Abstract
Cactin was originally identified as an interactor of the Drosophila IκB factor Cactus and shown to play a role in controlling embryonic polarity and regulating the NF-κB signaling pathway. While subsequent studies have identified the roles for Cactin in the mammalian immune response, the immune function of Cactin in insects has not been described yet. Here, we identified a Cactin gene from the mealworm beetle, Tenebrio molitor (TmCactin) and characterized its functional role in innate immunity. TmCactin was highly expressed in prepupa to last instar stages, and its expression was high in the integument and Malpighian tubules of last instar larvae and adults. TmCactin was induced in larvae after infection with different pathogens and detectable within 3 hours of infection. The highest levels of TmCactin expression were detected at 9 hours post infection. TmCactin RNAi significantly decreased the survival rates of larvae after challenge with Escherichia coli and Staphylococcus aureus, but had no significant effect after challenge with Candida albicans. Furthermore, TmCactin RNAi significantly reduced the expression of seven antimicrobial peptide genes (AMPs) after bacterial challenge. Our results suggest that TmCactin may serve as an important regulator of innate immunity, mediating AMP responses against both Gram-positive and Gram-negative bacteria in T. molitor.
Similar content being viewed by others
Introduction
Insects, unlike vertebrates, do not have an adaptive immune response and must rely on their innate immunity for pathogen defense. One important mechanism of innate immunity in insects is the production of antimicrobial peptides (AMPs). These peptides are induced through the activation of two key signaling pathways, namely the Toll and Immune deficiency (Imd) pathways1. In Drosophila and most other insects, the Toll pathway is activated mainly in response to fungal and Gram-positive bacterial infections2. This well-characterized pathway in Drosophila results in the phosphorylation and degradation of the inhibitor Cactus, which releases the NF-κB transcription factors Dif and Dorsal into the nucleus for activation of AMP genes such as Drosomycin3. In contrast, the Imd pathway responds mainly to Gram-negative bacterial infections and promotes nuclear translocation of the NF-κB Relish transcription factor to induce expression of AMP genes such as Diptericin4,5.
In addition to mediating innate immune responses in adult flies, the Toll pathway plays a role in directing Drosophila embryonic axis formation6. In both processes, the binding of Spätzle activates the Toll receptor, which together with adaptor proteins MyD88, Tube and protein kinase Pelle, causes degradation of Cactus7,8. Then, depending on the context, the NF-κB proteins Dif and/or Dorsal are released for nuclear translocation7,8. In the early embryo, degradation of Cactus, which arises in response to developmental cues that activate the Toll pathway, allows Dorsal to translocate into the nucleus and direct the expression of ventral genes, such as twist, snail, and rhomboid6. As mentioned above, the Toll pathway in the Drosophila immune response, however, involves the translocation of both Dif and Dorsal to the nucleus where they activate target genes, including AMP genes. It is evident that Dif and Dorsal function redundantly in the regulation of antifungal peptide gene expression in Drosophila larvae, but in adults, Dif alone is required for Toll-induced AMP gene transcription9,10,11.
Over the last twenty years, genetic and biochemical analyses in model systems, such as Drosophila, nematode, and mammals have led to the characterization of several Toll signaling components. As a result of these studies, it has become more and more apparent that although these components are highly conserved in their sequence and biochemical function, they can have diverse functions. One example of many such components is Cactin, which was initially identified in a yeast two hybrid screen as an interacting protein of Drosophila Cactus12. Overexpression of Cactin in Drosophila embryos causes strong ventralization12. Based on these observations and given that Cactus is an inhibitor of Dorsal and that Dorsal functions as a ventral activator, Cactin is suggested to promote nuclear translocation of Dorsal by negatively regulating the stability of the Cactus protein in developing embryos12.
Since its discovery in Drosophila, Cactin homologs have been identified and functionally characterized in other organisms. In C. elegans, Cactin has been shown to be required for normal distal tip cell migration and seam cell proliferation during larval development13,14. In Arabidopsis, Cactin is necessary for embryogenesis and has been suggested to play a role in splicing based on its interaction with splicesomal proteins in yeast and plant cells15. In zebrafish, knockdown of Cactin results in developmental defects and embryonic lethality16. In Toxoplasma gondii, Cactin is suggested to function in cell cycle progression based on the finding that a single mutation in Cactin causes G1 phase arrest17. Furthermore, the role of Cactin innate immune responses is suggested by a study showing that in human cells, Cactin overexpression inhibits Toll-like receptor-mediated NF-κB and interferon-regulatory factor (IRF) activation18. In further support of this role, the recently cloned Cactin from Litopenaeus vannamei has been shown to suppress the activities of Drosophila and shrimp AMP promoters in Drosophila S2 cells19.
