Abstract
C-reactive protein (CRP) is a heritable biomarker of systemic inflammation that is commonly elevated in depressed patients. Variants in the CRP gene that influence protein levels could thus be associated with depression but this has seldom been examined, especially in the elderly. Depression was assessed in 990 people aged at least 65 years as part of the ESPRIT study. A clinical level of depression (DEP) was defined as having a score of ⩾16 on The Center for Epidemiologic Studies Depression scale or a diagnosis of current major depression based on the Mini-International Neuropsychiatric Interview and according to Diagnostic and Statistical Manual of Mental Disorders-IV criteria. Five single-nucleotide polymorphisms spanning the CRP gene were genotyped, and circulating levels of high-sensitivity CRP were determined. Multivariable analyses adjusted for socio-demographic characteristics, smoking, ischemic pathologies, cognitive impairment and inflammation-related chronic pathologies. The minor alleles of rs1130864 and rs1417938 were associated with a decreased risk of depression in women at Bonferroni-corrected significance levels (P=0.002). CRP gene variants were associated with serum levels in a gender-specific manner, but only rs1205 was found to be nominally associated with both an increased risk of DEP and lower circulating CRP levels in women. Variants of the CRP gene thus influence circulating CRP levels and appear as independent susceptibility factors for late-life depression.
Similar content being viewed by others
Introduction
The link between depression and inflammation is well documented, and numerous studies have shown that C-reactive protein (CRP), a marker of systemic inflammation, is raised in depressed people, although this may be restricted to specific subgroups, for example, clinical samples or men,1 especially those with a late-onset depression.2 Some studies have suggested that the association between CRP levels and depression is no longer significant after adjustment for vascular factors, chronic illness or treatments,1 yet a recent meta-analysis found a weak but robust association even after covariate adjustment.3 However, features of this analysis limit the extent to which findings can be generalized to the wider population, such as the exclusion of people with chronic inflammatory conditions, the lack of control for specific illness and potential gender-specific effects were not considered.
The links between inflammation and depression thus remain unclear and may be bidirectional, as neurobiological correlates of depression may result in enhanced inflammation and CRP levels, and the latter, in turn, may increase the risk of depression. Both depression and CRP phenotypes are ~40% heritable, and twin studies suggest a common genetic pathway linking depression and inflammation.4 Environmental factors such as smoking, obesity and vascular pathologies, as well as gender could also be implicated and modulate the relationship between the CRP gene and depression.5 This may be especially important in the elderly who have a high prevalence of comorbid chronic disorders.
Several genetic polymorphisms in the CRP gene have been associated with CRP levels,6, 7, 8, 9 but their association with depression remains controversial. To date, there have only been two studies in adult populations that found no significant associations10, 11 and two others in the elderly reported inconsistent results.6, 12 However, none of these studies considered the involvement of potential mediating factors, such as cardiovascular and inflammation-related chronic pathologies, that are frequent in the elderly. In addition, gender-specific associations have not been examined specifically despite the reports of gender differences in the characteristics13 and risk factors for late-life depression (including genetic factors),14, 15, 16 and in the relationship between inflammation and depression,17, 18 as well as in the association between CRP genetic variants and CRP levels.8, 9
Thus, while there is some evidence to suggest that the CRP gene may constitute a susceptibility factor for late-life depression, this has yet to be examined within a large cohort in the elderly general population. In this study, we have taken into account possible gender differences and multiple independent and interactive factors, including various chronic physical disorders and antidepressant use. The aim is to more accurately describe the role of the CRP gene in late-life depression and determine whether circulating CRP levels could modulate this association.
Materials and methods
Participants
The data were derived from a longitudinal study of neuropsychiatric disorders in community-dwelling French elderly, the ESPRIT study.19 Community-dwelling participants ⩾65 years old were recruited by random selection from the electoral rolls between 1999 and 2001. Approval for the study was given by the national ethics committee. After obtaining written informed consent from all participants, interviews were administered by trained staff at baseline (wave 1) and 2, 4, 7 and 10 years (wave 2–5, respectively) of follow-up. Of the 2199 non-demented elderly recruited, participants were excluded from this analysis if they were not assessed for current psychiatric symptomatology (n=29) or had missing covariate data (n=48). Of the remaining participants, 990 provided buccal samples for genotyping. Compared with the participants included in the analysis, those excluded had a lower educational level, were more likely to have cognitive dysfunction, cardiovascular pathologies and depressive symptomatology, and were more likely to be treated with antidepressants (P⩽0.0001).
