Abstract
Alterations in central serotonin (5-hydroxytryptamine, 5-HT) neurotransmission and peripheral immune activation have been linked to multiple neuropsychiatric disorders, including depression, schizophrenia and autism. The antidepressant-sensitive 5-HT transporter (SERT, SLC6A4), a critical determinant of synaptic 5-HT inactivation, can be regulated by pro-inflammatory cytokine signaling. Systemic innate immune system activation via intraperitoneal lipopolysaccharide (LPS) injection rapidly elevates brain SERT activity and 5-HT clearance. Moreover, the pro-inflammatory cytokine interleukin (IL)-1β rapidly stimulates SERT activity in raphe nerve terminal preparations ex vivo, effects that are attenuated by pharmacological p38 MAPK inhibition. To establish a role of serotonergic p38α MAPK signaling in LPS/IL-1β-induced SERT regulation and attendant behavioral responses, we pursued studies in mice that afford conditional elimination of p38α MAPK in 5-HT neurons (p38α5HT−). We found p38α5HT− and control (p38α5HT+) littermates to be indistinguishable in viability and growth and to express equivalent levels of SERT protein and synaptosomal 5-HT transport activity. Consistent with pharmacological studies, however, IL-1β fails to increase SERT activity in midbrain synaptosomes prepared from p38α5HT− animals. Moreover, although LPS elevated plasma corticosterone and central/peripheral pro-inflammatory cytokines in p38α5HT− animals, elevations in midbrain SERT activity were absent nor were changes in depressive and anxiety-like behaviors observed. Our studies support an obligate role of p38α MAPK signaling in 5-HT neurons for the translation of immune activation to SERT regulation and 5-HT-modulated behaviors.
Similar content being viewed by others
Introduction
Depression remains the leading cause of disability worldwide.1 Although the etiology of depression and other mood disorders is complex, multiple studies have reported that depressed subjects display an elevation of pro-inflammatory cytokines (see Raison et al.2 for review). Experimental and/or therapeutic immune system manipulations, as with administration of cytokines or viral/bacterial mimetics such as poly I:C and lipopolysaccharide (LPS), result in mood alterations in humans.3 We4 and others2, 3, 5 have hypothesized that inappropriate activation of immune signaling mechanisms may also contribute to risk for mood disorders in the absence of environmental triggers. Significant evidence points to a bidirectional interaction between the immune system and serotonin (5-hydroxytryptamine, 5-HT) signaling in both the brain and periphery.2, 6 Peripheral immune system stimulation and/or inflammatory cytokines have been found to modulate 5-HT neuron activation, 5-HT synthesis and 5-HT release, and alter levels and/or signaling of various 5-HT receptor subtypes.7, 8, 9, 10, 11, 12, 13, 14, 15, 16
We have provided evidence using cell and animal models that local and systemic immune modulation can influence the antidepressant-sensitive 5-HT transporter (SERT).4 SERT proteins are critical for efficient clearance of the neurotransmitter after release, and represent the most common target for the pharmacological treatment of mood disorders. Using cells derived from rodent mast cells (RBL-2H3) or 5-HT neurons (RN46A), as well as transfected cells, we demonstrated a role for p38 MAPK in the regulation of SERT, with evidence supportive of rapid (minutes), trafficking-independent effects.17, 18, 19 Subsequently, we demonstrated the engagement of p38 MAPK signaling in the stimulatory actions of interleukin (IL)-1β and tumor necrosis factor (TNF)-α on SERT, findings gathered both in the RN46A model and mouse brain synaptosomes.20 Most remarkably, we found that peripheral activation of the innate immune system with LPS leads to a rapid (1 h) stimulation of central nervous system (CNS) SERT activity, accompanied by an acceleration of 5-HT clearance rate and alterations in SERT-dependent behaviors.4 These effects were lost in mice treated with the p38 MAPK inhibitor SB203580, and were absent in interleukin-1 receptor type I (IL-1R) knockout (KO) mice.4 Although these efforts drew attention to SERT as a mediator of behavioral changes linked to peripheral immune activation, our reliance on pharmacological methods and constitutive KO models left unsettled the sites of IL-1R/p38 MAPK expression involved. Peripheral LPS increases CNS IL-1β at a time coinciding with SERT upregulation.21 In addition, the stimulatory effects of systemic LPS on SERT are blocked by in vitro incubation of synaptosomes with a p38 MAPK inhibitor,4 providing evidence that p38 MAPK signaling within serotonergic terminals, downstream of presynaptic IL-1Rs, translates immune activation to changes in SERT and SERT-modulated behaviors.
In the current report, we describe studies examining the impact of systemic LPS in mice exhibiting a selective elimination of p38α MAPK in 5-HT neurons. We find that these mice fail to translate acute peripheral LPS injections into increased CNS SERT activity, despite normal peripheral stress responses and CNS cytokine induction. Moreover, raphe p38α MAPK excision resulted in behavioral resilience to acute LPS administration, supporting p38α MAPK-modulated 5-HT signaling as a key determinant in the behavioral manifestations of innate immune activation.