In the mealworm beetle Tenebrio molitor, the upstream mechanisms involved in Toll activation have been elucidated, although relatively little is known about the intracellular signaling events of the Tenebrio Toll pathway. So far, it is known that the Tenebrio Toll signaling pathway is activated as a result of a three-step proteolytic cascade initiated by pathogen recognition20,21. Upon binding to lysine-type peptidoglycan from Gram-positive bacteria and diaminopimelic-type peptidoglycan from Gram-negative bacteria, peptidoglycan-recognition protein-SA (PGRP-SA) and Gram-negative binding protein 1 (GNBP1) form a complex that can activate the serine protease cascade and cause cleavage of Spätzle to its active Toll ligand form20,21. To understand the signaling pathway downstream of the Toll receptor, we recently cloned a myeloid differentiation factor 88 homolog, TmMyD88, from T. molitor and characterized its gene structure and function in antimicrobial responses22. From this study, we found that TmMyD88 contains a typical death domain and a conserved Toll-like interleukin-1 receptor (TIR) domain that can potentially interact with the intracellular TIR domains of TLRs22. Furthermore, we found that depletion of TmMyD88 in T. molitor larvae reduced resistance to infection by S. aureus, demonstrating that TmMyD88 is required for survival against Staphylococcus infection22. At the same time as this previous study, Johnston et al. conducted an RNA-seq time course analyses of T. molitor over a 7-day period in response to bacterial challenge23. By comparing their transcriptomic data to the Tribolium castaneum predicted proteome, they were able to identify putative orthologs for the majority of the components of the Toll, IMD, and JAK/STAT pathways23. For example, among those identified, genes encoding β-1,3-glucan recognition protein and NF-κB transcription factor Relish were transiently induced after immune challenge, while genes encoding AMPs, such as four attacins, and several components of the Toll pathway, including GNBP1, Toll, and serine proteases, SPE and SAE, remained elevated 7 days after challenge23.
In this study, we cloned and identified a Cactin homolog from T. molitor. The temporal and spatial expression profiles of TmCactin as well as its response after bacterial and fungal infections were analyzed. Further, we investigated the effects of RNAi-mediated knockdown of TmCactin on larval survival and AMP gene expression. Our studies on TmCactin contribute to a better understanding of the Toll/NF-κB pathway regulation and innate immune response in T. molitor.
Results
Gene organization of TmCactin and inferred protein domains
The TmCactin gene was identified by performing local-tblastn searches against the Tenebrio RNAseq and EST database using the T. castanuem Cactin protein sequence as query. The genomic organization of the TmCactin gene was determined by local-blastn against the Tenebrio genome database. Comparison between the genomic sequence and cDNA revealed that TmCactin (Accession number; KY618833) has seven exons and six introns. The ORF of 2,833 bp encodes a 952 amino acid long polypeptide with a molecular weight of approximately 110 kDa (Figs S1–2). Conserved domain analysis using InterProScan and the NCBI Conserved Domain Database revealed that TmCactin has three conserved domains: a coiled-coil domain (also known as the Cactin mid-domain) (Fig. 1A), a C-terminal Cactus-binding domain (Fig.1B), and a C2HC zinc-finger domain conforming to the consensus sequence CX2–4CX3FX5LX2HX3–424. Furthermore, we performed a blastp search against the NCBI non-redundant protein database using the entire TmCactin protein sequence and identified numerous homologs in other insects and animals. Of the top 100 hits, we retrieved homologous sequences with amino acid identities in the 56% to 86% range from 57 insect species, including 4 from beetles, 28 from ants, 7 from bees, 6 from wasps, and 2 from Drosophila (see Supplementary Table 1). We also retrieved predicted Cactin sequences from five mammals (dolphin, whale, common shrew, prarie vole, and camel), two crustaceans (water flea and shrimp), two alligator species, and three birds. All of these proteins possess a coiled-coil domain and a Cactus-binding domain, but only three of the proteins (uncharacterized proteins KYM93587.1, KYM76580.1, and EFN62654.1) contain multiple zinc-finger domains.
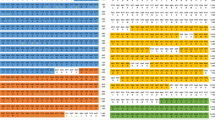
Multiple alignment of the conserved TmCactin mid-region (A) and C-terminal Cactus-binding region (B). Identical and chemically equivalent amino acid residues are labeled by black or grey underlay, respectively. (C) Phylogenetic analysis of TmCactin with representative Cactin from other insects. The analysis was performed using the Maximum Likelihood method based on the JTT matrix-based model and bootstrapped 1,000 times using MEGA6 program. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Homo sapiens Cactin sequence were used as an outgroup. (D) Percentage identity and distance analysis of TmCactin. TmCactin, T. molitor Cactin; TcCactin, T. castaneum Cactin (EFA07431.1); DmCactin, D. melanogaster Cactin (AAF50904.3); BtCactin, Bombus terrestris PREDICTED: uncharacterized protein C19orf29-like isoform 1 (XP_003395486.1); ApCactin; Acyrthosiphon pisum Predicted cactin (XP_001952287.2); AfCactin, Apis florea Predicted uncharacterized protein C19orf29-like (XP_003696278.1); AmCactin, Apis mellifera Predicted cactin isoform 2 (XP_624972.3); AaCactin, Aedes aegypti hypothetical protein AaeL_AAEL013167 (XP_001663347.1); CqCactin, Culex quinquefasciatus Cactin (XP_001844871.1); CfCactin; Camponotus floridanus Uncharacterized protein C19orf29 (EFN62654.1), AgCactin, Anopheles gambiae str. PEST AGAP006542-PA (XP_316580.4), DpCactin; Danaus plexippus hypothetical protein KGM_02089 (EHJ64422.1); HsCactin; H. sapiens Cactin (NP_067054.1).