Outcome measures
The diagnosis of lifetime depression was made using the Mini-International Neuropsychiatric Interview, a standardized psychiatric examination validated in the general population20 according to the Diagnostic and Statistical Manual of Mental Disorders-IV criteria.19 Positive cases were reviewed by a panel of psychiatrists. The Center for Epidemiologic Studies Depression Scale, validated in the elderly, was used to evaluate current depressive symptomatology.21 Participants with a Mini-International Neuropsychiatric Interview diagnosis of current major depression or high levels of depressive symptomatology (The Center for Epidemiologic Studies Depression Scale ⩾16) were defined as having a clinical level of depression (DEP), that is, levels of psychopathology that would warrant clinical intervention, as described.15, 22
CRP genotyping
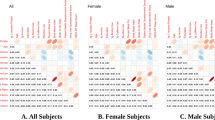
The CRP gene is located on chromosome 1 and consists of 2 exons and a long 3′ untranslated region. It encodes a 204 amino-acid protein. CRP polymorphisms were chosen based initially on common (minor allele frequency ⩾0.05) tag single-nucleotide polymorphisms (SNPs) identified using the Haploview program,23 and using Caucasian genotype data from the International HapMap Project (www.hapmap.org; version 3, release R2, ethnicity: CEU+TSI). We selected SNPs that have been associated with circulating CRP levels in prior studies, suggesting their potential functional significance24, 25 while ensuring adequate coverage across the gene. Chosen SNPs include the 5′ promoter region (rs3093059, at position −757), the intron (rs1417938, at position +29), exon 2 (rs1800947, at position +1059), the 3′ untranslated region (rs1130864, at position +1444) and the 3′ flanking region (rs1205, at position +1846; Figure 1). There is relatively high-linkage disequilibrium across the region estimated using D′ (>0.84 for all pairwise comparisons), although r2 values are very low, with the exception of rs1130864 and rs1417938. These values closely match those reported previously.24
(a) The C-reactive protein (CRP) gene region (chromosome 1q21–q23) and approximate locations of the tag single-nucleotide polymorphism (SNP) locations (dbSNP) relative to the transcription start site, as described previously.28 Representation of the linkage disequilibrium structure of the five SNPs genotyped across the CRP gene using r2 (b) and D′ (c). SNPs are listed in the order they appear on the NCBI database (that is, gene runs in the reverse direction). LD values are shaded on a gradient based on the strength of the correlation.
DNA was extracted from buccal samples collected during the follow-up and genotyping was performed by LGC Genomics, Hoddesdon, UK using their KASP SNP genotyping system as described previously.22, 26 The error rate for the KASP assay system is <0.3%.
Socio-demographic and clinical variables
The standardized interview included questions on socio-demographic characteristics, height, weight and smoking. Glycemia, lipids and CRP levels were measured from 12- h-fasting blood samples. Cognitive function was assessed using the Mini-Mental State Examination and those scoring <26 were considered as having cognitive impairment.27 Detailed medical questionnaires (with additional information from general practitioners) were used to obtain information on history of cardiovascular ischemic pathologies (angina pectoris, myocardial infarction, stroke, cardiovascular surgery and arteritis). Inflammation-related chronic pathologies corresponded to self-reported respiratory disorder (chronic bronchitis, asthma or dyspnea) as well as to taking regular treatment for chronic joint or back pain. All drugs used in the preceding month, including antidepressants and anti-inflammatory medication were recorded from medical prescriptions, drug packages and any other relevant information.