Materials and methods
Animals
Mouse experiments were performed under a protocol approved by the Vanderbilt Institutional Animal Care and Use Committee. Male animals of 8–12 weeks of age were housed on a 12:12 light cycle with food/water ad libitum, and were tested during the light period. Constitutive p38α KO mice are not viable.22 We therefore pursued two conditional strategies to selectively eliminate p38α MAPK within 5-HT neurons (p38α MAPK5HT−), both involving crosses to a p38α MAPKloxP/loxP line23, 24 that was maintained on a C57Bl/6J background. In one case, we crossed these mice to ePet::Cre, mixed C57Bl/6J;129 background,25 to afford constitutive, 5-HT neuron-specific deletion of p38α MAPK (p38α5HT−) or controls lacking Cre expression (p38α5HT+). To afford p38α MAPK excision in adult animals, we also crossed p38α MAPKloxP/loxP females to p38α MAPKloxP/loxP males that were transgenic for a BAC bearing an estrogen receptor (ER)-Cre fusion, inserted into the Slc6a4 (SERT, C57BL/6 background) gene locus.26 With the resulting progeny, we administered tamoxifen (20 mg ml−1; p38αER5HT−) or corn oil (p38αER5HT+) intraperitoneally (i.p.) for 5 consecutive days and performed biochemical assays 4 weeks later. This time period was chosen to allow for effective gene excision and elimination of kinase produced before tamoxifen injections, based on previous studies.27 As we detected elevations in serum corticosterone (CORT) in corn oil-injected animals, we evaluated brain 5-HT uptake regulation following LPS administration, but did not pursue behavioral studies in this model. To induce innate immune system activation, we administered LPS (i.p. 0.2 mg kg−1, 026:B6, ⩾10 000 eu mg−1 Sigma, St Louis, MO, USA, cat#L8274) or saline, followed 1 h later by killing by rapid decapitation, unless otherwise noted. The dose of LPS used was chosen to achieve a dose lower than that typically utilized for sickness models, and has been shown not to produce changes in locomotion in the open field assay.4
Immunohistochemistry
Mice were anesthetized with Nembutal (70 mg kg−1) and intracardially perfused with 4% paraformaldehyde. Brains were then harvested and maintained in 4% paraformaldehyde overnight at 4 °C. The following day, brains were placed in 10 ml of 30% sucrose overnight. Brains were sectioned (40 μm, Leica SM 200 R, Leica Biosystems, Nussloch, Germany) and stored at −20 °C in freezing medium (30% ethylene glycol, 25% glycerol in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4)) before analysis. Sections were stained free-floating with primary antibodies (rabbit P-p38 MAPK, 1:200 dilution, Cell Signaling Technologies, Danvers, MA, USA, #9211) or goat anti-5-HT, 1:1000 dilution, ImmunoStar, Hudson, WI, USA, #20079) overnight at 4 °C, and then with secondary antibodies (donkey anti-rabbit, 1:2000, Jackson Immunoresearch Laboratories, West Grove, PA, USA, cat#711-485-152, or donkey anti-goat, 1:200, Jackson Immunoresearch Laboratories, cat#705-025-00) for 1 h at room temperature. Antibody labeling was visualized on a Zeiss Axio Imager M2 (Thornwood, NY, USA) in the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126).
Neurotransmitter and mRNA assays
Brain samples obtained following rapid decapitation were assayed in the Vanderbilt Brain Institute Neurochemistry Core for biogenic amines, including 5-HT and metabolites, using high-performance liquid chromatography-based methods previously published by the Blakely laboratory.28 CORT levels were assayed from trunk blood using an ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA; cat#ADI-900-097) in the Vanderbilt Conte Center Bioanalytical Core. For mRNA analyses, dissected midbrain and spleen samples were flash-frozen using liquid nitrogen and stored at −80 oC until RNA extraction performed using Trizol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was conducted using a KAPA SYBR-FAST qRT-PCR One-Step Kit (KAPA Biosystems, Wilmington, MA, USA). Thermocycling conditions were as follows: 42 °C for 5 min for complementary DNA synthesis, followed by 95 °C for 5 min for denaturation. Samples were then subjected to 40 cycles of 95 °C for 3 s, followed by 30-s extension at 60 °C (Eco qRT-PCR machine, Illumina, San Diego, CA, USA). Oligonucleotide primer sequences are available on request. mRNA levels were quantified from real-time PCR curves using the ΔΔCt method29 normalized to Gapdh expression.
Western blot and synaptosome 5-HT uptake analyses
To quantify SERT protein levels, mice were killed by rapid decapitation. Midbrain and frontal cortex were dissected on ice and stored at −80 °C until use. Samples were homogenized in 25 mM HEPES, 25 mM sucrose, 1.5 mM MgCl2, 50 mM NaCl, pH=7.2, and protease inhibitor cocktail (Sigma, cat#P8346) before SDS-PAGE and were transfered to polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA, USA). Membranes were blocked in 5% nonfat dry milk in 1 × phosphate-buffered saline-0.1% Triton at room temperature for 1 h, washed twice with 1 × phosphate-buffered saline-0.1% Triton and incubated overnight at 4 °C with SERT antibody (1:3000 dilution, guinea pig anti-SERT; Frontier, Shinko-nishi, Ishikari, Hokkaido, Japan, cat#HTT-GP-Af1400-1) followed by a 1-h incubation at 4 °C with goat anti-guinea pig antibody (1:10 000 dilution; Jackson Immunoresearch Laboratories, cat#706-001-003). Bound antibody was detected on X-ray film (Kodak, Perkin Elmer, Boston, MA, USA, cat#NEF596) using enhanced chemiluminescence reagents (Perkin Elmer, Waltham, MA, USA, #NEL104001EA) and band density from digital scans used to quantified SERT levels. [3H] 5-HT uptake was measured in synaptosomes prepared from the midbrain, forebrain, hippocampus and striatum as previously described.28 Assays were conducted in 1-ml Krebs–Ringer's HEPES assay buffer (containing 130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.8 g l−1 glucose, 10 mM HEPES, pH 7.4, 100 μM pargyline and 100 μM ascorbic acid). After assessment of protein levels (Bradford assay, Bio-Rad, Hercules, CA, USA), 20–30 μg synaptosomes per sample (in a total volume of 200 μl) were pre-incubated at 37 °C in a shaking water bath for 5–10 min. Modifiers were then added for 10 min, and samples were incubated with 20 nM [3H] 5-HT 5 min at 37 °C. Uptake was terminated by adding 1 ml ice-cold Krebs–Ringer's HEPES buffer and by filtration through GF/B Whatman filters (soaked in 0.3% polyethylenimine for 1 h before experiment). Trapped radioactivity was eluted in scintillation liquid (Ecoscint H, National Diagnositics, Charlotte, NC, USA) overnight and quantified by scintillation spectrometry. Specific counts were obtained after subtraction of counts obtained from parallel samples assayed in the presence of 10 μM paroxetine.
