Abstract
Metabolic syndrome, an etiological factor in non-alcoholic fatty liver disease (NAFLD), is often present in hemodialysis patients. Little is known about the prevalence of, and factors associated with, liver fibrosis in hemodialysis populations. We used transient elastography (TE) to investigate these phenomena. 659 patients treated with chronic hemodialysis were enrolled. We excluded those with excess alcohol intake, liver stiffness measurement (LSM) failure, or unreliable LSM values. LSM ≥8.0 kPa was used as a cutoff suggesting clinically relevant fibrosis. Controlled attenuation parameter (CAP) ≥ 232.5 dB/m was used as a cutoff suggesting steatosis. 333 patients (50.5%) had steatosis, 159 (24.1%) had hepatitis B or C, and 149 (22.6%) had LSM ≥8.0 kPa. In multivariable analyses, male gender (OR: 2.16; 95% CI: 1.29–3.63; P = 0.004), overweight body habitus (OR:2.31; 95% CI: 1.35–3.94; P = 0.002), high AST level (OR:1.08; 95% CI: 1.04–1.12; P < 0.001), low albumin level (OR: 0.25; 95% CI: 0.12–0.53; P < 0.001), low creatinine level (OR: 0.89; 95% CI: 0.79–1.00; P = 0.05) and low platelet count (OR: 0.99; 95% CI: 0.99–1.00; P < 0.001) were associated with LSM ≥8.0 kPa. We thus conclude that hemodialysis patients have a high prevalence of NAFLD and clinically relevant fibrosis. NAFLD may be an important determinant of clinically relevant fibrosis in hemodialysis populations.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, affecting 15–40% of the population worldwide1. Around 20–30% of patients with NAFLD have nonalcoholic steatohepatitis (NASH), which will eventually progress to cirrhosis and hepatocellular carcinoma in 10–20% of patients2,3,4. NAFLD is strongly associated with the components of metabolic syndrome (central obesity, hypertension, hyperglycemia, and dyslipidemia) and is now regarded as the liver manifestation of metabolic syndrome5,6,7.
Moreover, the main risk factors responsible for the development of NAFLD (which are also the components of metabolic syndrome) are commonly observed in dialysis patients. Therefore, it is logical to expect that end-stage renal disease (ESRD) patients maintained on hemodialysis would also have a high prevalence of NAFLD.
Transient elastrography (TE) is a non-invasive test of liver fibrosis that is quick and easy to perform and causes no discomfort. It has high accuracy and reproducibility when used to detect advanced fibrosis and cirrhosis. The controlled attenuation parameter (CAP) is a measurement of the degree of ultrasound attenuation caused by hepatic fat at the central frequency of the FibroScan8. CAP measurements have been shown to be accurate in estimating the amount of liver fat9,10,11. It is thus now possible, using the non-invasive technique of TE, to measure liver fat and fibrosis simultaneously.
To date, only a limited number of studies have been performed focusing on the prevalence and risk factors for liver fibrosis in ESRD patients. Due to the fact that alanine aminotransferase (ALT) tends to be within the normal range in ESRD patients, we used one of the best available non-invasive tests (that is, TE) to evaluate liver fibrosis in this special population. The aim of our study was to investigate the prevalence of, and factors associated with, clinically relevant liver fibrosis, as measured by TE, in a large cohort of ESRD patients on maintained hemodialysis.
Methods
Patients
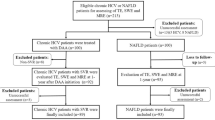
Patients treated at our center (Kaoshiung Chang Gung Memorial Hospital) for chronic hemodialysis between April and September of 2014 were considered for inclusion in our study. These patients received hemodialysis for at least 6 months. We excluded those patients with excess alcohol intake, meaning consumption of >21 alcoholic drinks per week in men and >14 alcoholic drinks per week in women over the preceding 2 years12, and also excluded others for technical reasons, including failure of the FibroScan or unreliable liver stiffness measurement (LSM) (i.e., interquantile range (IQR) > 0.3). The study protocol adhered to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethical committee of Chang Gung Memorial Hospital. Written informed consent was obtained from each of the participants in this study.
FibroScan examination
LSM and CAP results were obtained using a FibroScan device (Echosens, Paris, France). All the patients fasted for at least 2 h before the exam and all the exams were performed before dialysis. The LSM was represented by the median of 10 measurements and was considered reliable only if at least 10 successful acquisitions were obtained and the IQR-to-median ratio of the 10 acquisitions was ≤0.3. The CAP was represented by the median value. CAP measurements were considered reliable if 10 successful acquisitions were obtained. The M probe was used for all patients. Two operators who each had >6 years of experience with the FibroScan device and had performed >3000 procedures prior to the study performed the procedures. LSM ≥8.0 kPa and >13.0 kPa were taken as cutoffs suggesting clinically relevant liver fibrosis and cirrhosis, respectively13,14,15. CAP ≥ 232.5 dB/m was taken as the cutoff level suggesting hepatic steatosis16.