Circulating CRP
High-sensitivity CRP was measured on a subset of 608 participants in the ESPRIT study by means of particle enhanced immunonephelometry assay on thawed serum at the fifth wave of follow-up. The sensitivity and interassay coefficient variation of this assay were 0.17 mg l−1 and 2.3%, respectively. CRP values below the detection limit were assigned a value equal to half the detection limit (n=13). Persons with CRP levels >10 mg l−1 were excluded from the analyses, as this high level of CRP was considered to be owing to acute-phase response (n=23). Logarithmic transformation was applied to normalize raw score distributions of the CRP values and the geometric means and s.e. (confidence interval: m/s.e.1.96; m × s.e.1.96) are shown in the Tables.
Statistical analysis
χ2-Tests were used to compare the distribution of CRP genotypes with those predicted under the Hardy–Weinberg equilibrium. Linkage disequilibrium between the SNPs was calculated using Haploview version 4.2.23 Associations between CRP polymorphisms and prevalent DEP at wave 1 were assessed using multivariable logistic regression models adjusted for covariates that were found to be associated with prevalent DEP (P<0.15). Model M0 was adjusted for age only, whereas model M1 included additional variables such as gender, marital status, education level, smoking, cognitive impairment and inflammation-related chronic pathologies. Cardiovascular ischemic pathologies were also included owing to the potential association with CRP polymorphisms.28
The association between these SNPs and circulating CRP was investigated in the subsample of 608 participants who provided samples for analysis at wave 5. CRP levels at baseline were also available for some participants29 but were not examined, given the very small number having also provided buccal samples (n=136); however, there was a high correlation between both measurements (r=0.61, P<0.0001). The associations between CRP SNPs and circulating CRP were evaluated using analysis of covariance and were adjusted for age. Further adjustment was also made for depression and ischemic pathologies.
For two SNPs (rs1130864 and rs1417938) significant interactions were found with gender for DEP (P=0.04 for both SNPs) and circulating CRP levels (P=0.005 and P=0.004, respectively), consequently all analyses were stratified by gender. SAS (v9.4, SAS Institute, Cary, NC, USA) was used for the statistical analyses with a nominally significant level of P<0.05. Given that five SNPs were investigated in both males and females, the Bonferroni-corrected significance level was P<0.005.
Results
Population characteristics
At baseline, 26.2% of the 990 participants were identified as having DEP and antidepressant use was reported by 4.1% (Table 1). Depressed elderly were more frequently women and were living alone, had a lower education level, were more likely to have cognitive dysfunction, inflammation-related chronic disorders and a history of major depression, and were more frequently users of antidepressants compared with non-depressed elderly.
The CRP genotype frequencies were not significantly different from those predicted by Hardy–Weinberg equilibrium (P>0.15 for all SNPs). Owing to the very small number of homozygotes for the minor allele of both rs1800947 and rs3093059, these homozygotes were combined with the heterozygotes for analysis.
CRP polymorphisms and DEP
In women, significant associations were observed between DEP and three of the five SNPs, a trend level association was observed for a fourth (Table 2). The same pattern was observed in the age-adjusted and multivariate-adjusted logistic regression models (M0 and M1). Women homozygotes for the minor alleles of rs1130864 and rs1417938 had a 72% decreased odds of depression compared with homozygotes for the major allele, which remained significant after Bonferroni correction. Homozygotes for the T allele of rs1205 had an almost twofold increased odds of depression compared with women with the CC genotype, although the association was only significant at nominal P-values. Among men, none of the SNPs were significantly associated with DEP. Results remained unchanged after further adjustment for antidepressants (Supplementary Table S1).
CRP polymorphisms and circulating CRP levels
CRP levels adjusted for age were lower in women homozygous for the minor allele of rs1205 (Table 3). A number of associations were also observed in men with the minor alleles of rs1130864, rs1417938 and rs1800947 being associated with lower CRP levels compared with men homozygous for the major alleles. These results remained after further adjustment for depression and cardio-ischemic pathologies (Supplementary Table S2). However, none of the associations reached Bonferroni-corrected significance levels.
Discussion
In this study, we report that three of five variants of the CRP gene were strongly associated with late-life depression in older women but not in men. These associations were not reduced by the inclusion of potential mediating factors, such as lifestyle, physical and mental health. Highly significant associations were observed with rs1130864 and rs1417938 that appear as susceptibility factors for late-life depression in women independently of circulating CRP levels.