Acute midbrain slice recordings
Following rapid decapitation, midbrain slices (170 μm thickness from the midbrain) were prepared in oxygenated ice-cold sucrose-substituted artificial cerebrospinal fluid using a vibratome (VT1000S, Leica Biosystems) as previously described.30, 31 To measure basal firing activity of neurons in the dorsal raphe nuclei, cell-attached recordings were performed in artificial cerebrospinal fluid supplemented with 400 nM phenylephrine and 30 μM tryptophan at a perfusion rate of 1 ml min−1 at 32 °C. The glass pipettes (4 MΩ) were filled with HEPES solution (150 mM NaCl, 10 mM HEPES, 3.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2 and 10 mM D-glucose, pH 7.4) and voltage-clamped at 0 V. Putative serotonergic neurons in dorsal raphe (DR) were selected based on cell soma size, induction of firing by phenylephrine and inhibition of basal firing rate to 5-HT1A receptor agonist 8OH-DPAT (1 μM). Recordings were obtained with an Axopatch 200B amplifier connected to a Digidata 1322 A (both from Molecular Devices, Sunnyvale, CA, USA) interface connected to a Windows 7-based computer equipped with the Clampex 10.2 software (Molecular Devices).
Behavioral assays
All assays were preformed in the Vanderbilt Brain Institute Neurobehavior Core Facility (supported by NICHD Grant P30 HD15052 to the Vanderbilt Kennedy Center for Research on Human Development). All treatments and assays were performed blind to genotype. Animals were tested in the elevated plus maze (EPM) before either tail suspension test (TST) or forced swim test (FST) as the latter tests are viewed as more stressful. No randomization was used in subject assignment. Following EPM assays, mice were allowed to rest for 7 days before running either the TST or FST. EPM: EPM assays were performed by placing mice into a custom-built maze with four arms at right angles to each other at ~40 cm off of the ground. One pair of opposing arms of the apparatus is open and exposed to bright room light (302 lux), and the other pair contains a walled enclosure afforded dim light (162 lux). Mice were allowed to freely explore the apparatus for 5 min while being positioned in the maze. Total distance traveled and number of entries into open or closed arms of the apparatus were recorded using the AnyMaze video tracking software (San Diego Instruments, San Diego, CA, USA). TST: The TST was performed as described by Steru et al.32 Mice were tested 1 h after i.p. injections by securely fastening the proximal end of the tail to a flat metallic surface, suspended in a visually isolated area (40 × 40 × 40 cm white box) with movements video recorded for 6 min. Time spent immobile was recorded, with immobility hand-scored from videos as the absence of movement aside from passive swaying. FST: FST studies were performed by placing mice into transparent cylinders filled to approximately two-thirds with tap water maintained at approximately room temperature (23±1 °C). FST activity was hand-scored from videos for time spent immobile versus struggling. Immobility was defined as the swimming just enough to stay afloat or not moving at all.
Graphical and statistical analyses
We used Prism 6.0 (Graphpad Software, La Jolla, CA, USA) to perform statistical analyses and graph results. Sample sizes of experiments were chosen that minimized animal usage, that resulted in comparable variation between replicates and that insured detection of effects of ⩾25% difference as statistically significant. Grubb’s test was used to identify and eliminate potential outliers. Data were analyzed via one and two-way analysis of variance under an assumption of normality, assessing main effects of genotype, drug and genotype × drug interactions followed by Bonferonni post hoc comparisons. In all tests, P<0.05 was taken as statistically significant.
