synaptic dopamine appear to be more likely to be involved in smoking cessation.
Of these genes, DRD2, located on chromosome 11q22–q23, has received much attention.7, 8, 9 Many studies10, 11, 12, 13, 14, 15, 16 have reported that a growing number of variants in DRD2 are significantly associated with smoking behaviors. In particular, the Taq1A polymorphism (dbSNP rs1800497), located ~9.5 kb downstream from DRD2 and in exon 8 of ANKK1, has been investigated extensively. Since Noble et al.17 first showed that there was a significantly higher prevalence of Taq1A A1 allele among current smokers (P=0.024) and former smokers (P=0.044) than among nonsmokers in the Caucasian population, many genetic association studies15, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 were conducted to elucidate the association between the polymorphism of Taq1A and smoking-related phenotypes, including cessation. However, the results remain ambiguous. For example, two meta-analyses28, 29 showed no evidence of association between Taq1A polymorphism and smoking behaviors, whereas other two meta-analyses30, 31 showed significant associations of Taq1A polymorphism with smoking behaviors. In addition, there exists heterogeneity among the findings of reported studies.
Both in vivo and in vitro studies32, 33, 34, 35 have documented that the minor A1 allele (or T allele) of the Taq1A polymorphism is correlated with regulating the density and availability of dopamine D2 receptor, indicating that the variant potentially involves tailoring of the concentrations of synaptic dopamine. Therefore, it is rational to assume that the Taq1A polymorphism has an important part in regulating smoking cessation. A growing body of pharmacogenetic evidence36, 37, 38, 39 has suggested an association of Taq1A polymorphism with the pharmacotherapy of smoking cessation, although a few longitudinal treatment studies40, 41 have failed to reveal such an association. Many moderating factors could be causing these inconsistent results, such as gene × sex36, 42 and DRD2 × SLC6A3 interactions.38, 43
To date, there have been three meta-analyses28, 31, 44 regarding the association of Taq1A polymorphism with smoking cessation. On the basis of five cross-sectional studies with relatively small samples (N=1750 in total), the first reported meta-analysis by Munafo et al.28 found no evidence of an association between the variant and smoking cessation. Then Ohmoto et al.31 performed an independent meta-analysis by pooling six cross-sectional studies and one longitudinal treatment study, of which only the placebo arm was employed (N=4249). The authors demonstrated that smokers with one or more A1 alleles have more difficulty of quitting smoking than do those carrying homozygous A2 alleles in both the overall population (pooled odds ratio (OR) 1.19; 95% confidence interval (CI) 1.05–1.35; P=0.008) and the Caucasian population (pooled OR 1.27; 95% CI 1.10–1.45; P=0.001). Very recently, a meta-analysis by Choi and Shin44 integrating nine longitudinal treatment studies of which the placebo arms were excluded (N=2851), showed that there was no significant association between DRD2 Taq1A polymorphism and smoking cessation outcomes. Because no meta-analysis was conducted combining the effects from both longitudinal treatment and cross-sectional studies, and several related studies were recently reported, it is important to conduct an updated meta-analysis for the effect of Taq1A polymorphism on smoking cessation. Thus, we performed a stratified meta-analysis by considering the influence of the underlying sources of heterogeneity such as method variation, ancestral variation and sampling variation.
Materials and methods
Search strategy and inclusion criteria
We searched the reported studies on the association of DRD2/ANKK1 Taq1A polymorphism with smoking by interrogating the electronic database of PubMed. From the first date available in the database up to 17 May 2015, the search was performed using the following key terms: ‘dopamine D2 receptor,’ ‘DRD2,’ ‘smoking,’ ‘nicotine,’ ‘cigarette’ and ‘tobacco.’ Electronic abstracts were examined for potentially relevant articles in accordance with the standard inclusion and exclusion criteria suggested by Moher et al.45 Duplications were discarded. Once studies met the criteria for selection, the references were hand-checked for underlying additional studies. Articles that reported previously published data were excluded.
Data extraction
After a systematic review according to the Quality of Reporting of meta-analysis and PRISMA guidelines (Supplementary Figure S1), 29 studies were examined more closely for possible inclusion. After excluding the repeated data set, such as data from the study reported by Yudkin et al.,36 which were the same data used by Johnstone et al.,37 23 eligible studies, consisting of nine cross-sectional surveys,15, 17, 19, 20, 21, 22, 23, 46, 47 one longitudinal no-treatment study48 and 13 longitudinal treatment studies36, 38, 39, 40, 41, 43, 49, 50, 51, 52, 53, 54, 55 satisfied the inclusion criteria for this meta-analysis. As described in many previous studies,28, 31, 56, 57 we used a comparison of current smokers (non-abstinent group) with former smokers (abstinent group) to define the phenotype of smoking cessation for all acceptable studies. For each study, the following data were extracted by two authors (YM and MW) using standardized forms: authors and year of publication, language (English or other), country of origin, type of study, ancestry of sample, sample size, sex ratio, statement of Hardy–Weinberg equilibrium, diagnostic criteria, classification of smoking status and the number of participants having different smoking statuses categorized by the Taq1A genotype (Table 1; Supplementary Table S1).