Further phylogenetic analysis was carried out using amino acid sequences from 13 different insect species (with the human Cactin sequence as an outgroup). The phylogenetic tree was generated by MEGA6 using the neighbor-joining algorithm. As expected, insect Cactins from the same insect order were grouped together, with TmCactin and TcCactin on the same branch (Fig. 1C). Furthermore, percent identity and distance data revealed that TmCactin had the highest identity with TcCactin (84% identity) and more than 50% identity with Cactins from other insect orders (66% with Hymenoptera, 64% with Hemiptera, 51–56% with Diptera, and 51% with Lepidoptera) (Fig. 1D).
Temporal and spatial expression patterns of TmCactin
To understand the role of TmCactin, we measured its mRNA expression levels at different developmental stages and tissues by qRT-PCR. TmCactin was expressed at high levels in last instar larvae and prepupae, but at low levels during the pupal and adult stages (Fig. 2A). Tissue-specific expression analysis showed that TmCactin was expressed in all tissues examined, with particularly high expression in the integument and Malpighian tubules of both larvae and adults (Fig. 2B and C).
(A) The result of developmental expression patterns shows that TmCactin is highly expressed in last instar larval and prepupal stages. LL, last instar larva; PP, prepupa; P1-P7, 1–7 day old pupa and A1-A2, 1 and 2 day old adult (B) Tissue specific expression patterns indicate that TmCactin is highly expressed in integument and Malpighian tubules in both last instar larval and adult stages. Hc, hemocytes; MT, Malpighian tubule; GT, gut; FB, fat body; IT, integument; Ov, ovary and Te, testis. TmL27a was used as an internal control and the result was normalized in the last instar larval stage.
Induction pattern analysis of TmCactin
To determine whether TmCactin expression can be induced in larvae after fungal and bacterial infections, larvae were injected with either fungi (C. albicans), Gram-negative (E. coli), or Gram-positive (S. aureus, L. monocytogenes) bacteria, and TmCactin expression was measured by qRT-PCR at 3, 6, 9 and 12 hours post-infection (Fig. 3). PBS-injected larvae controls were used as a baseline to compare induction of TmCactin expression in larvae challenged with bacteria and fungi. We found that after infection with all four microorganisms, TmCactin mRNA levels increased between 3 and 9 h post infection and then decreased by 12 h. At the 9 h time point, TmCactin expression induced by C. albicans and L. monocytogenes was 3-fold and 2-fold, respectively, higher than that induced by E. coli and S. aureus.
Effects of TmCactin gene silencing on Tenebrio larval mortality
Because significant changes in TmCactin gene expression were observed following bacterial and fungal infections in Tenebrio larvae, we next wanted to test the relevance of TmCactin involvement in immune responses. To assess for this function, we knocked down TmCactin expression in 12–15th instar larvae by using dsRNA corresponding to a 599-bp portion of the TmCactin gene. After confirming effective knockdown (Fig. 4A), we then examined the survival rates of TmCactin RNAi larvae after infection with Gram-negative bacteria (E. coli), Gram-positive bacteria (S. aureus and L. monocytogenes), and fungi (C. albicans), respectively, reasoning that if TmCactin were acting as a positive regulator, loss of TmCactin would result in increased susceptibility and decreased survival when compared to the control group, while the opposite would be expected if TmCactin was acting as a negative regulator. We observed that knock-down of TmCactin leads to reduced larvae survival rates (~50%) compared to the control dsEGFP larvae (>80%) six days after infection with E. coli (Fig. 4B) or S. aureus (Fig. 4C); however, no difference was detected after infection with C. albicans (Fig. 4D) and L. monocytogenes (Fig. 4E).
(A) Down-regulated TmCactin transcripts by injection of dsTmCactin were measured by qPCR. dsEGFP-injected larvae were used as a negative control for RNAi. Survival rates against microbial injection including (B) E. coli, (C) S. aureus, and (D) C. albicans (E) L. monocytogenes were investigated until 7 days.
Effects of TmCactin gene silencing on Tenebrio AMPs expression
To investigate the effects of TmCactin on AMP gene expression, we measured changes in the mRNA levels of several AMPs in TmCactin knockdown larvae following bacterial challenge. As before, we injected larvae with control EGFP or TmCactin dsRNAs. Then, after 2 days of dsRNA treatment, the larvae were further injected with E. coli, S. aureus or PBS, and collected one day later for AMP gene expression analysis (Fig. 5). We found that among the 11 AMPs analyzed, the expression of 9 AMPs was significantly increased (greater than 1,000-fold) in dsEGFP-injected larvae after challenge with E. coli and S. aureus as compared to the PBS control. The fold induction ranged from 1,614-fold (Att-1a) to 13,358-fold (Cole-1) in response to E. coli infection and from 1,585-fold (Def-1) to 9,915-fold (Cole-1) in response to S. aureus infection. Of the 9 AMPs induced by bacterial challenge in dsEGFP-injected control larvae, 7 (Tene-1, Tene-4, Def-1, Def-2, Cole-1, Cole-2, and Att-1b) were significantly reduced in dsTmCactin-injected larvae: 45–98% after E. coli infection and 75–98% after S. aureus infection. Interestingly, in contrast to these observations, we found that TmCactin RNAi increased the induction of Tene-2 (Fig. 5B) and Atta-1a (Fig. 5I) by 13.4-fold and 2.4-fold, respectively, after E. coli infection. To rule out possible off-target effects, we used a different dsRNA to TmCactin and obtained similar results (Figs S3–S4).
