Abstract
The filamentous fungus Aspergillus nidulans primarily reproduces by forming asexual spores called conidia and produces the mycotoxin sterigmatocystin (ST), the penultimate precursor of aflatoxins. It has been known that asexual development and ST production are tightly co-regulated by various regulatory inputs. Here, we report that the novel regulator AslA with a C2H2 domain oppositely regulates development and ST biosynthesis. Nullifying aslA resulted in defective conidiation and reduced expression of brlA encoding a key activator of asexual development, which indicates that AslA functions as an upstream activator of brlA expression. aslA deletion additionally caused enhanced ST production and expression of aflR encoding a transcriptional activator for ST biosynthetic genes, suggesting that AslA functions as an upstream negative regulator of aflR. Cellular and molecular studies showed that AslA has a trans-activation domain and is localized in the nuclei of vegetative and developing cells but not in spores, indicating that AslA is likely a transcription factor. Introduction of the aslA homologs from distantly-related aspergilli complemented the defects caused by aslA null mutation in A. nidulans, implying a functional conservancy of AslA. We propose that AslA is a novel regulator that may act at the split control point of the developmental and metabolic pathways.
Similar content being viewed by others
Introduction
The ascomycete fungus Aspergillus nidulans serves as one of the best model organisms for investing many aspects of cell biology and genetics of filamentous fungi, owing to the extensive available background information on its genetic and biochemical properties. A. nidulans has two major reproductive cycles, asexual and sexual, involving a number of developmental events, including spatiotemporal control of transcription for many genes, specialized cellular differentiation and intercellular communication.
During the asexual life cycle, conidial germination and vegetative growth yield undifferentiated networks of interconnected hyphal cells termed mycelium. After acquisition of developmental competency, asexual development is driven by a series of morphogenetic differentiation processes triggered by environmental signals, such as exposure to air1,2,3 and nutrient starvation4, to yield a specialized conidia-bearing structure known as the conidiophore. The central developmental pathway (CDP) controlling the temporal and spatial expression of conidiation specific genes involves three major regulatory transcription factors (TFs), BrlA, AbaA and WetA5. BrlA functions as a key transcriptional activator of the central regulatory pathway by directing the expression of other genes required for conidiation5,6,7. AbaA mainly controls the genes required for the middle and terminal stages of conidiophore development, including phialide formation8,9. WetA is responsible for activating the genes involved in conidial wall assembly10,11.
Expression of brlA is regulated by the upstream developmental activator (UDA) pathway consisting of FluG12,13, suppressors of fluG (SFGs, including SfgA14,15), and fluffy low brlA expression (FLBs, such as FlbA, FlbB, FlbC, FlbD and FlbE3). FluG functions in the synthesis of the extracellular signaling molecule directing asexual sporulation and potentially other aspects of colony growth16. The signal generated by FluG suppresses the expression of sfgA encoding the upstream negative regulator, resulting in derepression of flb gene expression. FlbA inhibits vegetative growth signaling mediated by a heterotrimeric G protein composed of FadA and SfaD::GpgA17,18, and is indirectly involved in the positive regulation of asexual development mediated by brlA17. FlbC is a putative C2H2 TF that directly controls the expression of brlA19. FlbB and FlbD, bZIP- and cMyb-type TFs, respectively, function cooperatively to activate brlA expression20,21. In addition, FlbE, a UDA containing two conserved uncharacterized domains, physically interacts with FlbB, and both proteins activate flbD expression interdependently22,23. In addition to the FluG-initiated UDA network, several negative regulators are involved in CDP gene expression. NsdD, a major zinc finger GATA-type activator of sexual reproduction, functions as a key negative regulator of conidiation, potentially exerting a repressive role via downregulating brlA expression24. The velvet protein, VeA, which functions as a pivotal positive regulator of sexual development, is also involved in negative control of asexual development25.
Fungi produce a variety of secondary metabolites, such as mycotoxins, antibiotics, pigments and sporulation-activating compounds. The molecular mechanisms controlling secondary metabolism are frequently involved in the regulation of asexual and sexual development26. One of the predominant regulatory links is the heterodimeric complex VelB-VeA, which associates with LaeA in the nucleus27,28. The resulting heterotrimeric VelB-VeA-LaeA complex coordinates secondary metabolism and development in the dark. The nuclear protein LaeA was the first-identified transcriptional activator of several secondary metabolite gene clusters in A. nidulans and is well conserved across fungi29,30. LaeA contains an S-adenosylmethionine (SAM) binding motif and appears to methylate histone proteins differentially to alter the chromatin structure for promoting gene expression31. The secondary metabolite gene clusters subjected to LaeA-dependent regulation contains the genes involved in biosynthesis of sterigmatocystin (ST), such as aflR and stcA-X32,33,34; and terraquinone (TQ), such as tdiA-E30,35.
In the present study, we characterized aslA ( as exual differentiation with l ow-level conidiation, AN5583) gene encoding a C2H2-type zinc finger TF in relation to asexual development and secondary metabolism. We have previously analyzed the function of aslA with regard to K+ stress resistance and vacuolar morphology, and found that AslA attenuates the K+ stress-inducible expression of the genes involved in vacuolar sequestration of K+ ions and vacuolar biogenesis36. In this paper, we describe the characterization of the putative C2H2-type zinc finger transcription factor, AslA, in relation to both asexual differentiation and secondary metabolism. We provide evidence that AslA is required to activate asexual differentiation via positive regulation of the key conidiation-specific CDP gene, brlA. With regard to secondary metabolism, we found that AslA negatively controls the expression of the genes involved in secondary metabolite biosynthesis, such as ST and TQ. Introduction of the A. fumigatus or A. flavus orthologs of aslA into the ΔaslA strain led to recovery of the wild-type (WT) phenotypes related to asexual development and secondary metabolite production, implying functional conservation of aslA among the aspergilli.