Genotype and phenotype classification
In the accepted studies, the genetic model was mainly assumed to be an A1-dominant model saying that subjects carrying one or more A1 alleles were less likely to have achieved smoking cessation than were those who carry no such allele. We thus applied the A1-dominant model where the comparison is A1/* genotypes, including A1/A1 and A1/A2 genotypes, compared with the A2/A2 genotype. In the cross-sectional studies, the classification of smoking status was based on self-reported questionnaire responses, whereas except for a longitudinal treatment study by Swan et al.43 classifying smoking by self-report, in other longitudinal studies, both self-reported responses and biochemical verification, including the measurements of expiratory carbon monoxide or salivary cotinine concentration, were applied. Of note, only smokers who volunteer to stop smoking were recruited in the longitudinal treatment studies.
Because smoking cessation followed by relapses is a dynamic process, the cessation rate in the treatment study declines as the follow-up time increases after the pharmacotherapy. The included treatment studies consisted of a variety of time frames for smoking abstinence assessment, which may be one of the underlying limitations for this meta-analysis. Considering that former smokers were generally classified as those who had stopped smoking at least 12 months before the survey in cross-sectional studies, in order to harmonize the phenotypes between the cross-sectional and longitudinal treatment reports, we adopted the point-prevalent smoking checked at the 6-month or 12-month follow-up for these treatment studies, except for four studies in which Tashkin et al.50 reported the characteristics of the sustained quitters and continuing smokers at baseline. Stapleton et al.52 verified abstinence during weeks 3 and 4, Han et al.49 checked the smoking status at 4 weeks after bupropion administration and the smoking cessation rate of participants at 10 weeks as reported by Wilcox et al.55 were adopted.
Data analysis
In consideration of the sources of heterogeneity, including study designs, ethnicity and participants’ health conditions, stratified meta-analysis was performed first. Next, we pooled all the selected studies to implement a meta-analysis for the combined efforts. The significance of pooled OR is determined by a Z-statistical test, and exact P-values are presented throughout. All the pooled ORs were analyzed under both the fixed- and random-effects models with a 95% CI as described by DerSimonian and Laird.58 The hypothesis of between-study homogeneity was rejected if the P-value for the Q-statistical test was <0.05, and the I2 statistical test was used to determine the degree of variation across studies resulting from heterogeneity rather than chance. Heterogeneity among studies was assumed if the I2 value was >50%.59
Potential publication bias was evaluated graphically by a funnel plot, which assumes that studies with a larger sample size will be distributed near the average, whereas studies with a smaller sample will be equally distributed on both sides of the average. Deviation from this funnel-shaped distribution can indicate the presence of publication bias. The Egger regression test was applied to assess the significance of publication bias. Data were analyzed by the Comprehensive Meta-Analysis statistical software (version 2.0, Biostat, Englewood, NJ, USA).
Results
Description of the included studies
Through this stringent search strategy, we selected 23 eligible studies consisting of nine cross-sectional surveys15, 17, 19, 20, 21, 22, 23, 46, 47 and 14 longitudinal studies,36, 38, 39, 40, 41, 43, 48, 49, 50, 51, 52, 53, 54, 55 which were published between 1994 and 2014. A total of 11 151 participants were extracted from these papers, which included sample sizes from 76 to 2123 (Table 1). For the selected populations, 18 were predominantly of Caucasian origin, four concentrated on samples of East Asian ancestry and only one study, reported by Wu et al.,21 included persons of Mexican or African origin. The frequencies of A1/* genotypes showed large differences by ethnicity, being from 32.5 to 43.3% (mean=37.5%) in Caucasians and from 44.8 to 70.1% (mean=60.9%) in East Asians. In this meta-analysis, a total of 4411 participants were identified with A1/* genotypes with a frequency of 39.6%, which was in conformity with the frequencies in the Caucasian population. In addition, there was a wide distribution of cessation rates between 9.6 and 66.9%. The cessation rates of the cross-sectional studies and partial longitudinal studies, in which the smoking status was verified at end of treatment, ranged from 21.9 to 66.9%, whereas that of the longitudinal studies checked at 6- or 12-month follow-up were relatively lower (9.6–33.1%).
Meta-analysis of cross-sectional studies
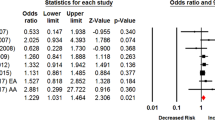
We initially conducted a meta-analysis to calculate the pooled effect of Taq1A A1/* genotypes on smoking cessation by combining 10 data sets extracted from nine cross-sectional studies in that the data set of the study by Wu et al.21 was divided into two parts according to ancestral origin. The pooled OR was 1.12 (P=0.07) with 95% CI ranging from 0.99 to 1.27 under the fixed-effects model and 1.03 (P=0.84) with 95% CI from 0.81 to 1.30 under the random-effects model (Table 2). Although results from the fixed-effects model suggested a trend to the genotypes of Taq1A A1/* correlating with a lower possibility of obtaining smoking cessation, there existed statistically significant heterogeneity among studies (I2=53.8%; P=0.02). By taking into account the variation of ethnicity, we repeated the meta-analysis on the basis of predominant Caucasian ancestry, which revealed a significant association of Taq1A polymorphism with smoking cessation under the fixed-effects model (pooled OR 1.19; 95% CI 1.04–1.36; P=0.01; see Figure 1) and no significant association under the random-effects model with a moderate heterogeneity among studies (I2=57.0%; P=0.05; Table 2).