Results
Activation and conditional elimination of p38α MAPK in raphe neurons
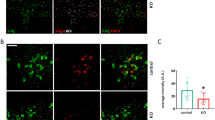
To determine whether peripheral LPS administration activates p38 MAPK specifically within 5-HT neurons of the DR, we used immunohistochemistry to label phospho-p38 MAPK (P-p38 MAPK), the activated form of p38 MAPK, in conjunction with a 5-HT antibody to co-label DR 5-HT neurons. The P-p38 MAPK antibody we used does not discriminate among p38 MAPK isoforms; however, as noted earlier, prior pharmacological, viral and short interfering RNA manipulations support an involvement of p38α MAPK in SERT regulation.17, 18, 20, 33, 34 Figure 1a demonstrates a low level of activated P-p38 MAPK in DR 5-HT cell bodies and surrounding neuropil 1 h post i.p. saline injections. Following LPS administration (0.2 mg kg−1, 1 h), a noticeable enhancement in P-p38 MAPK immunoreactivity was detected in 5-HT neurons. Constitutive p38α MAPK KO mice are not viable,22 and, regardless, the global deletion of the protein in multiple cell types would limit interpretation of results and preclude attribution of any observed effect to an effect in 5-HT neurons. Therefore, we pursued a conditional strategy, breeding p38α MAPKloxP/loxP mice24 to p38α MAPKloxP/loxP;ePet::Cre,27 to selectively eliminate p38α MAPK within 5-HT neurons (p38α MAPK5HT−). Immunofluorescence imaging of p38α MAPK expression in DR-containing sections confirmed the loss of kinase expression in 5-HT neurons of p38α MAPK5HT− animals compared with p38α MAPK5HT+ animals (Figure 1b).
Activation and elimination of p38 MAPK in dorsal raphe (DR) 5-hydroxytryptamine (5-HT) neurons. Images of 5-HT and p38 MAPK staining for lipopolysaccharide (LPS) studies were collected from the dorsomedial division of the DR nucleus55 from adult male C57BL/6J mice. Images of the conditional p38α MAPK elimination experiments were obtained in the same manner. (a) Peripheral administration of LPS (0.2 mg kg−1 1 h before being killed) elevates levels of phospho-p38 MAPK immunoreactivity in 5-HT-labeled neurons of the adult mouse dorsomedial division of the DR. Scale bar, 10 μm. (b) Conditional elimination of p38α MAPK immunoreactivity. Immunofluorescence for p38α MAPK is presented for floxed p38α MAPK mice without (p38α5HT−) or with (p38α5HT+) expression Cre recombinase in 5-HT neurons via ePET::Cre as described in Materials and Methods. Scale bar, 5 μm.
Impact of serotonergic p38α MAPK deletion on 5-HT biochemistry, physiology and SERT function
To determine whether p38α5HT− mice exhibit alterations in 5-HT signaling capacity, we assessed midbrain, forebrain, hippocampal and striatal levels of 5-HT and metabolites using high-performance liquid chromatography evaluation of tissue extracts as noted in the Materials and methods section. We detected a small, but statistically significant effect of genotype on 5-HT levels, with a modest, region-independent reduction (10–20%) seen in the p38α5HT− mice (Figure 2a). No significant alterations were found in the levels of dopamine or norepinephrine or the 5-HT metabolite, 5-hydroxyindoleacetic acid (data not shown). Whole-cell recordings of DR 5-HT neurons in acute brain slices of p38α5HT+ and p38α5HT− mice revealed no statistically significant alterations in basal firing rates (Figure 2b). p38α5HT− mice also demonstrated no changes in either midbrain or forebrain SERT levels (Figure 2c) nor was basal 5-HT uptake in midbrain synaptosomes influenced (Figure 2d). These findings indicate little or no effect of loss of p38α MAPK expression on basal serotonergic measures. However, when we queried the contribution of p38α MAPK to cytokine stimulation of SERT, a different picture emerged. Thus, whereas IL-1β, as previously published,4 rapidly stimulated 5-HT uptake in midbrain synaptosomes of control, p38α5HT+ animals, no stimulation was found with synaptosomes from p38α5HT− mice (Figure 2d).
Serotonergic expression of p38α MAPK is required for acute interleukin (IL)-1β-induced SERT activation. (a) High-performance liquid chromatography (HPLC) analysis of 5-hydroxytryptamine (5-HT) levels in the midbrain (MB), forebrain (FB), hippocampus (Hip) and striatum (Str) from male p38α5HT+ and p38α5HT− mice. Two-way analysis of variance (ANOVA) indicates significant region (F3,67=71.75, P<0.0001) and genotype (F1, 67,=4.12, P=0.001) effects and a nonsignificant interaction (F3,67=0.53, P=0.67). N for all regions=10 for p38α5HT+, N=8 for p38α5HT−. (b) Basal firing rate of dorsal raphe 5-HT neurons as assessed by cell-attached recordings of serotonergic neurons in acute midbrain slices from p38α5HT+ and p38α5HT− mice, N=10 neurons from three p38α5HT+ mice, N=15 neurons from three p38α5HT− mice, with data derived from two to three slices per animal. Student’s two-tailed t-test reveals no significant genotype effect. (c) SERT protein expression in MB and FB of p38α5HT+ and p38α5HT− mice assessed by western blotting. Two-way ANOVA indicates no significant region or genotype effects. N=7 for all groups. (d) Transport of [3H]-5HT (50 nM) in MB synaptosomes of p38α5HT+ and p38α5HT− mice following treatment with IL-1β (10 ng ml−1), compared with vehicle. Two-way ANOVA indicates significant drug × genotype interaction (F1, 20=6.68, P=0.02), drug (F1, 20=5.62, P=0.02), and genotype (F1, 20=6.68, P=0.02). Bonferroni post hoc shows a significant effect of IL-1β in lipopolysaccharide (LPS)-treated p38α5HT+, but not p38α5HT− mice, compared with vehicle control counterparts. *P<0.05; **P<0.01, N=6 for all groups.