Results
aslA is required for proper asexual development
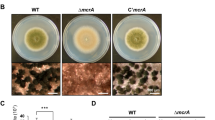
To clarify the role of aslA gene in asexual development, we monitored the growth and development of the ΔaslA mutant (MCBA103) on glucose minimal medium (MMG) plates, compared with those of WT (MCBA003) and aslA complementation (C’aslA, MCBA203) strains. After 5-day culture on MMG, the ΔaslA mutant showed a similar rate of radial growth as the WT and C’aslA strains, but formed a fluffy-looking colony distinct from the two other strains that generated well-conidiated colonies (Fig. 1A). Accordingly, the number of conidia produced by the ΔaslA mutant was less than a quarter of those generated by the WT and C’aslA strains (Fig. 1B).
(A) Colonies of the WT (MCBA003), ΔaslA (MCBA103) and aslA complementation (C’aslA, MCBA203) strains grown for 4 days after point-inoculation on solid MMG. Entire colonies and close-up views of the center of individual colonies are shown. Bar, 100 μm. (B) Quantitative analyses of conidia formation by the strains shown in (A) performed in triplicate (***P < 0.001). (C) Colonies of the WT and OEaslA (MCBA303) strains grown for 4 days after point-inoculation on solid MMG (Un-induction) and MMT (Induction). Entire colonies and close-up views of the center of individual colonies are shown. Bar, 100 μm. The result of Southern blot verifying a single copy integration of aslA-overexpression cassette into the pyroA locus of the OEaslA strain is presented in Supplementary Figure S1A-C online. The result of RT-qPCR supporting overexpression of aslA in the OEaslA strain is presented in Supplementary Figure S1D. (D) Quantitative analyses of conidia formation by the strains shown in (C) performed in triplicate (***P < 0.001). (E) Photomicrographs of the WT and OEaslA hyphae at 12 h post-transfer to MMG (Un-induction) and MMT (Induction). Bar, 20 μm.
The effect of aslA overexpression on growth and development was determined by analyzing the phenotype of the OEaslA (MCBA303) strain. Following point inoculation and culture growth for 5 days on threonine minimal medium (MMT) plates, whereby aslA expression was induced by threonine, the OEaslA strain formed a fully conidiated colony while the WT strain formed an insufficiently developed colony (Fig. 1C). The number of conidia formed in the induced colony of the overexpression strain was more than two-fold higher than that of the WT strain (Fig. 1D). On the other hand, no significant phenotypic differences were observed between the aslA overexpression and WT strains on MMG. The effect of aslA overexpression was additionally assessed in liquid submerged culture, which normally suppresses sporulation. Interestingly, when mycelia of the overexpression strain grown for 18 h in liquid MMG were shifted for 12 h to liquid MMT, almost fully developed conidiophores were generated, in contrast to the WT strain (Fig. 1E). Taken together, these results clearly suggest that aslA contributes to the process of asexual development.
AslA positively controls expression of the key CDP gene, brlA
The above phenotypic traits of the ΔaslA mutant support the view that AslA transcriptionally regulates CDP genes controlling the conidiation-specific gene expression and the order of gene activation during conidiophore development and spore maturation. We thus monitored the changes of brlA, abaA and wetA mRNA levels during asexual development in the ΔaslA and OEaslA strains, compared with the WT strain, via real-time reverse transcription polymerase chain reaction (RT-qPCR). When mycelia grown in liquid MMG were shifted to solid MMG to induce synchronized asexual differentiation, the ΔaslA strain showed significantly reduced transcript levels of brlA, abaA and wetA compared to the WT strain (Fig. 2A). Upon inducing aslA expression by transferring mycelia of the OEaslA strain grown in liquid MMG to MMT, mRNA levels of the CDP genes were significantly increased (Fig. 2B). Considering that BrlA consecutively activates the downstream CDP genes, abaA and wetA5, the collective results suggest that AslA functions as a positive regulator of brlA expression, either directly or indirectly via interaction with other specific regulators.
(A and B) RT-qPCR analyses of brlA, abaA and wetA mRNA levels in the WT (MCBA003), ΔaslA (MCBA103) and OEaslA (MCBA305) strains performed in triplicate. Mycelia of the strains grown in liquid MMG for 18 h were shifted to solid MMG (A) or MMT (B), and total RNAs were extracted after the time intervals indicated. Primers used for RT-qPCR: brlA, PbrlA-qf and PbrlA-qr; abaA, PabaA-qf and PabaA-qr; wetA, PwetA-qf and PwetA-qr; 18S rRNA (internal control), P18S-rRNA-qf and P18S-rRNA-qr. (C) Colony and hyphal morphology of the WT, ΔaslA, OEaslA and OEbrlA ΔaslA (MCBA553) strains. Colonies were grown for 3 days after point-inoculation on solid MMG (Un-induction) and MMT (Induction). For DIC microscopic observation of hyphae, each strain was coverslip-cultured on a block of MMG and MMT for 4 days. Bar, 20 μm. The results of Southern blot verifying single copy integrations of brlA-overexpression cassettes into the pyroA loci of the OEbrlA and OEbrlA ΔaslA strains are presented in Supplementary Figure S2A–C. The results of RT-qPCR supporting overexpression of brlA in the OEbrlA and OEbrlA ΔaslA strains are presented in Supplementary Figure S2D.