Forest plot of the meta-analysis results for the association between Taq1A A1/* genotypes and smoking cessation in cross-sectional studies based on predominantly Caucasian subjects. The Z-value and P-value of each eligible study are displayed by rows. The central vertical solid line stands for ORs that equal 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot was calculated under the fixed-effects model. CI, confidence interval; OR, odds ratio.
Because only one study, reported by Gordiev et al.,23 recruited cancer patients, and this study also deviated from the funnel-shaped distribution (Supplementary Figure S5b), it is reasonable to postulate that the study of Gordiev et al. might contribute to the observed heterogeneity in the meta-analysis for the Caucasian population. After we excluded this study23 from the meta-analysis, the P-value of the Q-test increased from 0.05 to 0.97, and the I2 value was reduced from 57.4% to 0, indicating absence of between-study heterogeneity. There was a statistically significant association between Taq1A A1/* genotypes and smoking cessation for both fixed- and random-effects models (pooled OR 1.27; 95% CI 1.10–1.46; P=0.001 for both models). The forest plot is presented in Supplementary Figure S2.
Meta-analysis of longitudinal studies
By pooling all the longitudinal studies, the OR in the fixed-effects model was 1.20 (P=0.003) with 95% CI of 1.06 and 1.36. In the random-effects model, the OR was 1.20 (P=0.007) with 95% CI of 1.05 and 1.37. There was no evidence of significant heterogeneity among studies (I2=11.0%; P=0.33). For this part of the analysis, the results from the fixed-effects model seem to be more encouraging, indicating a statistically significant association of Taq1A A1/* genotypes with smoking cessation (Figure 2). When we performed the meta-analysis on samples with predominantly Caucasian ancestry, the results showed consistently that Taq1A A1/* genotypes were significantly associated with smoking cessation under both the fixed-effects (pooled OR 1.18; 95% CI 1.04–1.34; P=0.01) and the random-effects (pooled OR 1.17; 95% CI 1.03–1.34; P=0.01) models. The P-value of the Q-statistical test was 0.39, and the value of the I2 statistical test was 6.1%, indicating no evidence of between-study heterogeneity. These data are shown in Table 2, and the forest plot is presented in Supplementary Figure S3.
Forest plot of the meta-analysis results for the association between Taq1A A1/* genotypes and smoking cessation across all longitudinal studies. The Z-value and P-value of each eligible study are displayed by rows. The central vertical solid line stands for ORs that equal 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot was calculated under the fixed-effects model. CI, confidence interval; OR, odds ratio.
Meta-analysis of combined studies
Considering there were no significant differences in the pooled effects between the cross-sectional surveys and the longitudinal studies, we combined these studies to conduct a meta-analysis, as previously reported.56 For the overall population, the pooled OR was 1.16 (P=0.0008) with 95% CI of 1.06 and 1.27 under the fixed-effects model (Figure 3). There was evidence of marginally significant between-study heterogeneity (I2=33.9%; P=0.055), but when repeating the meta-analysis under the random-effects model, we found that evidence for association remained significant (pooled OR 1.13; 95% CI 1.00–1.27; P=0.04).
Forest plot of the meta-analysis results for the association between Taq1A A1/* genotypes and smoking cessation across all studies. The Z-value and P-value of each eligible study are displayed by rows. The central vertical solid line stands for ORs that equal 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot was calculated under the fixed-effects model. CI, confidence interval; OR, odds ratio.
Similarly, we carried out stratified meta-analyses for East Asian and Caucasian ancestry. Other ancestries, namely Mexicans or Africans, were excluded because there was only one study.21 With regard to East Asians, there was no evidence of significant association of Taq1A A1/* genotypes with smoking cessation (Table 2). When studies that contained subjects of primarily Caucasian origin were analyzed, the results indicated a statistically significant association between Taq1A A1/* genotypes and smoking cessation under the fixed-effects model (pooled OR 1.18; 95% CI 1.08–1.30; P=3 × 10−4). The forest plot is presented in Supplementary Figure S4. There was no evidence for significant heterogeneity among studies (I2=23.0%; P=0.18). When we performed the meta-analysis under the random-effects model, the pooled OR was 1.16 (P=0.009) with 95% CI of 1.04 and 1.30. Again, after excluding the study of Gordiev et al.,23 the I2 value is reduced to 0, and the P-value of the Q-test increased to 0.63, indicating that the residual between-study heterogeneity has been eliminated. The results showed a more significant effect of Taq1A A1/* genotypes on smoking cessation under the fixed- and random-effects models (pooled OR 1.22; 95% CI 1.11–1.34; P=3.9 × 10−5 for both models; see Table 3).