LPS elevation of central and peripheral pro-inflammatory cytokine mRNA, as well as plasma CORT, occurs independently of serotonergic p38α MAPK expression
Peripheral LPS administration activates the innate immune system, stimulating the release of multiple inflammatory cytokines, including IL-1β and TNF-α, in the brain and periphery,35 and producing a systemic stress response reflected in elevations in plasma CORT.36 To ensure that our conditional targeting strategy did not alter these responses, we quantified mRNA levels of IL-1β and TNF-α in periphery (spleen) and midbrain, as well as serum CORT 1 h following the administration of LPS (0.2 mg kg−1 i.p.) to p38α5HT+ mice and their p38α5HT− littermates. Neither the LPS-induced increases in splenic and midbrain IL-1β and TNF-α mRNA expression nor the elevation of plasma CORT were influenced by serotonergic loss of p38α MAPK (Figure 3a–e).
Lipopolysaccharide (LPS) induction of central and peripheral pro-inflammatory cytokines, as well as plasma corticosterone, occurs independently of serotonergic p38α MAPK expression. Quantitative PCR (qPCR) analyses of mRNA expression were assessed 1 h post saline (i.p.) or LPS (0.2 mg kg−1, i.p.) injection. (a, b) Two-way analysis of variance (ANOVA) demonstrates significant LPS-induced elevation of spleen interleukin (IL)-1β (F1, 21=20.43, P<0.001) and tumor necrosis factor (TNF)-α (F1, 28=29.20, P<0.001) independent of genotype (nonsignificant genotype and interaction). N=5–11 per group. (c, d) Two-way ANOVA demonstrates significant LPS-induced elevation of midbrain IL-1β, (F1, 24=8.70, P<0.01) and TNF-α (F1, 21=42.63, P<0.01) independent of genotype. N=5–8 per group. (e) Two-way ANOVA demonstrates significant LPS-induced elevation of serum corticosterone (CORT); drug effect F1, 24=15.56, P<0.01, independent of genotype. N=4–8 per group. *P<0.05; **P<0.01; ***P<0.001, Bonferroni post hoc tests.
Serotonergic p38α MAPK is required for peripheral LPS stimulation of CNS SERT activity as well as LPS-induced depressive/anxiety-like behaviors
Observing that SERT in midbrain synaptosomes from p38α5HT− mice lacked sensitivity to acute stimulation with IL-1β, we next asked whether SERT activity in these animals would also lack responsiveness to peripheral LPS administration. Consistent with previous studies of C57BL/6 mice,4 LPS (0.2 mg kg−1) stimulated [3H]-5-HT uptake (50 nM) in midbrain synaptosomes of p38α5HT+ mice, but failed to increase SERT activity in p38α5HT− mice (Figure 4a). Together with our ex vivo IL-1β studies, these findings provide strong support for an essential role of p38α MAPK in translating acute innate immune activation to changes in SERT activity. We observed a similar inability of LPS to stimulate SERT activity when p38α MAPK excision was induced in adults using tamoxifen-treated p38α MAPKloxP/loxP mice positive for an Slc6a4::ER-Cre transgene26 (Figure 4b).
Serotonergic expression of p38α MAPK is required for lipopolysaccharide (LPS) stimulation of SERT activity and depressive/anxiety-like behaviors. (a) [3H]-5-HT (5-hydroxytryptamine) uptake (50 nM) in midbrain synaptosomes of p38α5HT+ and p38α5HT− males 1 h post intraperitoneal (i.p.) saline or LPS (0.2 mg kg−1) injection. LPS increased SERT activity in midbrain synaptosomes of p38α5HT+, but not p38α5HT–, mice. Two-way analysis of variance (ANOVA) significant effect of genotype (F1, 23=4.31, P<0.05) and LPS (F1, 23=7.12, P<0.05), Bonferroni post hoc *P<0.05 in p38α5HT+ group only. N=6–8 per group. (b) Assessment of SERT activity in mice with adult excision of p38α MAPK. Slc6a4-ER-Cre;p38α MAPKloxP/loxP mice were treated for 5 days with corn oil or tamoxifen as described in Methods. Four weeks later, mice were administered saline or LPS (0.2 mg kg−1 i.p.) 1 h before being killed. SERT activity was measured in synaptosomes prepared from midbrain (MB), forebrain (FB), hippocampus (Hip) and striatum (Str). The percentage change of 5-HT uptake activity for LPS versus saline controls is plotted for each condition. Two-way ANOVA demonstrates significant LPS effect on SERT activity in p38αER5HT+ (N=8 for MB and FB; N=9 for Hip; N=4 for Str)-treated animals (F3, 55=8.56, P=0.005) but not in p38αER5HT− (N=12 for MB; N=10 for FB; N=7 for Hip; N=4 for Str) animals (P<0.05). (c) Percent time immobile in the forced swim test (FST) for p38α5HT+ and p38α5HT- mice 1 h post saline or LPS (0.2 mg kg−1 i.p). Two-way analysis of variance (ANOVA) shows significant interaction between LPS and genotype (F1, 15=7.01; P=0.02). Immobility time was increased in p38α5HT+, LPS-treated mice (*P<0.05, Bonferroni post hoc; N=5 per for saline and LPS-treated animals), but not p38α5HT- mice (N=5 for saline-treated and N=4 for LPS-treated animals). (d) Percent time immobile in the tail suspension test (TST) for p38α5HT+ and p38α5HT− mice 1 h post saline or LPS (0.2 mg kg−1 i.p.). Two-way ANOVA shows significant interaction between treatment and genotype (F1, 17=7.83; P=0.01). Immobility time was increased in p38α5HT+ (N=5 per for saline and LPS-treated animals), LPS-treated mice (*P<0.05, Bonferroni post hoc), but not in p38α5HT− mice (N=6 for saline-treated and N=5 for LPS-treated animals). (e) Open, closed and total number of arm entries in the EPM in p38α5HT+ (+) and p38α5HT− (−) mice treated with saline or LPS (0.2 mg kg−1 i.p.). There was no significant effect of genotype or drug. (f) Percent time spent in the open arms of the EPM (6-min test). Two-way ANOVA shows significant effects of LPS (F1, 17=8.81; P<0.01) and genotype (F1, 17=4.71; P=0.04). LPS reduced time spent in open arms in p38α5HT+ (N=5 in saline- and LPS-treated animals) but not in p38α5HT− (N=6 and 4 in saline- and LPS-treated animals, respectively) mice (*P<0.05, Bonferroni post hoc).