CRP polymorphisms and late-life depression
To date, only four studies have investigated CRP variants and depression with inconsistent results.6, 10, 11, 12 Two adult studies found no associations with depressive10 or affective symptoms.11 Luciano et al.12 examined the associations between depressive mood state and CRP SNPs in two elderly cohorts while adjusting for age and gender. They only reported a nominally significant association with rs1800947 in a subsample of one of the cohorts, the oldest 229 participants assessed at age 87, and they found no association with rs1205.12 In contrast, Almeida et al.6 reported an association between rs1205 and depression in 3700 men aged 70 years and over. In our study, all associations were specific to women, the strongest associations being that with rs1130864 and the closely linked rs1417938, although there was also some evidence of an association with both rs1205 and rs1800947. In contrast with Almeida et al.,6 we did not observe an association with rs1205 in men both in unadjusted analysis and after considering possible mediating factors. Possible explanations for differences in the findings of their study and ours could involve decreased power due to a 2.6-fold lower number of depressed men in our study and/or differences in cohort characteristics. Our sample including younger and healthier European men with lower mean CRP levels compared to that of the Australian cohort. None of the previous studies reported significant associations with rs1130864 and they did not examine rs1417938, both of which we found to be highly significantly associated (P=0.002) with depression in elderly women even after correction for multiple testing. Two of the SNPs we identified, rs1130864 and rs1205 have potential functional significance, as they are found in the 3′ untranslated region of the CRP gene, a regulatory region which plays a key role in controlling gene expression. The third SNP, rs1417938 is intronic with the potential that it influences gene function through alternative splicing.30 However, the associations found with this SNP, may also be due to its strong linkage disequilibrium with rs1130864.
CRP polymorphisms and circulating CRP
The association between SNPs and serum CRP levels was comparable in women and men for rs1205, rs1800947, and rs 3093059, but only for rs1205 in women and rs1800947 in men were the minor alleles associated with lower CRP levels. We observed clear gender differences for rs1130864 and rs1417938; homozygotes for the minor alleles (TT and AA, respectively) had lower CRP levels in men (-28%), while in women there was only a very weak in the reverse direction. Only a few population-based studies have examined men and women separately.6, 7, 8, 9 Globally, similar trends have been reported for rs1205 and rs1800947 in a Finnish study of older women and men,8 as well as a cohort of Australian men6 and American women.7 For rs1130864 the TT homozygote has also been associated with higher CRP levels in women but lower levels in men,8, 9 whereas Almeida et al.6 reported a reverse association in men. A study of 968 postmenopausal women showed similar associations of these five SNPs with CRP levels as in our study.31
CRP polymorphisms, circulating CRP and late-life depression
Although we found CRP gene variants to be associated with both depression and CRP levels, the direction and strength of these associations are inconsistent with the assumption that CRP levels are an intermediate between gene variants and depression. A similar lack of correlation has been reported for cardiovascular disease: several variants associated with blood levels are not constituting a risk for cardiovascular disease.28, 32 In our study, the CRP variants that were associated with CRP levels in men were not significantly associated with depression. In women, the polymorphisms significantly associated with a decreased risk of depression, tended to be associated with higher CRP levels, but this was only nominally significant for rs1205. Almeida et al.6 suggested that CRP may be an adaptive compensatory response to external insults and poor physical health that predispose to depression.33 Our analysis further showed that adjusting for depression and cardiovascular ischemic pathologies did not modify the association between CRP variants and CRP levels (cf. Supplementary Table S2). In the reverse sense, further adjusting for CRP levels did not attenuate the association between rs1130864 or rs1417938 and depression (odds ratio=0.10 (0.02−0.45), P=0.003 for both SNPs). The lack of a mediating effect by CRP levels is consistent with a meta-analysis of 51 cross-sectional studies that reported a weak positive association between CRP levels and depression in the general population, especially after adjusting for vascular mediators and comorbidities or treatments.1 Common CRP variants have been reported to account for only 2–4% of the total variation in plasma CRP concentration.31, 34 This suggests that both CRP concentration and genotype could be independently associated with depression. CRP is known to interact with a variety of cytokines and immune cells. Genome-wide association studies have already identified some 20 CRP-associated loci explaining ~5% of the variance.25, 35, 36, 37 Many of these genes involve pathways related to either innate immunity (CRP, IL6R, IL6, NLRP3, IL1RN and IRF1) or lipid and glucose metabolism, and have been associated with a variety of diseases as well as biochemical traits.5, 38