Sensitivity and accumulative analysis
We conducted a sensitivity analysis for the overall population of the combined studies under the fixed-effects model to examine whether the observed association of Taq1A polymorphism with smoking cessation was prominently influenced by leaving one individual study out at a time. As shown in Figure 4, the pooled ORs fluctuated faintly between 1.14 and 1.19, indicating the results of current meta-analysis were not significantly affected by any individual study. All the P-values for each examination of sensitivity were statistically significant (Supplementary Table S2). To determine whether the effect of the Taq1A polymorphism on smoking cessation changes with the publication year, we also carried out an accumulative analysis for overall population of combined studies under the fixed-effects model. The pooled OR decreased from 1.26 in 1994 to 1.19 in 2006 and achieved relatively steady values after that (Figure 5). The P-values and other detailed data are presented in Supplementary Table S3.
Plots of sensitivity analysis results for the meta-analyses across all studies. The y axis stands for the pooled OR, and the x axis for the individual study that was left out in sequence from the included studies. The diamond symbols represent the pooled OR, and the top and bottom horizontal bars mark the 95% CIs. CI, confidence interval; OR, odds ratio.
Plots of accumulative analysis results. The pooled effect size of the Taq1A polymorphism for the possibility of smoking cessation was plotted against the publication year across all studies. The y axis stands for the pooled OR and the x axis for the publication year relative to the pooled effect size. The diamond symbols indicate the pooled effect size, and each vertical line with horizontal bars labels the 95% CI. CI, confidence interval; OR, odds ratio.
Assessment of possible publication bias
We applied both statistical and graphic approaches to assess the potential publication bias that might exist in the stratified and combined meta-analyses. As displayed in Table 2, the P-values from all Egger regression tests were more than 0.05, suggesting there was no significant publication bias in our meta-analyses. Also, the funnel plots showed a consistent result with no evidence for possible bias. For longitudinal studies, the funnel plots were symmetrical, indicating there was no evidence of ascertainment bias (Supplementary Figures S6a and b). For cross-sectional and combined studies, there was one or two studies of several plots deviating from the funnel-shaped distribution (Supplementary Figures S5a, S5b, S7a and S7b). By considering the influence of ethnicity and subjects’ health, we removed studies for a more reliable effect of Taq1A polymorphism on smoking cessation. As for the meta-analyses based on predominantly Caucasians without the data of the Gordiev study,23 the funnel plots achieved symmetry (Supplementary Figures S5c and S7c).
Discussion
In this study, we observed an effect of Taq1A polymorphism on smoking cessation. For the cross-sectional studies, the meta-analysis showed a statistically significant association of Taq1A A1/* genotypes with smoking cessation in the Caucasian population, which is consistent with the results of a previous meta-analysis reported by Ohmoto et al.22 Thus, the nonsignificant association for the first meta-analysis reported by Munafo et al.28 was probably attributable to the insufficient number of studies with relatively small samples. In the longitudinal treatment studies, we extended previous work in the pharmacogenetic field of smoking cessation, as current meta-analysis provided evidence for the significant association, although Choi and Shin44 did not observed a significant association of Taq1A polymorphism with smoking cessation therapy in nine longitudinal treatment studies. This difference might have been attributable to the fact that only the treatment subjects were employed, which limited the number of samples. With respect to the meta-analysis of the combined studies, a much stronger association signal of Taq1A A1/* genotypes with smoking cessation was detected after excluding the underlying heterogeneity. On the basis of previous and current scientific evidence, we conclude that DRD2 Taq1A polymorphism is statistically significantly associated with smoking cessation (pooled OR 1.22; 95% CI 1.11–1.34; P=3.9 × 10−5 for both models), indicating that individuals with the homozygous Taq1A A2/A2 genotype are more likely to be successful in abstinence from cigarette smoking than those with A1/A1 or A1/A2 genotypes.