Previously, we demonstrated that acute, 1 h systemic LPS treatment increased immobility in the FST and TST, a depressive-like effect that was not seen in SERT KO animals.4 In addition, treatment with the p38α MAPK inhibitor SB203580 mitigated LPS-mediated increases in immobility, although systemic drug administration precluded determination of critical sites of kinase expression. To determine whether p38α MAPK signaling in 5-HT neurons is required for LPS-mediated depressive-like behavior, p38α5HT+ and p38α5HT− littermates were treated with LPS as in our previous report4 (0.2 mg kg−1 i.p. 1 h before testing). As expected, we observed increased immobility time in both the FST (Figure 4c) and TST (Figure 4d) in p38α5HT+ mice. In contrast, p38α5HT− littermates failed to exhibit changes in these behaviors in response to LPS treatments. In the EPM, we detected no genotype- or treatment-related differences in the number of open, closed or total number of arm entries (Figure 4e), consistent with the dose of LPS used not influencing global locomotor activity in either p38α5HT+ or p38α5HT− mice. However, LPS injections reduced time spent in the open arms of the maze in p38α5HT+ but not in p38α5HT− mice (Figure 4f), consistent with an anxiety response in the former but not the latter animals.
Discussion
That immune activation can lead to CNS-mediated physiological and behavioral changes has been clear for decades.37, 38 Such changes likely arise in part from a subsequent elevation of CNS pro-inflammatory cytokines, including IL-1β. Canonical signaling by the IL-1R engages a MAPK kinase signaling cascade that ultimately activates JNK and p38 MAPK signaling pathways.39 We found that within 1 h after peripheral immune activation with LPS, SERT activity in synaptosomes ex vivo and SERT-mediated 5-HT clearance in vivo were significantly elevated,4 effects abolished by pre-administration of SB203580,4, 17, 40, 41, 42 an inhibitor of the p38α and β MAPK isoforms.40 Supporting the idea that these effects were mediated through the p38α MAPK isoform, increases in SERT activity induced by anisomycin, a non-selective activator of p38 MAPKs, were suppressed using short interfering RNAs derived selectively from p38α MAPK sequences.41 Moreover, this stimulation led to behavioral changes known to be sensitive to acute selective serotonin reuptake inhibitor administration, suggesting that they might involve modulation of CNS 5-HT signaling pathways and be supported by modulation of SERT activity. In support of this idea, peripheral LPS administration has been found to activate c-Fos expression in raphe 5-HT neurons43, 44 and microdialysis studies indicate enhanced elevations in 5-hydroxyindoleacetic acid levels, consistent with elevated 5-HT uptake and metabolism, following LPS administration.45, 46 Finally, the behavioral significance of 5-HT neural p38α MAPK was demonstrated by Bruchas et al.34 as being critical to the translation of social defeat stress to depressive-like behavior.
We used two genetic approaches to compromise p38α MAPK signaling in 5-HT neurons. The primary approach used in this study involved elimination of p38α MAPK expression via ePet::Cre expression in animals homozygous for a floxed allele of Mapk14.23, 24, 25, 27 The ePET::Cre approach we implemented has been shown to produce efficient excision of floxed genes in the majority of CNS 5-HT neurons,25 with Cre expression initiating in raphe neurons by e12.5, before elaboration of serotonergic traits. Serotonergic p38α MAPK excision did not result in overt effects in morphology, physiology or behavior. Thus, we found no differences in viability, growth rates, gross physical appearance or reproduction with these animals, nor did we observe alterations in spontaneous locomotor activity, as evaluated in open field tests (data not shown). Slight changes were found in 5-HT levels across brain regions, although raphe serotonergic neuron number and size appeared grossly normal and basal firing rates recorded ex vivo were unchanged. In contrast, p38α MAPK excision completely eliminated the ability of peripheral LPS in vivo or IL-1β ex vivo to elevate SERT activity, supporting an essential contribution of kinase activation to SERT regulation by these inflammatory stimuli. We also induced Cre expression in adult, SERT-expressing cells via a cross of p38α MAPKloxP/loxP animals to a line expressing Cre via an ER-SERT BAC construct.26 Our studies with the ER-SERT BAC line add evidence for an ongoing requirement for 5-HT neuron p38α MAPK activity in LPS-induced SERT regulation.