CRP is a more general marker of inflammatory processes compared, for instance, with interleukins (ILs) that have direct effects on the brain and other inflammatory markers, as well as other indirect mechanisms leading to neuro-inflammation and neuropsychiatric disorders.39, 40 A recent meta-analysis reported a small significant association between CRP levels and depression risk.3 They suggested that this small contribution to depression could be related to (i) the heterogeneity of depression with inflammation being etiologically related to some, but not all cases, (ii) a variable contribution of inflammation to the symptoms of depression between individuals and (iii) fluctuating or cumulative effects only observed after a long follow-up. Together, this may explain why CRP levels are not the most appropriate biomarker for depression.3
Potential mechanisms linking CRP variants and depression and gender differences
Our data suggest that CRP variants can influence circulating levels differently in older men and women but appear as independent susceptibility factors for late-life depression in women, even after controlling for factors known to alter inflammatory processes (smoking, cardiovascular and inflammation-related pathologies, and antidepressants).
Twin studies suggest the existence of a common genetic pathway linking depression and inflammation.4 The relationship is reciprocal and could involve various cytokines, for example, IL-6, which together with IL-1β activates transcription factors that induce CRP transcription.5, 28 Conversely, depression can elevate circulating levels of IL-6 through the influence of catecholamine release and the sympathetic nervous system. A dysfunctioning of the corticotropic hypothalamic-pituitary-adrenal (HPA) axis has also been reported as both a consequence and cause of inflammatory processes and depression. IL-6 can induce production of CRH resulting in hypercortisolemia, which can contribute to depression.41 Conversely, stress-related system pathways, for example, sympathetic nervous system, coupled with the HPA axis can contribute to chronic activation of inflammatory responses.42 Cortisol, in particular, can exert diverse actions influencing the central nervous system, the immune system, and glucose and lipid metabolism,43 and this can also result in depression. Glucocorticoid resistance and associated hypercortisolemia can contribute to the inflammatory profile observed in major depressive disorder.44 The two CRP variants, rs1205 and rs1417938, have been shown to affect CRP concentrations but also cortisol levels after awakening, suggesting a negative feedback of CRP on the HPA axis.45 It is also possible that the association between the CRP gene and depression occurs via its ability to modulate the glucocorticoid receptor.44
The mechanisms for gender-specific differences are multifactorial and may involve not only CRP-related genetic variation but also the hormonal environment. Inflammation levels are known to fluctuate with the female menstrual cycle, menopause status and use of hormone treatment.46, 47, 48 We have already shown that hormonal fluctuation can modulate depressive symptomatology and interact with genetic vulnerability.16, 49, 50 Estrogen receptors and steroids have been involved in depression and participate in physiological regulation of brain inflammation16, 18 and both have also been involved in sex differences in HPA axis function.51 Sexual dimorphism of stress responses and HPA axis functioning could influence the relationship between depression and inflammation,17, 52, 53 and opposite effects of sexual steroids on the HPA axis and associated immune system functioning have been reported; androgenic steroids having milder suppressive actions on the HPA axis and smaller peripheral immunosuppressive and anti-inflammatory actions than estrogens.17 We have already shown in this elderly cohort the persistence of an abnormal cortisol secretion, especially in women, even after recovery from major depression.54 Whether the HPA axis could act as a mediating factor between CRP and depression in women remains to be examined.