To further elucidate the functional relationship between aslA and brlA, we observed the effect of brlA overexpression on the asexual differentiation-related phenotype induced by aslA deletion using the OEbrlA strain with ΔaslA or aslA+ background. On solid MMT, the OEbrlA ΔaslA (MCBA553) strain showed similar morphological features to the OEbrlA (MCBA353) strain, generating a characteristic colony bearing conidia at the tips of both branched aerial and substrate mycelium (Fig. 2C). Our results are consistent with previous reports that overexpression of brlA leads to termination of vegetative growth and formation of conidial spores from hyphae in submerged culture3. It appears that the fluffy phenotype resulting from aslA deletion is suppressed by brlA overexpression, in keeping with the view that brlA is located downstream of and positively controlled by aslA.
The fluffy phenotype of ΔaslA is suppressed by ΔnsdD or veA1 mutation
NsdD, a pivotal activator of sexual reproduction, also acts as a key negative regulator of conidiation, possibly exerting its repressive role through downregulation of brlA24. To assess the relationship between aslA and nsdD, we compared the phenotypes of the ΔaslA ΔnsdD double mutant (MCBA403) related to asexual differentiation, with those of the ΔaslA, ΔnsdD (TNJ108) and WT strains. When grown on solid MMG, the ΔaslA ΔnsdD and ΔnsdD strains formed well-conidiated colonies (Fig. 3A). Quantitative estimation of conidia formation disclosed that the ΔnsdD mutant forms more than twice as many conidia as WT while the ΔaslA ΔnsdD mutant produces similar amounts of conidia as the WT strain (Fig. 3B). We also analyzed the expression of brlA in the ΔaslA ΔnsdD mutant during asexual development in comparison with those of the ΔaslA, ΔnsdD and WT strains via RT-qPCR. During the period of synchronized asexual differentiation, the ΔnsdD mutant exhibited obviously increased and accelerated brlA expression compared to the WT strain. The ΔaslA ΔnsdD double mutant showed significantly increased level of brlA mRNA compared to the ΔaslA strain, hence up to more than 50% of that observed in the WT strain (Fig. 3C). Thus, we suggest that nsdD deletion suppresses the impaired conidiation phenotype caused by aslA deletion through derepression of brlA expression.
(A) Colony morphology of the WT (MCBA003), ΔaslA (MCBA103), ΔnsdD (TNJ108), ΔaslA ΔnsdD (MCBA403), veA1 (MCBA004), ΔaslA veA1 (MCBA104), ΔnsdD veA1 (TNJ111) and ΔaslA ΔnsdD veA1 (MCBA404) strains. Colonies were grown for 4 days after point-inoculation on solid MMG. Entire colonies and close-up views of the center of individual colonies are shown. Bar, 100 μm. (B) Quantitative analyses of conidiation by the strains shown in (A) performed in triplicate (***P < 0.001). (C) RT-qPCR analyses of brlA mRNA levels in the strains shown in (A) performed in triplicate. Mycelia of the strains grown in liquid MMG for 18 h were shifted to solid MMG, and total RNAs were extracted after the time intervals indicated. Primers used for RT-qPCR: brlA, PbrlA-qf and PbrlA-qr; 18S rRNA (internal control), P18S-rRNA-qf and P18S-rRNA-qr.
VeA, a major light-dependent regulator governing development in A. nidulans, also negatively controls brlA expression27. The relationship between aslA and veA was investigated by characterizing the asexual differentiation-related phenotype of the ΔaslA veA1 (MCBA104) strain relative to those of the ΔaslA, veA1 (MCBA004) and WT strains. The ΔaslA veA1 strain produced a relatively well-conidiated colony bearing a similar number of conidia as WT, but generated half of the conidia formed by the veA1 strain (Fig. 3A and B). Additionally, the level of brlA expression in the ΔaslA veA1 mutant were apparently higher compared to that in the ΔaslA strain, although significantly lower than that observed in the WT strain (Fig. 3C). We suggest that, similarly as in the case of ΔnsdD, veA1 mutation suppresses the effect of ΔaslA via derepression of brlA expression.
AslA negatively regulates the expression of the genes necessary for ST and TQ biosynthesis
In general, the molecular mechanism of A. nidulans development is closely related to secondary metabolism. To ascertain whether aslA plays a role in secondary metabolism, we initially assessed the effect of aslA deletion on ST biosynthesis via thin-layer chromatography (TLC) analysis. In both veA+ and veA1 background, the ΔaslA mutant produced increased amount of ST, compared with the WT and C’aslA strains inoculated and grown on solid MMG for 5 days (Fig. 4A). Additionally, we monitored the time-course profile of ST production and expression levels of genes involved in ST biosynthesis, such as aflR and stcU. When mycelia grown in liquid MMG were shifted to solid MMG, the ΔaslA mutant showed significantly increased aflR and stcU expression (Fig. 4B) as well as ST production (Fig. 4D) throughout asexual development compared to the WT strain. The effects of aslA overexpression on ST biosynthesis were assessed using the OEaslA strain. Significantly decreased levels of aflR and stcU expression were observed in the OEaslA mutant compared to the WT strain following transfer of liquid cultured mycelia to liquid MMT (Fig. 4C). ST production was similarly decreased by aslA overexpression (Fig. 4E). Accordingly, we propose that AslA is an important negative regulator of aflR expression at the transcriptional level in both veA and veA1 backgrounds.