E. coli and S. aureus were injected into dsTmCactin-teated T. molitor larvae and whole body samples were collected at 24 h post injection. Expression of AMP genes including TmTene-1 (A) TmTenecin-1), TmTene-2 (B) TmTenecin-2), TmTene-3 (C) TmTenecin-3), TmTene-4 (D) TmTenecin-4), TmDef-1 (E) TmDefensin-1), TmDef-2 (F) TmDefensin-2), TmCole-1 (G) TmColeoptericin-1), TmCole-2 (H) TmColeoptericin-2), TmAtt-1a (I) TmAttacin-1a), TmAtt-1b (J) TmAttacin-1b), and TmCec-2 (K) TmCecropin-2) were investigated by using qPCR. EGFP dsRNA was used as knock down control and TmL27a was used as an internal control. (C) PBS-injected control; Ec, E. coli injected; Sa, S. aureus injected.
Discussion
In this study, we identified a Cactin homolog in the coleopteran beetle T. molitor and characterized its expression patterns and potential function in innate immunity. TmCactin mRNA was found to be highly expressed in late larval and prepupal stages, but at very low levels in the pupal and adult stages. Our tissue expression results indicated that TmCactin expression was detectable in all larval tissues examined, but with notable expression in the integument and Malpighian tubules. As in larvae, TmCactin expression in adults also appeared to be relatively high in the integument and Malpighian tubules compared with other tissues (gut, fat body, hemocytes, ovaries, and testes). We presume that these differences in TmCactin expression between tissues and developmental stages might have to do with hormonal regulation.
We suggest this possibility since several studies in Drosophila and other insects have shown that the developmental hormones ecdysone and juvenile hormone regulate the expression of a number of genes involved in innate immune responses25,26,27,28,29. Interestingly, one of these studies showed that in the Drosophila Malpighian tubules of both larvae and adults, AMPs respond to ecdysone very quickly in the absence of infection28. In a previous study, these authors showed constitutive expression of AMPs in the Malpighian tubules that persisted from larvae to adults independent of infection30. This observation might be explained by the fact that during metamorphosis, Malpighian tubules do not undergo ecdysone-induced destruction as other larval tissues31. It is possible that we are seeing a similar hormonal effect on TmCactin expression, perhaps resulting from either ecdysone or another developmental hormone, in the Malpighian tubules. Considering the above reasons, and given that components of the IMD32 and Toll33 pathways, and AMPs28,34 are expressed in the Drosophila Malpighian tubules, it is not surprising that TmCactin is highly expressed in these tissues of T. molitor larvae and adults. However, it is less clear why TmCactin is highly expressed in the larvae and adult integuments. Given that the Toll pathway is required epidermally for muscle development35 and that Cactus is necessary for normal neuromuscular function in Drosophila36, it is possible that TmCactin could be involved in these processes in T. molitor. These possibilities need to be examined further.
Furthermore, our expression analysis indicated increased TmCactin mRNA expression in the Tenebrio larvae after the injection of E. coli, S. aureus, C. albicans, and L. monocytogenes, with peak levels occurring at 9 hours post-infection. Given this observation, and given that Cactin acts as a positive NF-κB regulator in Drosophila development, but as a negative NF-κB regulator of innate immune signaling in shrimp and humans12,18,19, and since there is no existing data on its immune function in insects, we were interested in determining whether TmCactin might be involved in the regulation of innate immune responses in T. molitor. We observed that the survival rates of TmCactin RNAi larvae were lower compared to the control groups, especially those infected with E. coli and S. aureus, but this decrease was less significant in those infected with C. albicans. These results suggest that TmCactin might have a positive, rather than a negative, role in resistance against Gram-negative and Gram-positive bacterial infections in T. molitor. It has been proposed that Cactin in Drosophila may play a positive role in the dorsal-ventral pathway in regulating Cactus degradation and Dorsal nuclear translocation12. Since the Toll pathway that regulates dorsal-ventral patterning in Drosophila embryos also controls the immune response in larvae and adults37, we can imagine that Cactin may also positively regulate Dorsal nuclear targeting after immune challenge.
By analogy, we predict that TmCactin could have a similar role in Toll signaling as Drosophila Cactin and may regulate the translocation of Tenebrio NF-κB-like proteins to the nucleus to activate AMP expression. This hypothesis may be supported by the fact that out of the 213 orthologs identified, several genes related to putative components of the Toll pathway from the beetle, T. castaneum were found in T. molitor, including Cactus, Dif1, and Dif223. We should also mention that in this same study, an ortholog of Cactin was detected, and although we cannot say for sure, it is possible that the Cactin we isolated might be the very same one that Johnston et al. (2014) identified in their RNA-seq analyses, since in both cases, they were identified as homologs of T. castaneum Cactin, although different search methods were used23.