Fibrosis 4 calculator (FIB-4) as a comparator for fibrosis assessment
We used FIB-4 as a comparator for liver fibrosis assessment. FIB-4 is an accepted, non-invasive procedure for the identification of cases at risk of advanced fibrosis as recommended by the European Association for the Study of the Liver (EASL) guideline17. FIB-4 ≥2.67 was defined as advanced fibrosis in NAFLD patients18.
Biological parameters
The same day as the TE measurements were conducted, the following clinical parameters were recorded: age, gender, body mass index (BMI), diabetes mellitus (DM) status, fasting lipid profile, fasting sugar level, hepatitis B surface antigen (HBsAg), hepatitis C virus antibody (anti-HCV), liver function tests, complete blood count, and creatinine. Biological parameters were measured before the hemodialysis.
Statistical analysis
The baseline characteristics and clinical variables were summarized as mean ± standard deviation, median (25th–75th percentile), or percentage, and the Student’s t test, chi-square test, or Fisher’s exact test were used as appropriate to assess the significance of differences in the distributions of the characteristics and variables. Multivariate logistical regression analyses were conducted to identify patient characteristics independently associated with clinically relevant fibrosis. A univariate analysis was first performed on each of the considered independent variables to select candidate variables for the multivariate analyses. The performance of the LSM for diagnosing clinically relevant fibrosis compared with that of FIB-4 was assessed using the area under the receiver operating characteristic curve (AUROC). In all analyses, a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using Stata software version 11.0.
Results
Study population
Six hundred and fifty-nine patients were enrolled in this study. The baseline characteristics of the patients are shown in Table 1: 51.8% of the patients were women, the mean age of the patients was 61.9 ± 11.8 years, and the mean BMI of the patients was 22.3 ± 3.4 kg/m2. In addition, the median LSM was 5.9 kPa, 50.5% of the patients had hepatic steatosis, and 159 (24.1%) had positive viral serology (72 were HBsAg positive and 95 were anti-HCV positive, while 8 were both HBsAg and anti-HCV positive).
Factors associated with clinically relevant fibrosis
LSM ≥8.0 kPa, suggesting the presence of clinically relevant fibrosis, was detected in 149 participants (22.6%). The distribution of LSM values in our cohort of 659 patients is illustrated in Fig. 1. Multivariate analyses showed that male gender (odds ratio [OR]: 2.16, 95% confidence interval [CI]: (1.29–3.63), P = 0.004), overweight body habitus (i.e., 24 ≤ BMI < 30 (OR: 2.31, 95% CI: 1.35–3.94, P = 0.002)), high AST level (OR: 1.08, 95% CI: 1.04–1.12, P < 0.001), low albumin level (OR: 0.25, 95% CI: 0.12–0.53, P < 0.001), low creatinine level (OR: 0.89. 95% CI: 0.79–1.00, P = 0.05) and low platelet count (OR: 0.99. 95% CI: 0.99–1.00, P < 0.001) were independent factors associated with LSM ≥8.0 kPa (Table 2).
Diagnostic performance of the LSM for FIB-4-identified clinically relevant fibrosis
Using FIB-4 ≥ 2.67 as a surrogate gold standard for clinically relevant fibrosis, the diagnostic performance of LSM ≥8.0 kPa in all patients was as follows: AUROC = 0.807, 95% CI = 0.64–0.97, sensitivity = 83.33%, specificity = 78.09%, positive predictive value (PPV) = 3.29%, and negative predictive value (NPV) = 99.81%. The diagnostic performance of LSM ≥8.0 kPa in patients without chronic hepatitis B or C was as follows: AUROC = 0.821, 95% CI = 0.66–0.99, sensitivity = 83.33%, specificity = 80.87%, PPV = 4.90%, and NPV = 99.76%.