Dopamine receptors consist of two subfamilies with different functions: D1-like, including D1 and D5, and D2-like, including D2, D3 and D4. In particular, the dopamine D2 receptor (encoded by DRD2) is one of the important components of the dopaminergic reward pathway. Many studies have concentrated on determining whether variants in DRD2 confer susceptibility to addictive disorders including smoking-related phenotypes and other psychiatric disorders. Multiple lines of evidence10, 11, 12, 13, 14, 15, 16 have demonstrated that numerous variants in DRD2 are significantly associated with smoking behaviors. The polymorphism of rs1800497, which historically has been referred to as ‘DRD2 Taq1A’, although it is located in ANKK1, was widely investigated in many genetic association studies9, 15, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 for smoking behaviors, including cessation. Furthermore, the Taq1A A1 allele has been associated with regulation of the functions of the dopamine D2 receptor by reducing the densities and binding affinity,32, 33, 34, 35 which may be one of the underlying molecular mechanisms of the association of the Taq1A A2/A2 genotype with the higher likelihood of abstaining from smoking. Nevertheless, there is a question remaining to be addressed: how a variant in Taq1A residing ~9.5 kb downstream from DRD2 could influence the expression of DRD2. One speculative hypothesis is that the variant acts as a proxy marker in linkage disequilibrium (LD) with causative variant(s) within DRD2. Zhang et al.60 documented that Taq1A was in strong LD with rs2283265 and rs1076560 of DRD2 (D′=0.86). Those two intronic single-nucleotide polymorphisms have been significantly associated with addictions,15, 61, 62, 63, 64, 65, 66 schizophrenia67, 68, 69, 70, 71 and reduced density of D2S relative to D2L and D2 receptors.60
Another possible mechanism is that the Taq1A polymorphism may exert its effort on the dopaminergic reward pathway by directly altering the function of ANKK1 itself. The product of ANKK1, which contains a single serine/threonine kinase domain and 11 ankyrin repeats and is a member of a protein family involved in signal transduction, was suggested to affect the dopaminergic circuitry through signal transduction or other cellular responses.72, 73 In addition, the function of many proteins could be modulated by phosphorylation of key amino-acid residues, which would cause a change of affinity of the protein for ligands or other aspects of activity. Thus, ANKK1 might act on the dopamine transporter to affect its activity.74 The Taq1A polymorphism was reported to alter a glutamate-to-lysine substitution at amino-acid residue 713 in the putative binding domain of ANKK1, indicating the variant may directly tailor the function of ANKK1 and indirectly regulate the activity of the dopamine transporter. Consistent with this idea, several family-based association studies have demonstrated that a significant association of ANKK1 with nicotine addiction,10, 11, 13 as well as the association signal from variants in ANKK1 appears to be stronger than that in DRD2.9 Besides, the Taq1A polymorphism may serve as a surrogate in LD with causative variant(s) in ANKK1. For example, Gelernter et al.10 reported that Taq1A is in complete LD with rs11604671 (D′=1.0) and in moderate LD with rs4938015 (D′=0.73), which are two functional variants in ANKK1. Given that the function of ANKK1 in the dopaminergic reward pathway is still unclear, more related functional studies are warranted. Meanwhile, as next-generation sequencing technologies become available, gene-based deep-sequencing analysis of the DRD2/ANKK1 gene cluster can be used to reveal more novel functional variants, which may be in strong LD with the Taq1A polymorphism, conferring susceptibility to smoking cessation.
To avoid the influence of underlying heterogeneity among studies, several stepwise stratified analyses were carried out in our meta-analyses. We first implemented two subgroup meta-analyses by two methods before combining the two types of studies. There was no evidence that the observed effect for the cross-sectional studies and the longitudinal studies were heterogeneous, providing persuasive evidence for the robustness of the finding of our meta-analysis of combined studies. In light of the differences in the frequencies of the A1/* genotype among different ethnic groups, we speculated that the influence of ethnicity might be attributable to the detected heterogeneity among studies. After excluding the subjects of East Asian and other non-Caucasian origins, the between-study heterogeneity was obviously reduced even though not erased. The results of the meta-analysis showed a more significant association in the Caucasian population. Further, we excluded the study of cancer patients23 and performed another meta-analysis. The residual heterogeneity among studies was eliminated, indicating that the findings of our meta-analysis for the Caucasian population were much more reliable. Unfortunately, the current study had an insufficient number of studies to incorporate East Asian subjects in the estimate of pooled effect. In addition, it is worth noting that there were several whole-genome-wide association studies75 where the Taq1A polymorphism was not revealed as a variant significantly associated with smoking cessation at the genome-wide significant level. To some extents, this is not surprising to us at all as so far only limited loci were identified by genome-wide association studies even in a study with a sample size of >140 000.75 Many factors might have contributed to this phenomenon; for example, inconsistent definition of smoking cessation phenotype and high heterogeneity among different studies, and very conservative threshold for significance with stringent Bonferroni correction.
Although a statistically significant association of Taq1A A1/* genotypes with smoking cessation was revealed, the results of our meta-analysis should be interpreted with caution in view of the following potential limitations. First, although reconciling the phenotypes of the chosen studies to the degree we could, the longitudinal treatment studies had different time frames for abstinence assessment, which might influence the observed effect of Taq1A polymorphism on smoking cessation. Furthermore, various medicines were used in these treatments for smoking abstinence interventions, which might also affect the detected association of the variant with smoking cessation, even though this study was concentrated on the main effect of DRD2 genotypes on smoking cessation. Second, there were different sex ratios and mean ages of participants among these studies, which may be contributable to limitations, as suggested by many previous studies.24, 31, 34, 48, 76 Finally, because there are an insufficient number of studies on other polymorphisms, we could not examine the gene-by-gene interactions by which the pathogenesis of complex addictive disorders, including cessation, could be influenced. For example, Lerman et al.38 indicated that there was a significant statistical interaction of SLC6A3 × DRD2 with smoking cessation at the end of treatment, such that smokers with SLC6A3 9-repeat and DRD2 A2A2 genotypes were more likely to abstain from cigarette smoking than were those who carry SLC6A3 non-9-repeat and DRD2 A2A2 genotypes. Further, Swan et al.43 consistently reported that subjects with both A1/* and non-9-repeat genotypes were less likely to be abstinent from smoking at 12-month follow-up. Recently, a meta-analysis57 by our group documented that a significant effect of SLC6A3 3′-UTR VNTR 9-repeat genotypes on smoking cessation in Caucasians, with an estimated effect of a 9-repeat allele being a 17% increase in the likelihood of abstinence. We thus postulate that DRD2 Taq1A A2/A2 and SLC6A3 9/* genotypes could contribute interactively to the process of quitting smoking.