Early studies of TollD (gain-of function) mutant and infected wild-type flies revealed that the NF-kB transcription factors Dorsal and Dif are constitutively nuclear and active even in the presence of high levels of Cactus inhibitor38,39,40. To account for this paradox, several explanations have been proposed, one of which is that the nuclear translocation of NF-kB factors is not only correlated to the level of Cactus proteins, but also to the intensity of Cactus degradation40. As suggested by Nicolas et al, this implies that once dissociated from the Cactus-NF-kB (Dif and/or Dorsal) complex, the NF-kB proteins cannot be inhibited by free Cactus (e.g. because of structural modifications to either or both of the NF-kB and Cactus proteins)40. Expanding on this idea, we consider that there may be instances in which Cactus dissociates from the NF-kB complex without being degraded. After dissociating from Dif and Dorsal, a possible scenario might be that Cactus changes its conformation upon binding to Cactin so that it can no longer bind to Dif and Dorsal. We raise this possibility because it has been shown that Cactus expression peaks after 3 hours and levels off thereafter40, which suggests to us that Cactus is not fully degraded. Alternatively, Cactus degradation by proteasomes may be promoted by Cactin, which has already been suggested by Lin et al. (2000)12. Time course experiments could be performed to determine if cactin and cactus gene expression levels are correlated and whether increased levels of Cactin would lead to degradation of Cactus.
Our results, together with previous studies12,18, indicate that Cactin acts in both Drosophila and T. molitor as a positive regulator of Toll signaling while in humans, Cactin functions as a negative regulator. While these observations suggest that Cactin is functionally more conserved between Drosophila and Tenebrio than with human, pairwise sequence alignments of Drosophila Cactin (DmCactin) with Tenebrio Cactin or human Cactin (hCactin) generates similar identity scores (55% and 52%, respectively), indicating that, sequence-wise, DmCactin is not considerably more similar to TmCactin than it is to hCactin. Yet, despite their overall similarities, close comparison of all three Cactin sequences reveals ten identical residues: N196/127, S198/129, R255/186, N341/268, Q352/279, G404/333, A406/335, S419/348, E512/449, and E571/495 (numbering based on DmCactin and TmCactin, respectively; underlined in Fig. S2) that are shared by DmCactin and TmCactin, but not by hCactin. It is possible that these residues might be of functional importance in distinguishing DmCactin and TmCactin from hCactin.
Finally, we tested if the expression of fourteen Tenebrio AMPs could be induced after E. coli and S. aureus challenge and whether RNAi knockdown of TmCactin had any effect on the transcription of these AMPs. Bacterial challenge showed that ten of the fourteen AMPs were induced by both E. coli and S. aureus infections, indicating that these AMPs are involved in the immune responses of T. molitor against these two bacteria. Furthermore, of the nine AMPs that responded to bacterial infection, seven of them (Tene1, Tene4, Def1, Def2, Cole1, Cole2, and Atta1b) showed significantly reduced expression after TmCactin knockdown. These results, and given that Cactin is an interacting protein of Cactus12, and that the Tenebrio PGRP-SA can activate the Toll pathway in response to peptidoglycans from both Gram-negative and -positive bacteria21, lead us to suggest that in T. molitor, TmCactin plays a positive role in the Toll pathway in regulating the expression of these seven AMPs in response to Gram-negative E. coli and Gram-positive S. aureus (Fig. 6).
(A) Upon activation of Toll signaling by Gram-positive and Gram-negative bacteria infection, Cactin in the cytosol binds to Cactus and mediates the release of Dif and Dorsal from Cactus, allowing their translocation into the nucleus and activation of AMP genes. (B) Depletion of TmCactin mRNA causes Dif and Dorsal to be retained in the cytoplasm by Cactus, preventing their entry into the nucleus and activation of AMP gene expression.
Interestingly, however, we also found that TmCactin RNAi resulted in increased expression of two AMPs (Tenecin2 and Attacin1a) following E. coli challenge, but not S. aureus challenge. This suggests the possibility that TmCactin are negative regulators of these two AMPs. In the case of Tenecin2, since it has been demonstrated that production of this AMP is triggered by the Toll pathway through recognition of Gram-negative peptidoglycans20,21, but shown here to be elevated after TmCactin knockdown, it is possible that Tenecin2 may also be activated by other signaling pathways such as the Imd pathway. This leads us to ask if Tenecin2 and other AMPs in T. molitor are induced synergistically by both Toll and Imd pathways. Although, as of yet, the Imd pathway in T. molitor has not been fully defined, putative orthologs for several components of Imd pathway have been identified in T. molitor23. It will be interesting to determine in future studies the knockdown effects of these putative Tenebrio orthologs on AMP gene expression. This information will help us understand the extent to which the Toll and Imd pathways contribute to the induction of AMP genes in mealworm beetles.
Materials and Methods
Insect rearing
The model insect, Tenebrio molitor, was maintained at 26 ± 1 °C, 60% ± 5% relative humidity, and under dark conditions with an artificial diet (4.4 g of NeoVita, 0.5 g of chloramphenicol, 0.4 g of L-ascorbic acid, 0.5 g of sorbic acid, 0.5 ml of propionic acid, 2.2 g of yeast extract, 2.2 g of bean powder, 7.6 g of agar, 4.4 g of wheat powder and 73.3 g of wheat bran in 200 ml of D.W; autoclaved at 121 °C for 15 min)41. Healthy 12–15th instar larvae were used for all experiments.