(A) TLC analyses of ST in the chloroform extracts of the WT (MCBA003), ΔaslA (MCBA103), C’aslA (MCBA203), veA1 (MCBA004) and ΔaslA veA1 (MCBA104) strains. Colonies were grown for 5 days after point-inoculation on solid MMG, and 0.2 g (wet weight) of mycelium was used to prepare 100 μl of chloroform extract as described in Methods section. Approximately 20 μl of each sample and ST standard (5 mg) were loaded onto a TLC silica plate. ST, ST standard. Full-length TLC plates are presented in Supplementary Figure S3A. (B) and (C) RT-qPCR analyses of aflR and stcU mRNA levels in the WT (MCBA003), ΔaslA (MCBA103) and OEaslA (MCBA303) strains performed in triplicate. Mycelia of the strains grown in liquid MMG for 18 h were shifted to solid MMG (B) or MMT (C), and total RNAs were extracted after the time intervals indicated. Primers used for RT-qPCR: aflR, PaflR-qf and PaflR-qr; stcU, PstcU-qf and PstcU-qr; 18S rRNA (internal control), P18S-rRNA-qf and P18S-rRNA-qr. (D and E) TLC analyses of ST in the chloroform extracts of the WT, ΔaslA and OEaslA strains. Mycelia of the strains grown in liquid MMG for 18 h were shifted to solid MMG (D) or MMT (E), and 0.2 g (wet weight) of mycelium was used to prepare 100 μl of chloroform extract as described in Methods section after the time intervals indicated. Approximately 20 μl of each sample and ST standard (5 mg) were loaded onto a TLC silica plate. ST, ST standard. Full-length TLC plates are presented in Supplementary Figure S3B and C. (F) and (G) RT-qPCR analyses of tdiA and tdiB mRNA levels in the WT, ΔaslA and OEaslA strains performed in triplicate. Mycelia of the strains grown in liquid MMG for 18 h were shifted to solid MMG (F) or MMT (G), and total RNAs were extracted after the time intervals indicated. Primers used for RT-qPCR: tdiA, PtdiA-qf and PtdiA-qr; tdiB, PtdiB-qf and PtdiB-qr; 18S rRNA (internal control), P18S-rRNA-qf and P18S-rRNA-qr.
To address whether AslA additionally affects the synthesis of secondary metabolites other than ST, we evaluated the effect of deletion and overexpression of aslA on expression of the genes involved in TQ biosynthesis, tdiA and tdiB. During asexual differentiation induced by shifting mycelia from liquid to solid MMG, the mRNA levels of both tdiA and tdiB were significantly higher in the ΔaslA than the WT strain (Fig. 4F). On the other hand, aslA overexpression induced by shifting mycelia of the OEaslA strain from liquid MMG to liquid MMT resulted in dramatically lower levels of tdiA and tdiB expression, relative to the WT strain (Fig. 4G). These results suggest that AslA functions as a negative regulator of TQ gene transcription.
aslA is differentially expressed during late growth and early developmental stages, and AslA is localized in nuclei of vegetative hyphae and developmental structures except spores
To determine the expression profile of aslA through the life cycle, Northern blot analysis was performed for total RNAs extracted from spores, vegetative mycelia and developmentally induced cultures of A. nidulans FGSC4. As shown in Fig. 5A, aslA mRNA accumulation was negligible during the early and middle stages (6, 12 and 18 h) of vegetative growth while considerably high levels were detected during the late stages (24 and 48 h). Moderate to low levels of aslA transcript were detected throughout asexual development, with an expression peak at 12 h post-asexual induction. During sexual differentiation, aslA transcript was most abundant during the early stages (0–12 h post-sexual induction) and decreased thereafter. No signs of aslA transcript was detected in the samples from conidia and ascospores. Thus, we conclude that aslA is mainly expressed during the early stages of asexual and sexual development as well as the late vegetative stages, and possibly plays a role during both asexual and sexual development, but not maturation of spores.
(A) Northern blot analysis of aslA mRNA through the lifecycle of A. nidulans FGSC4. Vegetative mycelia were harvested from the culture grown in liquid MMG inoculated with 1.0 × 105 conidia/ml conidia and shake cultured at 120 rpm. Asexual development was induced by shifting the vegetative mycelia grown for 18 h in liquid MMG onto solid MMG followed by incubation under normoxic conditions. For induction of sexual development, the shifted vegetative mycelia were subjected to hypoxia for 24 h followed by incubation under normoxic conditions. Total RNAs were extracted from the vegetative and differentiating mycelia harvested from the cultures after the time intervals indicated. Conidia are indicated as Co, and ascospore as AS. Equal loading of total RNA was confirmed by ethidium bromide staining of rRNA. Full-length gels are presented in Supplementary Figure S4A and B. (B) Intracellular localization of AslA-YFP fusion protein. The C’aslA::YFP strain (MCBA253) was coverslip-cultured on solid MMG for 2–3 days and observed by DIC and fluorescence microscopy. Bar, 20 μm.
The deduced amino acid sequence of AslA comprises 306 amino acids (Mr 35.6 kDa) containing tandem C2H2 zinc fingers near the N-terminus and a Gln-rich domain in the posterior region (Supplementary Figure S6)36. Accordingly, we hypothesized that AslA function as a TF and is mainly localized in nuclei. To determine the intracellular localization of AslA, the C’aslA::YFP strain (MCBA253) expressing YFP-tagged AslA (AslA-YFP) was coverslip-cultured on solid MMG, which supported vegetative mycelial growth and asexual development. After 2–3 days of coverslip culture, YFP fluorescence was observed in the nuclei of vegetative hyphae and most components of conidiophores, i.e., stalks, vesicles, metulae and phialides, but not nuclei of mature conidia (Fig. 5B). Our findings suggest that AslA is present in the majority of vegetative cells as well as developmental structures, except spores, and localized to their nuclei, where it functions as a transcriptional activator.