Factors associated with liver cirrhosis
Forty-three (6.5%) of the 659 cases were found to have LSM >13.0 kPa. Of those patients, 16 (37.2%) were female, the mean age was 64.6 ± 9.7 years, 13 (30.2%) were overweight (BMI 24–30), 2 (4.7%) were obese (BMI > 30), 11 (25.6%) had hepatic steatosis, 18 (41.9%) had DM, and 18 (41.9%) were HBsAg and/or anti-HCV positive. Multivariate analyses showed that male gender (OR: 2.75, 95% CI: 1.16–6.52, P = 0.02), obesity (i.e., BMI ≥30 (OR: 13.64, 95% CI: 2.06–90.50, P = 0.007)), high AST level (OR: 1.06, 95% CI: 1.02–1.11, P = 0.007), low creatinine level (OR: 0.76, 95% CI: 0.62–0.94, P = 0.01) and low platelet count (OR: 0.99, 95% CI: 0.98–0.99, P < 0.001) were independent factors associated with LSM >13.0 kPa.
Factors associated with steatosis
Multivariate analyses showed that old age (OR:1.03, 95% CI: 1.01–1.05, P < 0.001), overweight body habitus (i.e., 24 ≤ BMI < 30 (OR: 2.12, 95% CI: 1.35–3.33, P = 0.001)), DM (OR: 2.58, 95% CI: 1.70–3.92, P < 0.001), high creatinine level (OR: 1.18, 95% CI: 1.06–1.30, P = 0.002), HDL–C <40 mg/dL (for males) or <50 mg/dL (for females) (OR: 1.82, 95% CI: 1.21–2.73, P = 0.004), and triglyceride >150 (OR: 3.46, 95% CI: 2.13–5.61, P < 0.001) were independent factors associated with steatosis (Table 3).
Factors associated with clinically relevant fibrosis in patients with NAFLD
A subgroup analysis was performed to investigate the factors associated with LSM in patients with NAFLD. Using CAP ≥232.5 dB/m as the definition of hepatic steatosis and excluding patients who were HBsAg or anti-HCV positive, 248 patients were diagnosed with NAFLD. Among those patients, the mean age was 60.8 ± 10.9 years, the mean BMI was 23.9 ± 3.5 kg/m2, the median LSM was 5.9 kPa (4.7–7.5), and 50 (20.2%) patients had LSM ≥8.0 kPa. Multivariate analyses showed that overweight body habitus (i.e., 24 ≤ BMI < 30 (OR = 4.13, 95% CI: 1.69–10.10, P = 0.002)), obesity (i.e., BMI ≥30 (OR = 9.44. 95% CI: 1.80–49.49, P = 0.008)), high AST level (OR = 1.21, 95% CI: 1.11–1.31, P < 0.001), and low ALT level (OR = 0.93. 95% CI: 0.87–0.99, P = 0.02) were independent factors associated with LSM ≥8.0 kPa in NAFLD patients (Table 4).
Discussion
The prevalence of NAFLD in patients undergoing dialysis remains unknown.
The main risk factors responsible for the development of NAFLD (which are also the components of metabolic syndrome), however, are commonly observed in dialysis patients. Therefore, it is logical to expect that ESRD patients would also have a high prevalence of NAFLD. In this study, the prevalence of NAFLD in ESRD patients was 50.5%, which is higher than the incidence in the general population (15–40%)1.
Serologic testing has clearly demonstrated that HCV infection is highly prevalent among ESRD patients and is a serious cause of increased morbidity and mortality in this group. In 2002, the prevalence of HCV infection across dialysis centers in the United States was approximately 8%19,20. In some European dialysis centers, the yearly incidence of HCV infection reportedly ranges from 0.4 to 16.0%21. The prevalence of HCV infection in this study was 14.4%, which is comparable to the rates reported in previous studies. Since Taiwan is an endemic country for HBV infection, the prevalence of HBV in Taiwan is 17.3%22, and in this study, the prevalence of HBV infection was found to be 10.9%. For the above reasons, ESRD patients have a higher prevalence of chronic liver disease. Therefore, it is logical to survey liver disease in this population. To the best of our knowledge, this is the first large-scale study to use TE to survey liver disease in an ESRD population. In this large hospital cohort, 22.6% of the ESRD patients had LSM ≥8.0 kPa. Male patients, overweight patients, and patients with higher AST levels and lower albumin, creatinine, and platelet levels were more likely to have LSM ≥8.0 kPa.