Microorganisms
The Escherichia coli K12, Staphylococcus aureus RN4220, Candida albicans and Listeria monocytogenes ATCC7644 were used for this experiment. E. coli and S. aureus were cultured in Luria-Bertani (LB) broth at 37 °C. C. albicans and L. monocytogenes were cultured in Sabouraud Dextrose broth and in Brain-heart infusion (BHI) broth at 37 °C, respectively. The microorganisms were harvested and washed in phosphate-buffered saline (PBS; pH 7.0) by centrifugation at 3,500 rpm for 10 min. The washed microorganisms were then suspended in PBS and the concentrations were measured at OD600. Finally, 106 cells/μl of E. coli, S. aureus and L. monocytogenes and 5 × 104 cells/μl of C. albicans were used for injection.
Identification and in silico analysis of TmCactin
The TmCactin was identified by local-tblastn analysis42 with T. castanuem Cactin amino acid sequences (EFA07431.1) as query and T. molitor nucleotide database derived from Tenebrio RNAseq/EST (unpublished). To understand the genomic organization of TmCactin, local-blastn analysis was performed with obtained nucleotide sequences of TmCactin as query and Tenebrio genome database (unpublished). Next, the ORF sequences of TmCactin were mapped in the genomic sequences. The specific domains were analyzed by using the InterProScan 543,44,45 and blastp programs. Domain specific multiple alignment was performed with representative Cactin from other insects obtained from Genbank using Clustal X246 and then visualized by using Genedoc software. Phylogenetic analysis and percentage identity and distance analysis were conducted by using the Clustal X2 and MEGA 6 programs47. Amino acid sequences of human cactin were used as an outgroup.
Expression and induction patterns of TmCactin
Transcriptomic expression profiles of TmCactin were examined by relative quantitative PCR method using the Exicycler Real-Time PCR Quantification System (Bioneer co., Daejon, South Korea). Total RNA was isolated from different developmental stages (last instar larva, prepupa, 1–7 day old pupa, and 1–2 day old adult) and tissues (integument, gut, fat body, Malpighian tubules and hemocytes of last instar larvae and 5-day old adult; ovary and testis of 5-day old adult).
To obtain induction patterns of TmCactin, 106 cells/μl of E. coli, S. aureus and L. monocytogenes and 5 × 104 cells/μl of C. albicans were injected into 12–15th instar larvae, respectively, and PBS was used as an injection control. Samples were collected at 3, 6, 9 and 12 h. Total RNA from each sample was isolated by using FavorPrep™ Tri-RNA Reagent (Favorgen biotech corp., Ping-Tung, Taiwan), and 2 μg of total RNAs were used for cDNA synthesis using AccuPower® RT PreMix (Bioneer) with Oligo (dT) 12–18 primer on a MyGenie 96 thermal block (Bioneer). Relative quantitative PCR was performed by using AccuPower® 2X GreenStar qPCR Master Mix (Bioneer) with synthesized cDNAs and specific primers, TmCactin_qPCR_Fw and TmCactin_qPCR_Rv (Table 1) at an initial denaturation of 95 °C for 20 sec, followed by 40 cycles at 95 °C for 5 sec, and 60 °C for 20 sec. T. molitor ribosomal protein L27a (TmL27a) was used as an internal control and results were analyzed by using ∆∆Ct methods.
TmCactin RNAi
599 bp of PCR product was amplified by Ex Taq™ Polymerase (Takara, Japan) with specific primers, TmCactin_Temp_Fw and TmCactin_Temp_Rv (Table 1) at 98 °C for 5 min, followed by 30 cycles at 98 °C for 10 sec, 55 °C for 30 sec and 72 °C for 1 min. Subsequently, the 419 bp PCR product containing the T7 promotor sequences was amplified by Ex Taq™ Polymerase (Takara) (Table 1) in the same condition as mentioned above. Double-stranded RNA (dsRNA) for TmCactin was synthesized by using AmpliScribe T7-Flash Transcription Kit (Epicentre, Madison, Wisconsin, USA) and was purified by PCI (Phenol: Chloroform: Isopropyl alcohol mixture) purification, ammonium acetate purification and ethanol precipitation. 2 μg of synthesized dsTmCactin was injected into 12–15th instar larvae for gene silencing and the dsEGFP was used as a control.
Mortality assay
To measure mortality, diluted microorganisms (106 cells/μl of E. coli, S. aureus, L. monocytogenes and 5 × 104 cells/μl of C. albicans) were injected into TmCactin silenced T. molitor larvae. 10 insects were used for each set of mortality assay and the experiments were triplicated.
AMP expression patterns
Microorganisms were challenged into TmCactin silenced 12–15th instar larva and the samples were collected 12 h post injection. PBS was used as injection control. Total RNAs were isolated and cDNAs were synthesized. A relative quantitative PCR was performed as mentioned above with AMP specific primers (Table 1).
Statistical analysis
All experiments were performed in triplicate and all data are shown as means ± S.E. The one-way analysis of variance (ANOVA) and Tukey’s multiple range tests were used to evaluate the difference between groups (p < 0.05).