The glutamine-rich region of AslA functions as a transcriptional activation domain
To verify the speculation that AslA acts as a transcriptional activator, we assessed its transactivational capacity and identify the transactivation domain using β-galactosidase and His3 reporters in S. cerevisiae. First, we constructed yeast transformants of pTLex-derived plasmids that ectopically express fusion proteins containing various lengths of AslA partial segments led by the LexA DNA-binding domain (LexADBD): LexADBD-AslAF (full-length 1–306 aa), LexADBD-AslAN160 (1–160 aa), LexADBD-AslAC167 (140–306 aa), LexADBD-AslAM111 (140–250 aa) and LexADBD-AslAC107 (200–306 aa) (Fig. 6A). To assess the ability to activate the β-galactosidase reporter, we observed the colony colors of transformants grown on SCD-U plates containing X-gal. Yeast strains expressing LexADBD-AslAC167, LexADBD-AslAM111 and LexADBD-AslAC107 exhibited blue color within 1 day after inoculation, similar to the strain expressing the Gal4DBD-AflR positive control37 (Fig. 6B). Data obtained from quantitative analysis of β-galactosidase activity corroborated with transactivation activities of the three fusion proteins. We further evaluated transactivation activity using the His3 reporter. Cells of each transformant were spotted in serial dilution on SCD-UH containing 1 mM or 5 mM 3-AT. As expected, strains expressing one of the three fusion proteins exhibiting positive results in X-gal and β-galactosidase assays were able to grow under these conditions (Fig. 6C). On the other hand, yeast strains expressing LexADBD-AslAN160 and LexADBD-AslAF exhibited negligible signs of tansactivation in both β-galactosidase and His3 reporter assays (Fig. 6A–C). The results collectively indicate that a transactivation domain centered by a glutamine-rich region (aa 209–245) resides within positions 200–250 of AslA (marked by star in Fig. 6A) and that the N-terminal half is involved in modulatory or inhibitory effects on transactivation. A similar phenomenon has been reported for the developmental regulators, FlbB20 and FlbE23, in A. nidulans.
(A) Fusion proteins containing various length of AslA partial segments led by LexADBD. Individual PCR amplicons of AslAF (full-length 1-306 aa), AslAN160 (1-160 aa), AslAC167 (140-306 aa), AslAM111 (140-250 aa) and AslAC107 (200-306 aa) were cloned in the pTLex vector and fused with LexADBD. A region crucial for transactivation ability of AslA is marked by ★. (B) β-Galactosidase reporter assay of transactivation capacity of AslA partial segments. Two-fold serial dilutions of each yeast strain expressing AflR, LexADBD-AslAF, LexADBD-AslAN160, LexADBD-AslAC167, LexADBD-AslAM111 and LexADBD-AslAC107 were spotted on SCD-U supplemented with X-gal (SCD-U/X-gal), and color of the spots was observed after 2-day culture at 30 °C. These strains were also tested for β-galactosidase activity using ONPG (right). Values are the mean ± SE of five independent experiments. (C) His3 reporter assay of transactivation capacity of AslA partial segments. Two-fold serial dilutions of each yeast strain listed in (B) were spotted on SCD-U, SCD-UH, SCD-UH/5 mM 3-AT and SCD-UH/10 mM 3-AT, and the plates were incubated for 3 days at 30 °C.
AslA may be functionally conserved in aspergilli
To determine the functional conservation of AslA among members of the genus Aspergillus, we examined whether the aslA orthologs from A. fumigatus (AfuaslA) and A. flavus (AflaslA) complement the phenotype of ΔaslA mutation in A. nidulans. Introduction of AfuaslA and AflaslA at the pyroA locus yielding C’AfuaslA (MCBA605) and C’AflaslA (MCBA615) strains fully rescued the defects in asexual differentiation induced by ΔaslA mutation (Fig. 7A and B). We additionally determined whether AfuaslA and AflaslA complement the ST-overproducing phenotype of the ΔaslA mutant. Similar to the WT strain, the C’AfuaslA and C’AflaslA strains showed lower level of ST production compared to the ΔaslA strain when grown on solid MMG for 5 days (Fig. 7C). This result indicates that both AfuaslA and AflaslA negatively regulate ST biosynthesis in A. nidulans similar to aslA. Accordingly, we propose that the functions of AslA in asexual development as well as secondary metabolism are conserved among the three aspergilli, A. nidulans, A. fumigatus and A. flavus.
(A) Colony morphology of WT (MCBA003), ΔaslA (MCBA103), C’AfuaslA (MCBA605) and C’AflaslA (MCBA615) strains. Colonies were grown for 4 days after point-inoculation on solid MMG. Entire colonies and close-up views of the center of individual colonies are shown. Bar, 100 μm. (B) Quantitative analyses of conidiation by the strains shown in (A) performed in triplicate (***P < 0.001). (C) TLC analyses of ST in the chloroform extracts of the strains shown in (A) grown on plates for 5 days. ST, ST standard. Full-length TLC plates are presented in Supplementary Figure S5.
Discussion
While numerous studies over several decades have focused on the processes of development and accompanying metabolic alterations in the model filamentous fungus, A. nidulans, the molecular networks modulating the expression of genes required for asexual differentiation and secondary metabolism remain to be established. In the present study, we characterized the putative C2H2-type zinc finger TF, AslA, shown to attenuate K+ stress-inducible expression of genes encoding vacuolar K+ pumps and proteins involved in vacuolar biogenesis36, in relation to both asexual differentiation and secondary metabolism.