We4 and others34 have demonstrated that acute LPS administration generates increased immobility in both the FST and the TST. At the dose of LPS used in our tests, immobility effects in these tests are not accounted for by changes in general locomotor activity, which can be suppressed at higher doses.4, 46, 47 These tests have predictive utility related to antidepressant efficacy in humans, many of which target SERT.48, 49 Moreover, we previously found that SERT KO mice exhibit resiliency with respect to LPS-induced immobility in the TST.4 Our studies demonstrate for the first time a requirement for p38α MAPK expression by 5-HT neurons in LPS-induced immobility in the FST and TST. We detected no alteration in the induction of peripheral or CNS IL-1β expression following LPS administration nor were there difference in serum CORT responses, supporting the hypothesis that a p38α MAPK signaling cascade in 5-HT neurons drives LPS-induced despair behavior in the TST/FST. Finally, LPS injections have been reported to reduce time spent by mice in the open arms of the EPM,50 commonly inferred as an anxiety response. We observed a dependence on serotonergic p38α MAPK expression for LPS-induced reduction in time spent in the open arms, suggesting a critical contribution of altered 5-HT signaling for immune activation-triggered anxiety responses.51, 52
The specific target(s) of p38α MAPK within 5-HT neurons underlying the behavioral effects of LPS remain to be definitely elucidated. Here we provide correlative evidence that a plausible target as a trigger for behavioral changes is SERT. Additional studies are needed to determine whether SERT regulation through the p38α MPAK pathway has a role in biochemical and behavioral changes brought about by chronic inflammatory states. SERT is a phosphoprotein under basal conditions53 with evidence that a significant portion of this phosphorylation is achieved via p38 MAPK-linked pathways18, 33 and that basal p38 MAPK-induced SERT phosphorylation contributes to transporter surface density,33 whereas 5-HT affinity and transport rates are influenced by activated p38 MAPK.41 Studies are needed that utilize animals in which SERT is rendered insensitive to p38α MAPK modulation. Although p38α MAPK activity supports basal phosphorylation of SERT in nerve terminal preparations,33 specific sites that support this activation remain to be identified. A site in the cytoplasmic C terminus of human SERT (Thr616) has been proposed as a potential site for p38α MAPK regulation based on in vitro studies using synthetic human SERT peptides and purified p38α MAPK,54 although these findings are yet to be validated with the intact SERT protein, or in a cellular context. Our studies suggest that p38α MAPK-dependent SERT regulatory mechanisms may harbor risk determinants for mood disorders and targeting these pathways could provide a novel route to therapeutics, one that buffers against inappropriate SERT activation versus the current strategy of totally eliminating SERT-mediated 5-HT clearance.
References
Organization WH. Depression: A Global Public Health Concern, 2012.
Raison CL, Capuron L, Miller AH . Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27: 24–31.
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW . From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56.
Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA . Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 2010; 35: 2510–2520.
Wohleb ES, McKim DB, Sheridan JF, Godbout JP . Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 2014; 8: 447.
Baganz NL, Blakely RD . A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci 2013; 4: 48–63.
Gemma C, Ghezzi P, De Simoni MG . Activation of the hypothalamic serotoninergic system by central interleukin-1. Eur J Pharmacol 1991; 209: 139–140.
Shintani F, Kanba S, Nakaki T, Nibuya M, Kinoshita N, Suzuki E et al. Interleukin-1 beta augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus. J Neurosci 1993; 13: 3574–3581.
Linthorst AC, Flachskamm C, Muller-Preuss P, Holsboer F, Reul JM . Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci 1995; 15: 2920–2934.
Gemma C, Imeri L, de Simoni MG, Mancia M . Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol. 1997; 272 (2 Pt 2): R601–R606.
Barkhudaryan N, Dunn AJ . Molecular mechanisms of actions of interleukin-6 on the brain, with special reference to serotonin and the hypothalamo-pituitary-adrenocortical axis. Neurochem Res 1999; 24: 1169–1180.
Wu Y, Shaghaghi EK, Jacquot C, Pallardy M, Gardier AM . Synergism between interleukin-6 and interleukin-1beta in hypothalamic serotonin release: a reverse in vivo microdialysis study in F344 rats. Eur Cytokine Netw 1999; 10: 57–64.
Yang ZJ, Blaha V, Meguid MM, Laviano A, Oler A, Zadak Z . Interleukin-1alpha injection into ventromedial hypothalamic nucleus of normal rats depresses food intake and increases release of dopamine and serotonin. Pharmacol Biochem Behav 1999; 62: 61–65.
Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H . Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 2000; 22: 566–580.
Zhang J, Terreni L, De Simoni MG, Dunn AJ . Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochem Int 2001; 38: 303–308.
von Meyenburg C, Langhans W, Hrupka BJ . Evidence for a role of the 5-HT2C receptor in central lipopolysaccharide-, interleukin-1 beta-, and leptin-induced anorexia. Pharmacol Biochem Behav 2003; 74: 1025–1031.
Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD . Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol 2004; 65: 1462–1474.
Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA 2005; 102: 11545–11550.
Steiner JA, Carneiro AM, Blakely RD . Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic 2008; 9: 1393–1402.
Zhu CB, Blakely RD, Hewlett WA . The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006; 31: 2121–2131.
Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT . Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation 2008; 5: 10.
Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F . Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 2006; 88: 1091–1098.