Additional Information
How to cite this article: Jo, Y. H. et al. TmCactin plays an important role in Gram-negative and -positive bacterial infection by regulating expression of 7 AMP genes in Tenebrio molitor. Sci. Rep. 7, 46459; doi: 10.1038/srep46459 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hetru, C. & Hoffmann, J. A. NF-kappaB in the immune response of Drosophila. Cold Spring Harb Perspect Biol 1, a000232, doi: 10.1101/cshperspect.a000232 (2009).
Stokes, B. A., Yadav, S., Shokal, U., Smith, L. C. & Eleftherianos, I. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Front Microbiol 6, 19, doi: 10.3389/fmicb.2015.00019 (2015).
Silverman, N. et al. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev 14, 2461–2471 (2000).
Lemaitre, B. & Hoffmann, J. The host defense of Drosophila melanogaster. Annu Rev Immunol 25, 697–743, doi: 10.1146/annurev.immunol.25.022106.141615 (2007).
De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M. & Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J 21, 2568–2579, doi: 10.1093/emboj/21.11.2568 (2002).
Belvin, M. P. & Anderson, K. V. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol 12, 393–416, doi: 10.1146/annurev.cellbio.12.1.393 (1996).
Lindsay, S. A. & Wasserman, S. A. Conventional and non-conventional Drosophila Toll signaling. Dev Comp Immunol 42, 16–24, doi: 10.1016/j.dci.2013.04.011 (2014).
Valanne, S., Wang, J. H. & Ramet, M. The Drosophila Toll signaling pathway. J Immunol 186, 649–656, doi: 10.4049/jimmunol.1002302 (2011).
Manfruelli, P., Reichhart, J. M., Steward, R., Hoffmann, J. A. & Lemaitre, B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J 18, 3380–3391, doi: 10.1093/emboj/18.12.3380 (1999).
Meng, X., Khanuja, B. S. & Ip, Y. T. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev 13, 792–797 (1999).
Rutschmann, S. et al. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat Immunol 1, 342–347, doi: 10.1038/79801 (2000).
Lin, P. H., Huang, L. H. & Steward, R. Cactin, a conserved protein that interacts with the Drosophila I kappa B protein Cactus and modulates its function. Mech Develop 94, 57–65, doi: 0.1016/S0925-4773(00)00314-2 (2000).
Tannoury, H. et al. CACN-1/Cactin interacts genetically with MIG-2 GTPase signaling to control distal tip cell migration in C. elegans. Developmental biology 341, 176–185, doi: 10.1016/j.ydbio.2010.02.025 (2010).
LaBonty, M. et al. CACN-1/Cactin Plays a Role in Wnt Signaling in C-elegans. Plos One 9, doi: DOI 10.1371/journal.pone.0101945 (2014).
Baldwin, K. L., Dinh, E. M., Hart, B. M. & Masson, P. H. CACTIN is an essential nuclear protein in Arabidopsis and may be associated with the eukaryotic spliceosome. Febs Lett 587, 873–879, doi: 10.1016/j.febslet.2013.02.041 (2013).
Atzei, P., Yang, F., Collery, R., Kennedy, B. N. & Moynagh, P. N. Characterisation of expression patterns and functional role of Cactin in early zebrafish development. Gene expression patterns : GEP 10, 199–206, doi: 10.1016/j.gep.2010.03.003 (2010).
Szatanek, T. et al. Cactin is essential for G1 progression in Toxoplasma gondii. Mol Microbiol 84, 566–577, doi: 10.1111/j.1365-2958.2012.08044.x (2012).
Atzei, P., Gargan, S., Curran, N. & Moynagh, P. N. Cactin targets the MHC class III protein IkappaB-like (IkappaBL) and inhibits NF-kappaB and interferon-regulatory factor signaling pathways. The Journal of biological chemistry 285, 36804–36817, doi: 10.1074/jbc.M110.139113 (2010).
Zhang, S. et al. Molecular characterization and functional analysis of Cactin gene from Litopenaeus vannamei. Fish Shellfish Immunol 41, 608–617, doi: 10.1016/j.fsi.2014.10.014 (2014).
Roh, K. B. et al. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. The Journal of biological chemistry 284, 19474–19481, doi: 10.1074/jbc.M109.007419 (2009).
Yu, Y. et al. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. The Journal of biological chemistry 285, 32937–32945, doi: 10.1074/jbc.M110.144014 (2010).
Patnaik, B. B. et al. Gene structure, cDNA characterization and RNAi-based functional analysis of a myeloid differentiation factor 88 homolog in Tenebrio molitor larvae exposed to Staphylococcus aureus infection. Developmental and comparative immunology 46, 208–221, doi: 10.1016/j.dci.2014.04.009 (2014).
Johnston, P. R., Makarova, O. & Rolff, J. I nducible defenses stay up late: temporal patterns of immune gene expression in Tenebrio molitor. G3 4, 947–955, doi: 10.1534/g3.113.008516 (2014).
Zhang, W. et al. Identification and characterization of DPZF, a novel human BTB/POZ zinc finger protein sharing homology to BCL-6. Biochem Biophys Res Commun 282, 1067–1073, doi: 10.1006/bbrc.2001.4689 (2001).
Flatt, T. et al. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J Exp Biol 211, 2712–2724, doi: 10.1242/jeb.014878 (2008).