An initial clue into the regulatory function of AslA in asexual development was obtained from the finding that deletion of aslA leads to the formation of fluffy colonies with significantly decreased conidia (Fig. 1A and B), while its overexpression induces a hyper-conidiating phenotype (Fig. 1C–E). The data indicate that AslA plays an important role in asexual differentiation. Deletion and overexpression of aslA significantly modulated the mRNA levels of brlA, abaA and wetA (Fig. 2A and B). In addition, the fluffy phenotype of the ΔaslA mutant was suppressed upon brlA overexpression (Fig. 2C), leading to the hypothesis that aslA is located upstream of brlA in the genetic network controlling asexual development and positively regulates the pivotal CDP gene, brlA. As described in the introduction, activities of various UDAs, including FluG, FlbA, FlbB, FlbC, FlbD and FlbE, regulate brlA expression3,12,13. Three of the UDA members have been identified as TFs closely involved in activation of brlA expression, specifically, a bZIP-type TF, FlbB21,22,38, C2H2 zinc-finger TF, FlbC19, and cMyb-type TF, FlbD21. Thus AslA is probably a novel TF belonging to the UDA members. However, further studies are essential to determine the hierarchical relationship between AslA and other UDA members.
In contrast to members of the FluG-initiated UDA network, the two key activators of sexual reproduction, NsdD and VeA, play negative roles in asexual development by suppressing brlA expression24,25,27. Analysis of the phenotypes of the double mutants, ΔaslA ΔnsdD and ΔaslA veA1, revealed that either nsdD deletion or veA1 mutation suppresses the impaired conidiation phenotype triggered by aslA deletion (Fig. 3A and B). Accordingly, either nullifying nsdD or introducing veA1 mutation in the ΔaslA mutant at least partially restored the expression of brlA (Fig. 3C). Thus, both NsdD and VeA are suggested to function downstream of AslA to control brlA expression. In agreement with these results, deletion of nsdD suppressed all developmental defects caused by null mutations of the UDA genes, including fluG, flbA, flbB, flbC and flbE, but not ΔbrlA24. Although limited information is available on suppression of the flb mutations by veA1 or ΔveA mutations, similar results are expected as for nsdD.
We investigated whether AslA participates in the regulation of secondary metabolite production by analyzing the effects of deletion and overexpression of aslA on expression of ST biosynthetic genes. Our data indicate that AslA acts as a negative regulator of ST biosynthesis by suppressing the expression of aflR and stcU encoding a Zn(II)2Cys6 TF and ST biosynthetic enzyme, respectively (Fig. 4A–E). Additionally, AslA negatively controls the expression of TQ biosynthetic genes, tdiA and tdiB (Figs 4F and 6G). It has long been accepted that in filamentous fungi, the regulatory gene networks for secondary metabolism and morphological development are intimately associated via a considerable number of common regulators26,39,40. For example, the putative C2H2 zinc finger TF, MtfA, positively regulates conidiophore formation via activation of brlA expression as well as ST production via effects on aflR expression41. Interestingly, deletion of mtfA or its overexpression leads to reduction of aflR transcription, suggesting that only wild-type MtfA levels in balanced stoichiometry with other relevant factors are required for normal ST production. MtfA is also a positive regulator of other secondary metabolism gene clusters, including those responsible for TQ biosynthesis. Additionally, LaeA, the first methyltransferase discovered to associate with VeA, is not only required for activation of aflR expression but also involved in the transcription of gene clusters for other secondary metabolites29,30. In the absence of light, LaeA associates with the VelB-VeA dimer to form the heterotrimeric velvet complex, VelB-VeA-LaeA, which performs chromatin remodeling required for expression of aflR and other secondary metabolite biosynthesis genes28,31. LaeA also plays an important role in light-dependent conidia production, which requires the presence of intact VeA protein42. In contrast to MtfA and LaeA that play positive roles in both asexual differentiation and ST biosynthesis, AslA provides a prime example of a TF that positively controls asexual differentiation by activating brlA expression, but negatively affects ST biosynthesis by suppression of aflR expression. Correspondingly, in spite of its significant defects in asexual development, ΔaslA mutant showed increased production of ST (Figs 1 and 4). On the basis of the data presented here, we propose that AslA is a novel regulator that may act at the split control point of the developmental and metabolic pathways. However, for clear understanding of the branch point, it should be rigorously established whether AslA modulates the expression aflR and brlA directly or indirectly through unknown regulator(s).
On the basis of the deduced amino acid sequence of AslA containing tandem C2H2 zinc fingers near the N-terminus and a Gln-rich domain in the posterior portion (see Supplementary Figure S6A)36, we attempted to determine whether this protein meets the basic criteria for functioning as a TF, i.e. nuclear localization and transcriptional activation. The AslA-YFP fusion protein predominantly accumulated in nuclei of not only vegetative cells but also differentiating cells in conidiophores, vesicles, metulae and phialides, except conidia (Fig. 5B). However, we found no evidence of differential localization of AslA according to developmental status of the fungal cells or environmental conditions. Using β-galactosidase and His3 reporters in S. cerevisiae, we showed that AslA has transactivation ability, which is mediated by a putative transactivation domain (200–250 aa) centered by a glutamine-rich region (Fig. 6). Generally, transcriptional activation functions of TFs are based within glutamine-rich, acidic, or proline-rich domains localized outside DNA-binding regions, such as C2H2, Zn(II)2Cys6 and GATA zinc fingers43. Transactivational function of the glutamine-rich domain is conserved among a wide variety of eukaryotes, from yeast to human44. In the filamentous fungus Neurospora crassa, Nit4, a single Zn(II)2Cys6 binuclear-type zinc finger protein, contains a glutamine-rich domain that functions in transcriptional activation45. The transactivation domains of several developmental regulators, such as FlbB20, FlbC19 and FlbE23, have been mapped in A. nidulans, none of which harbor a glutamine-rich domain with transactivation capacity. Thus, AslA appears to provide a primary example for a developmental regulator carrying a glutamine-rich transactivation module.