In summary, the results of the current meta-analyses reveal a statistically significant association between the DRD2 Taq1A polymorphism and smoking cessation in a large Caucasian population. In comparison with smokers carrying one or more A1 alleles, smokers with homozygous A2 alleles are more likely to remain abstinent from cigarette smoking. Our results provide supportive evidence for further investigation of personalized medicine for smoking cessation according to individual genotypes. However, research on this problem remains in its infancy, so more well-designed genetic association, pharmacogenetic and molecular functional studies are warranted to reveal the function of ANKK1 in the dopaminergic reward system and the molecular mechanism of Taq1A polymorphism for the etiology of smoking cessation; examine the association of other variants located in DRD2/ANKK1, which are in LD with the polymorphism of Taq1A, with cessation; identify more novel causative variants associated with cessation; and explore the underlying molecular mechanism of their function on tailoring intervention in nicotine dependence.
References
WHO. WHO Tobacco Fact sheet N°339. http://www.who.int/mediacentre/factsheets/fs339/en/, 2014.
Li MD, Cheng R, Ma JZ, Swan GE . A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 2003; 98: 23–31.
True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N et al. Genetic and environmental contributions to smoking. Addiction 1997; 92: 1277–1287.
Sullivan PF, Kendler KS . The genetic epidemiology of smoking. Nicotine Tob Res 1999; 1: S51–S57; discussion S69–70.
Xian H, Scherrer JF, Madden PA, Lyons MJ, Tsuang M, True WR et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res 2003; 5: 245–254.
Hardie TL, Moss HB, Lynch KG . Genetic correlations between smoking initiation and smoking behaviors in a twin sample. Addict Behav 2006; 31: 2030–2037.
Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE . Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics 1995; 5: 121–141.
Li MD . The genetics of nicotine dependence. Curr Psychiatry Rep 2006; 8: 158–164.
Ma Y, Yuan W, Jiang X, Cui WY, Li MD . Updated findings of the association and functional studies of DRD2/ANKK1 variants with addictions. Mol Neurobiol 2014; 51: 281–299.
Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet 2006; 15: 3498–3507.
Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N et al. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology 2009; 34: 319–330.
Wei J, Chu C, Wang Y, Yang Y, Wang Q, Li T et al. Association study of 45 candidate genes in nicotine dependence in Han Chinese. Addict Behav 2012; 37: 622–626.
Ducci F, Kaakinen M, Pouta A, Hartikainen AL, Veijola J, Isohanni M et al. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol Psychiatry 2011; 69: 650–660.
De Ruyck K, Nackaerts K, Beels L, Werbrouck J, De Volder A, Meysman M et al. Genetic variation in three candidate genes and nicotine dependence, withdrawal and smoking cessation in hospitalized patients. Pharmacogenomics 2010; 11: 1053–1063.
Morton LM, Wang SS, Bergen AW, Chatterjee N, Kvale P, Welch R et al. DRD2 genetic variation in relation to smoking and obesity in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Pharmacogenet Genomics 2006; 16: 901–910.
Voisey J, Swagell CD, Hughes IP, van Daal A, Noble EP, Lawford BR et al. A DRD2 and ANKK1 haplotype is associated with nicotine dependence. Psychiatry Res 2012; 196: 285–289.
Noble EP St, Jeor ST, Ritchie T, Syndulko K St, Jeor SC, Fitch RJ et al. D2 dopamine receptor gene and cigarette smoking: a reward gene? Med Hypotheses 1994; 42: 257–260.
Johnstone EC, Yudkin P, Griffiths SE, Fuller A, Murphy M, Walton R . The dopamine D2 receptor C32806T polymorphism (DRD2 Taq1A RFLP) exhibits no association with smoking behaviour in a healthy UK population. Addict Biol 2004; 9: 221–226.
Wernicke C, Reese J, Kraschewski A, Winterer G, Rommelspacher H, Gallinat J . Distinct haplogenotypes of the dopamine D2 receptor gene are associated with non-smoking behaviour and daily cigarette consumption. Pharmacopsychiatry 2009; 42: 41–50.
Bierut LJ, Rice JP, Edenberg HJ, Goate A, Foroud T, Cloninger CR et al. Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. Am J Med Genet 2000; 90: 299–302.
Wu X, Hudmon KS, Detry MA, Chamberlain RM, Spitz MR . D2 dopamine receptor gene polymorphisms among African-Americans and Mexican-Americans: a lung cancer case-control study. Cancer Epidemiol Biomarkers Prev 2000; 9: 1021–1026.
Ohmoto M, Takahashi T, Kubota Y, Kobayashi S, Mitsumoto Y . Genetic influence of dopamine receptor, dopamine transporter, and nicotine metabolism on smoking cessation and nicotine dependence in a Japanese population. BMC Genet 2014; 15: 151.