Limitations and strength
Study limitations include bias from excluding participants with missing data who were in poorer health and more likely to be depressed, thus reducing the overall study power. French law prohibits from questioning participants about ethnicity, however, prior genotyping data from a subsample of these participants indicates that >99% are white Europeans.22 This is supported by the similarity of the CRP genotype frequencies with those published previously in white Europeans11, 55, 56, 57 (and http://www.ncbi.nlm.nih.gov/projects/SNP/). The CRP analyses were based on only one measurement at wave 5. To limit intraindividual and analytical variability in circulating CRP levels, we excluded subjects with CRP levels >10 mg l−1 to eliminate cases of falsely elevated concentrations owing to acute-phase inflammation response, a high-sensitivity CRP assay was performed and we observed good correlation with baseline measurement, which is consistent with stability of CRP over time.4, 58 It remains possible that other unknown factors may have influenced the associations, including subclinical disease, which was not detectable through the analysis of lipids, glycemia and hypertension.
Our study is strengthened by its design and a large population-based sample. This is the first study of late-life depression to investigate potential associations with several CRP polymorphisms in community-dwelling elderly men and women separately while also examining the impact on circulating levels. DEP was assessed by trained staff using two distinct measures validated in the general population, including a structured Diagnostic and Statistical Manual of Mental Disorders-IV-based diagnostic interview.20, 21 Five SNPs were chosen to ensure satisfactory coverage across the gene. We used a genotyping system with a very low error rate and were able to control for the accuracy through duplicate samples. The high-sensitivity CRP assay provided a sensitive measure of CRP with measurement that extends below that of most conventional CRP assays.59 We were able to consider gender differences and controlled for numerous potential mediating factors in contrast with previous studies.
Conclusion
Our findings provide epidemiological support for the involvement of CRP variants in late-life depression; the associations were specific to women and remained significant after controlling for pre-existing medical illnesses and correction for multiple testing. Variants of the CRP gene also influence circulating CRP levels differently in women and in men, but this does not appear to modulate the association with depression. Our data suggest that CRP variants could be a better marker of depression than CRP levels. To our knowledge, this is the first report of a significant association between rs1130864 and rs1417938, and depression. The exact functional significance of these genetic variants, however, remains speculative and further investigation is warranted. Replication of this finding in other populations is necessary to confirm these associations and their potential usefulness in predicting late-life depression.
References
Howren MB, Lamkin DM, Suls J . Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186.
Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry 2012; 2: e79.
Valkanova V, Ebmeier KP, Allan CL . CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013; 150: 736–744.
Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C et al. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med 2009; 71: 152–158.
Hage FG, Szalai AJ . The role of C-reactive protein polymorphisms in inflammation and cardiovascular risk. Curr Atheroscler Rep 2009; 11: 124–130.
Almeida OP, Norman PE, Allcock R, van Bockxmeer F, Hankey GJ, Jamrozik K et al. Polymorphisms of the CRP gene inhibit inflammatory response and increase susceptibility to depression: the Health in Men Study. Int J Epidemiol 2009; 38: 1049–1059.
Fan AZ, Yesupriya A, Chang MH, House M, Fang J, Ned R et al. Gene polymorphisms in association with emerging cardiovascular risk markers in adult women. BMC Med Genet 2010; 11: 6.
Kettunen T, Eklund C, Kahonen M, Jula A, Paiva H, Lyytikainen LP et al. Polymorphism in the C-reactive protein (CRP) gene affects CRP levels in plasma and one early marker of atherosclerosis in men: The Health 2000 Survey. Scand J Clin Lab Invest 2011; 71: 353–361.
Komurcu-Bayrak E, Erginel-Unaltuna N, Onat A, Ozsait B, Eklund C, Hurme M et al. Association of C-reactive protein (CRP) gene allelic variants with serum CRP levels and hypertension in Turkish adults. Atherosclerosis 2009; 206: 474–479.
Halder I, Marsland AL, Cheong J, Muldoon MF, Ferrell RE, Manuck SB . Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C-reactive protein. Brain Behav Immun 2010; 24: 160–167.
Gaysina D, Pierce M, Richards M, Hotopf M, Kuh D, Hardy R . Association between adolescent emotional problems and metabolic syndrome: the modifying effect of C-reactive protein gene (CRP) polymorphisms. Brain Behav Immun 2011; 25: 750–758.
Luciano M, Houlihan LM, Harris SE, Gow AJ, Hayward C, Starr JM et al. Association of existing and new candidate genes for anxiety, depression and personality traits in older people. Behav Genet 2010; 40: 518–532.