Koehler et al. reported that 5.6% of the participants in their large Caucasian population-based study of older adults had LSM ≥8.0 kPa. Old age, high ALT level, smoking, HBsAg, or anti-HCV positivity and the combined presence of DM and steatosis were associated with LSM ≥8.0 kPa in their multivariable analyses23. Roulot et al. reported that 7.5% of participants had LSM > 8 kPa in their large Caucasian population-based study of older adults. Age ≥ 57 years, BMI ≥ 30 kg/m2, elevated waist circumference, DM, gamma-glutamyl transpeptidase (r-GT) ≥ 45 IU/L, and ALT ≥ 40 IU/L were associated with LSM >8 kPa in their multivariate analyses14. The prevalence of clinically relevant fibrosis (LSM ≥8.0 kPa) was higher in our study compared with previous studies that enrolled older adult populations. This was possibly due to the higher prevalence of viral hepatitis B or C in our study (24%), whereas the prevalence was 0.8% in the Koehler et al.23 study and less than 1% in the Roulot et al.14 study. Using CAP ≥ 232.5 dB/m as the definition of steatosis, we found that 50.5% of the cases in our study had steatosis. In contrast, using ultrasound to diagnose steatosis, Koehler et al. found that only 35.5% the cases in their study had steatosis23. Therefore, the higher proportions of viral hepatitis and NAFLD in our study could explain the higher proportion of clinically relevant fibrosis in this study. Notably, BMI and aminotransferase were the same independent factors associated with LSM ≥8.0 kPa in both our study and previous studies14,23.
Although TE is easy to perform, it is unlikely that clinicians can apply it to all hemodialysis patients because of the large number of such patients. Therefore, it is important to identify those patients who are at risk of advanced liver disease. To that end, male gender, higher BMI, higher AST level, and lower albumin, creatinine, and platelet levels were the independent factors found to be associated with increased LSM in this study. As such, patients with these risk factors may benefit from TE assessment.
In this study, 43 (6.5%) of the 659 cases were found to have LSM >13.0 kPa. Of those patients, 27 were male, 15 were overweight or obese, 11 had steatosis, 18 had DM, and 18 were HBsAg and/or anti-HCV positive. In contrast, Koehler et al. reported that 19 (0.6%) of the participants in their population-based study of older adults had LSM >13.0 kPa. Among those patients, most were female, the mean BMI was 28.7 kg/m2, two participants had excess alcohol intake, 11 participants had steatosis, and 7 had DM23. Obesity, DM, and steatosis were risk factors for liver cirrhosis in both our study and in the Koehler et al. study23.
Two hundred and forty-eight cases were diagnosed with NAFLD in our study, and 20.2% of those cases had LSM ≥8.0 kpa. Multivariate analyses showed that BMI, AST and ALT were independent factors associated with LSM ≥8.0 kpa, and these factors were also the independent factors associated with LSM ≥8.0 kpa in the entire population in this study. These findings suggest that NAFLD may be an important determinant of clinically relevant fibrosis in hemodialysis populations. Since NAFLD has become the predominant cause of chronic liver disease in many parts of the world24, NAFLD will become a more urgent health issue in hemodialysis populations.
Fibrosis is the most important prognostic factor in NAFLD and is correlated with liver-related outcomes and mortality25. The use of the NAFLD fibrosis score (NFS) is suggested in patients with suspected NAFLD, as recommended by both the American Association for the Study of Liver Diseases (AASLD) and EASL guidelines12,17. A previous study used the combination of LSM with NFS, is able to accurately diagnose the presence of severe liver fibrosis26. Severe fibrosis was defined as fibrosis ≥ F3 according to the NASH Clinical Research Network system27. Patients with severe liver fibrosis should be screened with endoscopy for esophageal varices and ultrasonography for hepatocellular carcinoma28. According to these studies, we could use the combination of LSM with NFS to diagnose the presence of severe liver fibrosis. Patients with severe liver fibrosis should be screened with endoscopy for esophageal varices and ultrasonography for hepatocellular carcinoma.
Our study has the strength of a large sample size and the use of one of the best and most widely available non-invasive tests of liver steatosis and fibrosis; this is the first large-scale study to have used TE to survey liver disease in an ESRD population. However, our study also has some limitations. Liver biopsy is the gold standard to evaluate liver fibrosis. The cut-off value of LSM for diagnosing clinically relevant fibrosis in this study was obtained from non-uremic populations13,14,15. However, studies with liver biopsy and TE performed in hemodialysis patients are scarce, and all of the previous studies of this type involved HCV-infected patients29,30,31,32,33,34,35. Therefore, we used FIB-4 as a comparator test for clinically relevant fibrosis, and we found that the diagnostic performance of the LSM for FIB-4-identified clinically relevant fibrosis is relatively good (AUROC > 0.8).
In conclusion, hemodialysis patients have a high prevalence of NAFLD and clinically relevant liver fibrosis. Hemodialysis patients who are male, obese, and have higher AST levels and lower albumin, creatinine, and platelet levels are at particularly high risk and may be good targets for TE assessment. These findings suggest that NAFLD may be an important determinant of clinically relevant fibrosis in hemodialysis populations.