Tian, L. et al. Genome-wide regulation of innate immunity by juvenile hormone and 20-hydroxyecdysone in the Bombyx fat body. BMC Genomics 11, 549, doi: 10.1186/1471-2164-11-549 (2010).
Rus, F. et al. Ecdysone triggered PGRP-LC expression controls Drosophila innate immunity. EMBO J 32, 1626–1638, doi: 10.1038/emboj.2013.100 (2013).
Verma, P. & Tapadia, M. G. Early gene Broad complex plays a key role in regulating the immune response triggered by ecdysone in the Malpighian tubules of Drosophila melanogaster. Mol Immunol 66, 325–339, doi: 10.1016/j.molimm.2015.03.249 (2015).
Sun, W., Shen, Y. H., Zhou, L. X. & Zhang, Z. Ecdysone Titer Determined by 3DE-3beta-Reductase Enhances the Immune Response in the Silkworm. J Immunol 196, 1646–1654, doi: 10.4049/jimmunol.1500158 (2016).
Verma, P. & Tapadia, M. G. Immune response and anti-microbial peptides expression in Malpighian tubules of Drosophila melanogaster is under developmental regulation. Plos One 7, e40714, doi: 10.1371/journal.pone.0040714 (2012).
Shukla, A. & Tapadia, M. G. Differential localization and processing of apoptotic proteins in Malpighian tubules of Drosophila during metamorphosis. Eur J Cell Biol 90, 72–80, doi: 10.1016/j.ejcb.2010.08.010 (2011).
McGettigan, J. et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect biochemistry and molecular biology 35, 741–754, doi: 10.1016/j.ibmb.2005.02.017 (2005).
Chintapalli, V. R., Wang, J. & Dow, J. A. T. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nature Genetics 39, 715–720, doi: 10.1038/ng2049 (2007).
Davies, S. A. & Dow, J. A. Modulation of epithelial innate immunity by autocrine production of nitric oxide. Gen Comp Endocrinol 162, 113–121, doi: 10.1016/j.ygcen.2008.09.012 (2009).
Halfon, M. S. & Keshishian, H. The Toll pathway is required in the epidermis for muscle development in the Drosophila embryo. Developmental biology 199, 164–174, doi: 10.1006/dbio.1998.8915 (1998).
Beramendi, A., Peron, S., Megighian, A., Reggiani, C. & Cantera, R. The inhibitor kappaB-ortholog Cactus is necessary for normal neuromuscular function in Drosophila melanogaster. Neuroscience 134, 397–406, doi: 10.1016/j.neuroscience.2005.04.046 (2005).
Wu, L. P. & Anderson, K. V. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature 392, 93–97, doi: 10.1038/32195 (1998).
Lemaitre, B. et al. Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J 14, 536–545 (1995).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983, doi: 10.1016/S0092-8674(00)80172-5 (1996).
Nicolas, E., Reichhart, J. M., Hoffmann, J. A. & Lemaitre, B. In vivo regulation of the IkappaB homologue cactus during the immune response of Drosophila. The Journal of biological chemistry 273, 10463–10469 (1998).
Jo, Y. H. et al. Molecular characterization and expression analysis of target of rapamycin (TmTOR) in coleopteran insectTenebrio molitor. Entomol Res 46, 139–147, doi: 10.1111/1748-5967.12163 (2016).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. Journal of molecular biology 215, 403–410, doi: 10.1016/S0022-2836(05)80360-2 (1990).
Quevillon, E. et al. InterProScan: protein domains identifier. Nucleic Acids Research 33, W116–W120, doi: 10.1093/Nar/Gki442 (2005).
Zdobnov, E. M. & Apweiler, R. InterProScan - an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17, 847–848, doi: 10.1093/bioinformatics/17.9.847 (2001).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240, doi: 10.1093/bioinformatics/btu031 (2014).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948, doi: 10.1093/bioinformatics/btm404 (2007).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular biology and evolution 30, 2725–2729, doi: 10.1093/molbev/mst197 (2013).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (Grant No. 2015R1A2A2A01005301).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Yeon Soo Han and Yong Hun Jo. Conducted the experiments: Yong Hun Jo, Ki Beom Park, Jeong Hwan Seong, Soo Gon KIM, Soyi PARK, Mi Young NOH. Analyzed the data: Yong Hun Jo. Wrote the paper: Yong Hun Jo and Yu Jung Kim. Contributed English editing and data analysis: Yu Jung Kim and Yong Seok Lee.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jo, Y., Kim, Y., Park, K. et al. TmCactin plays an important role in Gram-negative and -positive bacterial infection by regulating expression of 7 AMP genes in Tenebrio molitor. Sci Rep 7, 46459 (2017). https://doi.org/10.1038/srep46459
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46459
This article is cited by
-
TmRelish is required for regulating the antimicrobial responses to Escherichia coli and Staphylococcus aureus in Tenebrio molitor
Scientific Reports (2020)
-
Regulation of the expression of nine antimicrobial peptide genes by TmIMD confers resistance against Gram-negative bacteria
Scientific Reports (2019)
-
TmDorX2 positively regulates antimicrobial peptides in Tenebrio molitor gut, fat body, and hemocytes in response to bacterial and fungal infection
Scientific Reports (2019)
-
Insect anal droplets contain diverse proteins related to gut homeostasis
BMC Genomics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.