Goldstein RD, Gruenberg AM . Major Depressive Disorder in the older adult: implications for women. J Women Aging 2007; 19: 63–77.
Altemus M, Sarvaiya N, Neill Epperson C . Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 2014; 35: 320–330.
Ancelin ML, Carriere I, Boulenger JP, Malafosse A, Stewart R, Cristol JP et al. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study). Biol Psychiatry 2010; 68: 125–132.
Ryan J, Ancelin ML . Polymorphisms of estrogen receptors and risk of depression: therapeutic implications. Drugs 2012; 72: 1725–1738.
Chrousos GP . Stress and sex versus immunity and inflammation. Sci Signal 2010; 3: pe36.
Gosselin D, Rivest S . Estrogen receptor transrepresses brain inflammation. Cell 2011; 145: 495–497.
Ritchie K, Artero S, Beluche I, Ancelin ML, Mann A, Dupuy AM et al. Prevalence of DSM-IV psychiatric disorder in the French elderly population. Br J Psychiatry 2004; 184: 147–152.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59: 22–33.
Radloff L . The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement 1977; 1: 385–401.
Ancelin ML, Carriere I, Scali J, Ritchie K, Chaudieu I, Ryan J . Angiotensin-converting enzyme gene variants are associated with both cortisol secretion and late-life depression. Transl Psychiatry 2013; 3: e322.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Eiriksdottir G, Smith AV, Aspelund T, Hafsteinsdottir SH, Olafsdottir E, Launer LJ et al. The interaction of adiposity with the CRP gene affects CRP levels: age, gene/environment susceptibilty-Reykjavik study. Int J Obes (Lond) 2009; 33: 267–272.
Okada Y, Takahashi A, Ohmiya H, Kumasaka N, Kamatani Y, Hosono N et al. Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum Mol Genet 2011; 20: 1224–1231.
Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW . DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet 2003; 33: 67–72.
Folstein MF, Folstein SE, McHugh PR . "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198.
Hage FG, Szalai AJ . C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol 2007; 50: 1115–1122.
Straczek C, Ducimetiere P, Barberger-Gateau P, Helmer C, Ritchie K, Jouven X et al. Higher level of systemic C-reactive protein is independently predictive of coronary heart disease in older community-dwelling adults: the three-city study. J Am Geriatr Soc 2010; 58: 129–135.
Wang GS, Cooper TA . Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet 2007; 8: 749–761.
Lee CC, You NC, Song Y, Hsu YH, Manson J, Nathan L et al. Relation of genetic variation in the gene coding for C-reactive protein with its plasma protein concentrations: findings from the Women's Health Initiative Observational Cohort. Clin Chem 2009; 55: 351–360.
Manace LC, Babyatsky MW . Putting genome analysis to good use: lessons from C-reactive protein and cardiovascular disease. Cleve Clin J Med 2012; 79: 182–191.
Bufalino C, Hepgul N, Aguglia E, Pariante CM . The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav Immun 2013; 31: 31–47.
Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA 2006; 296: 2703–2711.
Naitza S, Porcu E, Steri M, Taub DD, Mulas A, Xiao X et al. A genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulation. PLoS Genet 2012; 8: e1002480.
Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 2011; 123: 731–738.
Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE et al. Loci related to metabolic-syndrome pathways including LEPR,HNF1 A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet 2008; 82: 1185–1192.
Pepys MB, Hirschfield GM . C-reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812.
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW . From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56.
Capuron L, Miller AH . Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 2011; 130: 226–238.
Felger JC, Lotrich FE . Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013; 246: 199–229.
Miller AH, Maletic V, Raison CL . Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–741.
Chrousos GP . Stress and disorders of the stress system. Nat Rev Endocrinol 2009; 5: 374–381.
Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM . Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 722–729.
Veen G, Giltay EJ, van Vliet IM, Derijk RH, Klaassens ER, van Pelt J et al. C-reactive protein polymorphisms are associated with the cortisol awakening response in basal conditions in human subjects. Stress 2011; 14: 128–135.
Dreon DM, Slavin JL, Phinney SD . Oral contraceptive use and increased plasma concentration of C-reactive protein. Life Sci 2003; 73: 1245–1252.