Gordiev M, Engstrom PF, Khasanov R, Moroshek A, Sitdikov R, Dgavoronkov V et al. Genetic analysis of polymorphisms in dopamine receptor and transporter genes for association with smoking among cancer patients. Eur Addict Res 2013; 19: 105–111.
Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D . The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics 1996; 6: 73–79.
Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S et al. A genetic association for cigarette smoking behavior. Health Psychol 1999; 18: 7–13.
Singleton AB, Thomson JH, Morris CM, Court JA, Lloyd S, Cholerton S . Lack of association between the dopamine D2 receptor gene allele DRD2*A1 and cigarette smoking in a United Kingdom population. Pharmacogenetics 1998; 8: 125–128.
Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM et al. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst 1998; 90: 358–363.
Munafo M, Clark T, Johnstone E, Murphy M, Walton R . The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res 2004; 6: 583–597.
Munafo MR, Timpson NJ, David SP, Ebrahim S, Lawlor DA . Association of the DRD2 gene Taq1A polymorphism and smoking behavior: a meta-analysis and new data. Nicotine Tob Res 2009; 11: 64–76.
Li MD, Ma JZ, Beuten J . Progress in searching for susceptibility loci and genes for smoking-related behaviour. Clin Genet 2004; 66: 382–392.
Ohmoto M, Sakaishi K, Hama A, Morita A, Nomura M, Mitsumoto Y . Association between dopamine receptor 2 TaqIA polymorphisms and smoking behavior with an influence of ethnicity: a systematic review and meta-analysis update. Nicotine Tob Res 2013; 15: 633–642.
Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ . Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991; 48: 648–654.
Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK et al. D2 dopamine receptor gene (DRD2) Taq1A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997; 7: 479–484.
Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry 1998; 3: 256–260.
Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 1999; 4: 290–296.
Yudkin P, Munafo M, Hey K, Roberts S, Welch S, Johnstone E et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ 2004; 328: 989–990.
Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics 2004; 14: 83–90.
Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr, Pinto A et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol 2003; 22: 541–548.
David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafo MR et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine Tob Res 2007; 9: 821–833.
Berlin I, Covey LS, Jiang H, Hamer D . Lack of effect of D2 dopamine receptor TaqI A polymorphism on smoking cessation. Nicotine Tob Res 2005; 7: 725–728.
Munafo MR, Johnstone EC, Murphy MF, Aveyard P . Lack of association of DRD2 rs1800497 (Taq1A) polymorphism with smoking cessation in a nicotine replacement therapy randomized trial. Nicotine Tob Res 2009; 11: 404–407.
Lerman C, Shields PG, Wileyto EP, Audrain J, Pinto A, Hawk L et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics 2002; 12: 627–634.
Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ et al. Joint effect ofdopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychol 2007; 26: 361–368.
Choi HD, Shin WG . Lack of association between DRD2 Taq1A gene polymorphism and smoking cessation therapy: a meta-analysis. Int J Clin Pharmacol Ther 2015; 53: 415–421.
Moher D, Liberati A, Tetzlaff J, Altman DG . PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012.
Yoshida K, Hamajima N, Kozaki K, Saito H, Maeno K, Sugiura T et al. Association between the dopamine D2 receptor A2/A2 genotype and smoking behavior in the Japanese. Cancer Epidemiol Biomarkers Prev 2001; 10: 403–405.
Hamajima N, Ito H, Matsuo K, Saito T, Tajima K, Ando M et al. Association between smoking habits and dopamine receptor D2 taqI A A2 allele in Japanese males: a confirmatory study. J Epidemiol 2002; 12: 297–304.
Styn MA, Nukui T, Romkes M, Perkins K, Land SR, Weissfeld JL . The impact of genetic variation in DRD2 and SLC6A3 on smoking cessation in a cohort of participants 1 year after enrollment in a lung cancer screening study. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 254–261.
Han DH, Joe KH, Na C, Lee YS . Effect of genetic polymorphisms on smoking cessation: a trial of bupropion in Korean male smokers. Psychiatr Genet 2008; 18: 11–16.
Tashkin DP, Rabinoff M, Noble EP, Ritchie TL, Simmons MS, Connett J . Association of dopamine-related gene alleles, smoking behavior and decline in FEV1 in subjects with COPD: findings from the lung health study. COPD 2012; 9: 620–628.
Ton TG, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM . Genetic polymorphisms in dopamine-related genes and smoking cessation in women: a prospective cohort study. Behav Brain Funct 2007; 3: 22.
Stapleton JA, Sutherland G, O'Gara C, Spirling LI, Ball D . Association between DRD2/ANKK1 Taq1A genotypes, depression and smoking cessation with nicotine replacement therapy. Pharmacogenet Genomics 2011; 21: 447–453.
Cinciripini P, Wetter D, Tomlinson G, Tsoh J, De Moor C, Cinciripini L et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: evidence for a pharmacogenetic effect on mood. Nicotine Tob Res 2004; 6: 229–239.
Breitling LP, Twardella D, Hoffmann MM, Witt SH, Treutlein J, Brenner H . Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics 2010; 11: 527–536.