Heinrichsdorff J, Luedde T, Perdiguero E, Nebreda AR, Pasparakis M . p38 alpha MAPK inhibits JNK activation and collaborates with IkappaB kinase 2 to prevent endotoxin-induced liver failure. EMBO Rep 2008; 9: 1048–1054.
Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet 2007; 39: 750–758.
Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA 2005; 102: 16472–16477.
Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 2007; 27: 9817–9823.
Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES . Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci 2010; 13: 1190–1198.
Zhu CB, Steiner JA, Munn JL, Daws LC, Hewlett WA, Blakely RD . Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J Pharmacol Exp Ther 2007; 322: 332–340.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108.
Thompson BJ, Jessen T, Henry LK, Field JR, Gamble KL, Gresch PJ et al. Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. Proc Natl Acad Sci USA 2011; 108: 3785–3790.
Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci USA 2012; 109: 5469–5474.
Steru L, Chermat R, Thierry B, Simon P . The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985; 85: 367–370.
Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S . A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci 2005; 25: 29–41.
Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 2011; 71: 498–511.
Laye S, Parnet P, Goujon E, Dantzer R . Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res 1994; 27: 157–162.
Cabrera R, Korte SM, Lentjes EG, Romijn F, Schonbaum E, De Nicola A et al. The amount of free corticosterone is increased during lipopolysaccharide-induced fever. Life Sci 2000; 66: 553–562.
Dantzer R . How do cytokines say hello to the brain? Neural versus humoral mediation. Eur Cytokine Netw 1994; 5: 271–273.
Dunn AJ . Nervous system-immune system interactions: an overview. J Recept Res 1988; 8: 589–607.
Weber A, Wasiliew P, Kracht M . Interleukin-1 (IL-1) pathway. Sci Signal 2010; 3: cm1.
Zhang J, Shen B, Lin A . Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol Sci 2007; 28: 286–295.
Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD . p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2 A-dependent process. J Biol Chem 2005; 280: 15649–15658.
Carneiro AM, Cook EH, Murphy DL, Blakely RD . Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest 2008; 118: 1544–1552.
Hollis JH, Evans AK, Bruce KP, Lightman SL, Lowry CA . Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain Behav Immun 2006; 20: 569–577.
Kopf BS, Langhans W, Geary N, Asarian L . Serotonin 2C receptor signaling in a diffuse neuronal network is necessary for LPS anorexia. Brain Res 2010; 1306: 77–84.
van Heesch F, Prins J, Konsman JP, Westphal KG, Olivier B, Kraneveld AD et al. Lipopolysaccharide-induced anhedonia is abolished in male serotonin transporter knockout rats: an intracranial self-stimulation study. Brain Behav Immun 2013; 29: 98–103.
van Heesch F, Prins J, Konsman JP, Korte-Bouws GA, Westphal KG, Rybka J et al. Lipopolysaccharide increases degradation of central monoamines: an in vivo microdialysis study in the nucleus accumbens and medial prefrontal cortex of mice. Eur J Pharmacol 2014; 725: 55–63.
Dunn AJ, Swiergiel AH . Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav 2005; 81: 688–693.
Cryan JF, Mombereau C, Vassout A . The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 2005; 29: 571–625.
Detke MJ, Johnson J, Lucki I . Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol 1997; 5: 107–112.
Swiergiel AH, Dunn AJ . Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav 2007; 86: 651–659.
Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A . Modulation of anxiety circuits by serotonergic systems. Stress 2005; 8: 233–246.
Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ et al. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci 2008; 1148: 86–94.
Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD . Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem 1998; 273: 2458–2466.
Sorensen L, Stromgaard K, Kristensen AS . Characterization of intracellular regions in the human serotonin transporter for phosphorylation sites. ACS Chem Biol 2014; 9: 935–944.
Franklin KBJ, Paxinos G . The Mouse Brain in Stereotaxic Coordinates. 3rd edn, Academic Press: : New York, NY, USA, 2008.
Acknowledgements
We gratefully acknowledge support by NIH awards N5007491 (NLB and MJR), MH094527 (RDB), MH096972 (RDB and ESD) and the Brain and Behavior Research Foundation (NLB), and the Institute for Psychiatric Neuroscience (RDB). We also acknowledge excellent laboratory oversight support by Chris Svitek, Jane Wright, Qiao Han, Angela Steele and Tracy Moore-Jarrett. We also thank Dr Manolis Pasparakis, University of Cologne, for kindly providing the two conditional mouse lines generated to selectively eliminate p38α MAPK within 5-HT neurons.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Baganz, N., Lindler, K., Zhu, C. et al. A requirement of serotonergic p38α mitogen-activated protein kinase for peripheral immune system activation of CNS serotonin uptake and serotonin-linked behaviors. Transl Psychiatry 5, e671 (2015). https://doi.org/10.1038/tp.2015.168
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2015.168
This article is cited by
-
Serotonin Transporter Ala276 Mouse: Novel Model to Assess the Neurochemical and Behavioral Impact of Thr276 Phosphorylation In Vivo
Neurochemical Research (2022)
-
Intestinal Predictors of Whole Blood Serotonin Levels in Children With or Without Autism
Journal of Autism and Developmental Disorders (2022)
-
Systemic immunization with altered myelin basic protein peptide produces sustained antidepressant-like effects
Molecular Psychiatry (2020)
-
Neuronal MAP kinase p38α inhibits c-Jun N-terminal kinase to modulate anxiety-related behaviour
Scientific Reports (2018)