Previous reports have shown that proteins sharing significant sequence similarity with AslA are mainly present in members of the family Trichocomaceae, including the genera Aspergillus, Penicillium and Talaromyces (see Supplementary Figure S6B)36. Results from the present study indicate that the aslA orthologs from A. fumigatus (AfuaslA) and A. flavus (AflaslA) complement the ΔaslA phenotype related to asexual differentiation and ST production in A. nidulans (Fig. 7). Accordingly, the role of AslA in development and secondary metabolism seems to be conserved across the members of the genus Aspergillus.
Based on our findings in the present study, we propose that AslA is a novel regulator that oppositely regulates development and ST biosynthesis at the split control point of the developmental and metabolic pathways. Further studies on the hierarchical relationship between aslA and other UDA gens would contribute to expand our understanding of the regulatory networks governing the processes of fungal development and secondary metabolite production.
Methods
Strains, media and cultivation
A. nidulans strains used in this study are listed in Table 1. MMG was prepared as described previously36,46. For overexpression of selected genes driven by the promoter of alcA gene (alcA(p)), MMT which was identical to MMG except that it contained 0.5% yeast extract and 100 mM threonine instead of 2% glucose was used. For preparation of solid media, 1.5% agar was added to the liquid media. Unless otherwise indicated, cultures for all experiments described were grown at 37 °C.
To prepare vegetative mycelia, conidia of A. nidulans strains were inoculated into liquid MMG to a concentration of 1.0 × 105 conidia/ml and grown in a shaking culture at 120 rpm. Mycelia were then harvested by filtering the culture through a Miracloth filter (Calbiochem, USA). To induce synchronized asexual differentiation, vegetative mycelia grown for 18 h in liquid MMG were spread onto solid MMG and incubated under air-exposed conditions. The whole cells undergoing asexual differentiation were harvested from the plates at designated time points after transfer. For microscopic observation of the fungal hyphae, each A. nidulans strain was coverslip-cultured on a block of appropriate agar medium under a coverslip on a glass slide for several days. For plasmid amplification, Escherichia coli DH5α was cultured in Luria-Bertani medium supplemented with 50 μg/ml of ampicillin (Sigma-Aldrich, USA).
Molecular techniques
Standard DNA transformation procedures were used for A. nidulans47 and E. coli48. For PCR experiments, standard protocols were applied using a MyGenie 96/384 Gradient Thermal Block (Bioneer, Korea) for reaction cycles. Genomic DNA was extracted from the spores or mycelia of A. nidulans as described previously49,50. Primers for PCR are listed in Supplementary Table S1.
Total RNA was isolated with Trizol according to the manufacturer’s protocols (Invitrogen, USA). Northern hybridization was performed using standard techniques48. aslA probe for Northern blots were amplified by PCR from A. nidulans genomic DNA with the primers, PaslA-Nf and PaslA-Nr. For RT-qPCR, first strand cDNA was copied from a total RNA preparation using M-MLV reverse transcriptase (Enzynomics, Korea) according to the manufacturer’s protocol. RT-qPCR was performed using a Bio-Rad CFX96 Real-Time PCR System (Bio-Rad, USA) and a TOPreal™ qPCR 2X PreMIX Kit (Enzynomics) with the primers against the genes of interest and 18S rRNA (internal control) (see Supplementary Table S1). Expression levels of target genes versus the 18S rRNA gene were computed using the 2−ΔCt method described previously36.
Generation of fungal strains
The green-spored ΔaslA strains, MCBA103 (pyroA4 argB2 ΔaslA::argB) and MCBA104 (pyroA4 ΔaslA::argB; veA1), were generated by a sexual cross between MCBA101 (yA2 pyroA4 ΔaslA::argB) and FGSC26 (biA1 veA1) strains according to standard methods46. The reference strains, MCBA003 (pyroA4) and MCBA004 (pyroA4 veA1), were constructed by crossing TJ1 (yA2 pyroA4) with FGSC26.
For complementation of ΔaslA mutation with a hybrid gene encoding FLAG-tagged AslA, a 4.1-kb aslA(p)::aslAorf fragment containing the presumptive promoter aslA(p) (3.0 kb) and aslAorf (1.1-kb) was amplified from the genomic DNA of FGSC4 by PCR using the primers, PaslA4-f and PaslA4-r, and cloned into the TOPcloner (Enzynomics) to yield TOP-aslA4. The 4.1-kb aslA(p)::aslAorf fragment was excised from TOP-aslA4 by KpnI-HindIII digestion and cloned into KpnI-HindIII-digested pHS1351 to yield pHS-aslA-FLAG. The aslA complemented (C’aslA) strain, MCBA203 (pyroA::aslA(p)::aslA::FLAG3x::trpC(t)::pyroA ΔaslA::argB), was generated by transformation of the aslA-null strain (MCBA103) with pHS-aslA-FLAG plasmid. A hybrid gene encoding YFP-tagged AslA fusion protein was constructed as follows. First, a 2.3-kb 3 × YFP fragment was excised from pBS-3 × YFP52 by EcoRI digestion and cloned into pHS13 to yield pHS-YFP. The 4.1-kb aslA(p)::aslAorf fragment was similarly PCR amplified with the primers, PC’YaslA-4f and PC’YaslA-4r, and cloned into PvuII-cut pHS-YFP to yield pHS-aslA-YFP. The aslA-null strain (MCBA103) was then transformed with pHS-aslA-YFP to yield the C’aslA::YFP strain, MCBA253 (pyroA::aslA(p)::aslA::YFP3x::FLAG3x trpC(t)::pyroA ΔaslA::argB).