Wilcox CS, Noble EP, Oskooilar N . ANKK1/DRD2 locus variants are associated with rimonabant efficacy in aiding smoking cessation: pilot data. J Investig Med 2011; 59: 1280–1283.
Stapleton JA, Sutherland G, O'Gara C . Association between dopamine transporter genotypes and smoking cessation: a meta-analysis. Addict Biol 2007; 12: 221–226.
Ma YL, Yuan WJ, Cui WY, Li MD . Meta-analysis reveals significant association of 3'-UTR VNTR in SLC6A3 with smoking cessation in Caucasian populations. Pharmacogenomics J 2015. doi: 10.1038/tpj.2015.44.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA 2007; 104: 20552–20557.
Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC et al. Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology 2011; 36: 753–762.
Al-Eitan LN, Jaradat SA, Hulse GK, Tay GK . Custom genotyping for substance addiction susceptibility genes in Jordanians of Arab descent. BMC Res Notes 2012; 5: 497.
Sullivan D, Pinsonneault JK, Papp AC, Zhu H, Lemeshow S, Mash DC et al. Dopamine transporter DAT and receptor DRD2 variants affect risk of lethal cocaine abuse: a gene-gene-environment interaction. Transl Psychiatry 2013; 3: e222.
Sasabe T, Furukawa A, Matsusita S, Higuchi S, Ishiura S . Association analysis of the dopamine receptor D2 (DRD2) SNP rs1076560 in alcoholic patients. Neurosci Lett 2007; 412: 139–142.
Doehring A, Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G et al. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics 2009; 19: 407–414.
Clarke TK, Weiss AR, Ferarro TN, Kampman KM, Dackis CA, Pettinati HM et al. The dopamine receptor D2 (DRD2) SNP rs1076560 is associated with opioid addiction. Ann Hum Genet 2013; 78: 33–39.
Zheng C, Shen Y, Xu Q . Rs1076560, a functional variant of the dopamine D2 receptor gene, confers risk of schizophrenia in Han Chinese. Neurosci Lett 2012; 518: 41–44.
Chien YL, Hwu HG, Fann CS, Chang CC, Tsuang MT, Liu CM . DRD2 haplotype associated with negative symptoms and sustained attention deficits in Han Chinese with schizophrenia in Taiwan. J Hum Genet 2013; 58: 229–232.
Glatt SJ, Faraone SV, Lasky-Su JA, Kanazawa T, Hwu HG, Tsuang MT . Family-based association testing strongly implicates DRD2 as a risk gene for schizophrenia in Han Chinese from Taiwan. Mol Psychiatry 2009; 14: 885–893.
Blasi G, Lo Bianco L, Taurisano P, Gelao B, Romano R, Fazio L et al. Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci 2009; 29: 14812–14819.
Blasi G, Napolitano F, Ursini G, Taurisano P, Romano R, Caforio G et al. DRD2/AKT1 interaction on D2 c-AMP independent signaling, attentional processing, and response to olanzapine treatment in schizophrenia. Proc Natl Acad Sci USA 2011; 108: 1158–1163.
Hoenicka J, Quinones-Lombrana A, Espana-Serrano L, Alvira-Botero X, Kremer L, Perez-Gonzalez R et al. The ANKK1 gene associated with addictions is expressed in astroglial cells and upregulated by apomorphine. Biol Psychiatry 2010; 67: 3–11.
Garrido E, Palomo T, Ponce G, Garcia-Consuegra I, Jimenez-Arriero MA, Hoenicka J . The ANKK1 protein associated with addictions has nuclear and cytoplasmic localization and shows a differential response of Ala239Thr to apomorphine. Neurotox Res 2011; 20: 32–39.
Munafo MR, Matheson IJ, Flint J . Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry 2007; 12: 454–461.
Tobacco Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010; 42: 441–447.
Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol 1999; 18: 14–20.
Acknowledgements
We thank Dr David L Bronson for excellent editing of this manuscript. This study was supported in part by the Research Center for Air Pollution and Health of Zhejiang University, Ministry of Science and Technology of China (2012AA020405), National Natural Science Foundation of China grant 81273223, Young Scientists Fund of National Science Foundation of China (81301140) and NIH grant DA012844.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ma, Y., Wang, M., Yuan, W. et al. The significant association of Taq1A genotypes in DRD2/ANKK1 with smoking cessation in a large-scale meta-analysis of Caucasian populations. Transl Psychiatry 5, e686 (2015). https://doi.org/10.1038/tp.2015.176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2015.176
This article is cited by
-
DRD2 TaqIA polymorphism-related functional connectivity between anterior insula and dorsolateral prefrontal cortex predicts the retention time in heroin-dependent individuals under methadone maintenance treatment
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
D2 dopamine receptor gene (DRD2) Taq1A (rs1800497) affects bone density
Scientific Reports (2020)
-
Meta-analytic method reveal a significant association of theBDNF Val66Met variant with smoking persistence based on a large samples
The Pharmacogenomics Journal (2020)
-
Association of STAT4 polymorphisms with hepatitis B virus infection and clearance in Chinese Han population
Amino Acids (2016)