Additional Information
How to cite this article: Cheng, B.-C. et al. Transient elastography as a screening tool for liver fibrosis in a large hemodialysis population. Sci. Rep. 7, 46458; doi: 10.1038/srep46458 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wong, V. W. et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: A population study with paired proton-magnetic resonance spectroscopy. J Hepatol 62, 182–9 (2015).
Wong, V. W. et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 59, 969–74 (2010).
Ascha, M. S. et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 51, 1972–8 (2010).
Bhala, N. et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborativem study. Hepatology 54, 1208–16 (2011).
D’Adamo, E. et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33, 1817–1822 (2010).
Kotronen, A. & Yki-Järvinen, H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 28, 27–38 (2008).
Kotronen, A. et al. Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia 52, 684–90 (2009).
Wong, G. L. Update of liver fibrosis and steatosis with transient elastography (Fibroscan). Gastroenterol Rep (Oxf) 1, 19–26 (2013).
Sasso, M. et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36, 1825–35 (2010).
Sasso, M. et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan®: validation in chronic hepatitis C. J Viral Hepat 19, 244–53 (2012).
de Lédinghen, V. et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol 60, 1026–31 (2014).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55, 2005–23 (2012).
Wong, V. W. et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51, 454–462 (2010).
Roulot, D. et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 60, 977–984 (2011).
Castera, L., Forns, X. & Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 48, 835–847 (2008).
Shi, K. Q. et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol 29, 1149–58 (2014).
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64, 1388–402 (2016).
Shah, A. G. et al. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 7, 1104–12 (2009).
Finelli, L., Miller, J. T., Tokars, J. I., Alter, M. J. & Arduino, M. J. National surveillance of dialysis-associated diseases in the United States,2002. Semin Dial 18, 52–61 (2002).
Patel, P. R., Thompson, N. D., Kallen, A. J. & Arduino, M. J. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis 56, 371–378 (2010).
Zampieron, A. et al. European study on epidemiology and management of hepatitis C virus (HCV) infection in the haemodialysis population. Part 3: prevalence and incidence. EDTNA ERCA J 32, 42–44 (2006).
Chen, C. H. et al. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc 106, 148–55 (2007).
Koehler, E. M. et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology 63, 138–47 (2016).
Masuoka, H. C. & Chalasani, N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci 1281, 106–122 (2013).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Petta, S. et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 35, 1566–73 (2015).
Kleiner, D. E. et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–21 (2005).
Castera, L., Vilgrain, V. & Angulo, P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol 10, 666–675 (2013).
Agarwal, S. K. & Gupta, S. D. Liver biopsy in patients on hemodialysis with hepatitis C virus infection: An important tool. Indian J Nephrol 25, 152–7 (2015).
Liu, C. H. et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol 6, 1057–65 (2011).
Mysorekar, V. V., Rao, S. G. & Mahadeva, K. C. Liver histology in patients on hemodialysis with chronic hepatitis C viral infection. Indian J Pathol Microbiol. 51, 182–5 (2008).
Yildirim, B. et al. Liver steatosis in hepatitis C positive hemodialysis patients and factors affecting IFN-2a treatment. Scand J Gastroenterol 41, 1235–41 (2006).
Glicklich, D., Thung, S. N., Kapoian, T., Tellis, V. & Reinus, J. F. Comparison of clinical features and liver histology in hepatitis C-positive dialysis patients and renal transplant recipients. Am J Gastroenterol 94, 159–63 (1999).
Iotti, A. et al. Liver histology in chronic hemodialysis patients infected with hepatitis C virus. Medicina (B Aires) 57, 541–5 (1997).
Pol, S. et al. Hepatitis C virus RNA in anti-HCV positive hemodialyzed patients: significance and therapeutic implications. Kidney Int 44, 1097–100 (1993).
Acknowledgements
This work was supported by Grant CMRPG8B1171 from Chang Gung Memorial Hospital-Kaohsiung Medical Center; Taiwan. The funder of this study had no role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
Research idea and study design: Ben-Chung Cheng, Yi-Hao Yen, Tsung-Hui Hu, Ming-Chao Tsai; data acquisition: Jin-Bor Chen, Cheng-Kun Wu, Ming-Tsung Lin, Jung-Ting Lin; data analysis/interpretation: Po-Lin Tseng, Jung-Fu Chen; statistical analysis: Kuo-Chin Chang.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cheng, BC., Yen, YH., Chen, JF. et al. Transient elastography as a screening tool for liver fibrosis in a large hemodialysis population. Sci Rep 7, 46458 (2017). https://doi.org/10.1038/srep46458
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46458
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.