Jilma B, Dirnberger E, Loscher I, Rumplmayr A, Hildebrandt J, Eichler HG et al. Menstrual cycle-associated changes in blood levels of interleukin-6, alpha1 acid glycoprotein, and C-reactive protein. J Lab Clin Med 1997; 130: 69–75.
Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation 1999; 100: 717–722.
Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML . Lifetime hormonal factors may predict late-life depression in women. Int Psychogeriatr 2008; 20: 1203–1218.
Scali J, Ryan J, Carriere I, Dartigues JF, Tavernier B, Ritchie K et al. A prospective study of hormone therapy and depression in community-dwelling elderly women: the Three City Study. J Clin Psychiatry 2010; 71: 1673–1679.
Handa RJ, Weiser MJ . Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol 2014; 35: 197–220.
Kumsta R, Entringer S, Koper JW, van Rossum EF, Hellhammer DH, Wust S . Sex specific associations between common glucocorticoid receptor gene variants and hypothalamus-pituitary-adrenal axis responses to psychosocial stress. Biol Psychiatry 2007; 62: 863–869.
de Kloet ER, Joels M, Holsboer F . Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475.
Beluche I, Chaudieu I, Norton J, Carriere I, Boulenger JP, Ritchie K et al. Persistence of abnormal cortisol levels in elderly persons after recovery from major depression. J Psychiatr Res 2009; 43: 777–783.
Suchankova P, Holm G, Traskman-Bendz L, Brundin L, Ekman A . The +1444C>T polymorphism in the CRP gene: a study on personality traits and suicidal behaviour. Psychiatr Genet 2013; 23: 70–76.
Flex A, Pola R, Serricchio M, Gaetani E, Proia AS, Di Giorgio A et al. Polymorphisms of the macrophage inhibitory factor and C-reactive protein genes in subjects with Alzheimer's dementia. Dement Geriatr Cogn Disord 2004; 18: 261–264.
van Oijen M, de Maat MP, Kardys I, de Jong FJ, Hofman A, Koudstaal PJ et al. Polymorphisms and haplotypes in the C-reactive protein gene and risk of dementia. Neurobiol Aging 2007; 28: 1361–1366.
Chocano-Bedoya PO, Mirzaei F, O'Reilly EJ, Lucas M, Okereke OI, Hu FB et al. C-reactive protein, interleukin-6, soluble tumor necrosis factor alpha receptor 2 and incident clinical depression. J Affect Disord 2014; 163: 25–32.
Lopresti AL, Maker GL, Hood SD, Drummond PD . A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48: 102–111.
Acknowledgements
The ESPRIT project is financed by the regional government of Languedoc-Roussillon, Agence Nationale de la Recherche Project 07 LVIE 004, Fonds de coopération scientifique Alzheimer (2009-2012) and an unconditional grant from Novartis. JR is the holder of a National Health and Medical Research Council Early Career Fellowship (APP1012735). The funders had no role in the design and conduct of the study, in data collection, management, analysis and interpretation of the data, or writing the report preparation, review or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Ancelin, ML., Farré, A., Carrière, I. et al. C-reactive protein gene variants: independent association with late-life depression and circulating protein levels. Transl Psychiatry 5, e499 (2015). https://doi.org/10.1038/tp.2014.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.145
This article is cited by
-
Major Depressive Disorder in Older Patients as an Inflammatory Disorder: Implications for the Pharmacological Management of Geriatric Depression
Drugs & Aging (2021)
-
Sex differences in the association between self-rated health and high-sensitivity C-reactive protein levels in Koreans: a cross-sectional study using data from the Korea National Health and Nutrition Examination Survey
Health and Quality of Life Outcomes (2020)
-
C-reactive protein concentration in bipolar disorder: association with genetic variants
International Journal of Bipolar Disorders (2019)
-
Sex Difference in the Association between High-sensitivity C-reactive Protein and Depression: The 2016 Korea National Health and Nutrition Examination Survey
Scientific Reports (2019)
-
Crosstalk Between Inflammation and Glutamate System in Depression: Signaling Pathway and Molecular Biomarkers for Ketamine’s Antidepressant Effect
Molecular Neurobiology (2019)