For construction of aslA-overexpressing (OEaslA) strains, the aslAorf was amplified from the genomic DNA of FGSC4 using the primers, POEaslA-1f and POEaslA-1r. The resulting 1.1-kb aslAorf fragment was cloned into TOPcloner to yield TOP-aslAORF. The 1.1-kb HindIII fragment of aslAorf was excised and cloned into HindIII-digested pHS351 downstream of alcA(p) to yield pHS-alcA(p)-aslA-FLAG. The final recombinant plasmids were used to transform the reference strain, MCBA003, to yield the OEaslA strain, MCBA303 (pyroA::alcA(p)::aslA::FLAG::trpC(t)::pyroA).
To generate brlA-overexpressing strains, the brlAorf was amplified from the genomic DNA of FGSC4 using the primers, POEbrlA-1f and POEbrlA-1r. The resulting 1.3-kb brlAorf fragment was cloned into TOPcloner to yield TOP-brlAORF. The 1.6-kb BamHI-NotI fragment of brlAorf was excised and cloned into BamHI-NotI-digested pHS3 downstream of alcA(p) to yield pHS-alcA(p)-brlA-FLAG. The recombinant plasmid was introduced into the reference (MCBA003) and ΔaslA (MCBA103) strains to yield OEbrlA strain, MCBA353 (pyroA::alcA(p)::brlA::FLAG::trpC(t)::pyroA), and ΔaslA OEbrlA strain, MCBA553 (pyroA:: alcA(p)::brlA::FLAG::trpC(t)::pyroA ΔaslA::argB), respectively. The ΔaslA ΔnsdD double mutant strains, MCBA403 (ΔaslA::argB ΔnsdD::AfupyrG) and MCBA404 (ΔaslA::argB ΔnsdD::AfupyrG veA1), were generated by a cross between MCBA101 and TNJ111 (ΔnsdD::AfupyrG veA1) strains.
For complementation of ΔaslA mutation by the aslA orthologues from A. fumigatus (AfuaslA) and A. flavus (AflaslA), 5.0-kb DNA fragments of AfuaslA and AflaslA loci were amplified by PCR from the genomic DNAs of A. fumigatus (AF293) with the primers, PC’AfuaslA-f and PC’AfuaslA-r, and A. flavus (NRRL3375) with the primers, PC’AflaslA-f and PC’AflaslA-r, respectively. The resulting amplicons were cloned individually into TOPcloner to yield TOP-AfuaslA and TOP-AflaslA. The 5.0-kb HindIII-NotI fragment of AfuaslA gene excised from TOP-AfuaslA was cloned into HindIII-NotI-digested and pHS723 to yield pHS-AfuaslA. Similarly, the 5.0-kb BamHI-NotI fragment of AflaslA gene excised from TOP-AflaslA was cloned into BamHI-NotI-digested pHS7 to yield pHS-AflaslA. The aslA-null strain (MCBA103) was then transformed with pHS-AfuaslA and pHS-AflaslA to yield the C’AfuaslA and C’AflaslA strains, MCBA605 (ΔaslA::argB AfuaslA::pyroA) and MCBA615 (ΔaslA::argB AflaslA::pyroA), respectively.
Sterigmatocystin analysis
ST was extracted with chloroform from the mycelia of A. nidulans strains harvested from solid or liquid cultures24. Briefly, 0.2 g (wet weight) of mycelium was mixed 10 ml of chloroform and incubated for 30 min at room temperature with vigorous vortexing in about 5-min intervals. The organic phase (lower) was harvested and centrifuged (700× g, 5 min). The resulting chloroform layer was collected, dried and resuspended in 100 μl of chloroform. Approximately 20 μl of each sample and ST standard (5 mg; Sigma-Aldrich) were loaded onto a TLC silica plate (Silica gel 60 F254; Merck, Germany). The plate was then developed in a mobile phase composed of toluene:ethylacetate:acetic acid (80:10:10, v/v/v), and ST spots were visualized by spraying aluminum chloride (20% w/v in 95% ethanol) on the TLC plate followed by baking the plate at 70 °C for 5 min. Photographs of TLC plates were taken following exposure to UV of 320 nm.
Microscopy
Coverslip culture was performed as described previously36. The coverslips were stained with 1 mg/ml Hoechst 33342 (Sigma) for 10 min, briefly washed with distilled water and dipped in distilled water for 10 minutes. Then the coverslips were washed with ethanol, air-dried for 5 minutes and mounted with antifade mounting medium (H-1000; Vectashield, USA). For differential interference contrast (DIC) and fluorescence microscopy, an Olympus System microscope Model BX51 (Olympus, Japan) equipped with UPlanSApo 60× and UPlanFL 100× objective lenses (Olympus) were used. DAPI (High brightness) filter cube (Excitation filter: center wavelength 377 nm, Emission filter: center wavelength 447 nm; Olympus) and FITC filter cube (Excitation filter: center wavelength 483 nm, Emission filter: center wavelength 535 nm; Olympus) were used to observe the fluorescence of Hoechst and YFP, respectively. Images were captured with a DP71 digital camera (Olympus) and processed using the DP manager imaging software (Olympus) and Photoshop CS5.1 (Adobe Systems, USA).
Mapping a transactivation domain in AslA
The transactivating capacity of AslA was determined by using a modified yeast one hybrid system19. Briefly, cDNA fragments encoding the full-length and various partial segment of AslA were amplified by PCR from aslA cDNA using the primers as follows: AslAF (full-length 1–306 aa; PaslA-1f and PaslA-306r), AslAN160 (1–160 aa; PaslA-1f and PaslA-160r), AslAC166 (141–306 aa; PaslA-141f and PaslA-306r), AslAM110 (141–250 aa; PaslA-141f and PaslA-250r) and AslAC112 (195–306 aa; PaslA-195f and PaslA-306r). After SmaI-XhoI digestion, the amplicons were individually fused with the coding sequence of LexA DNA-binding domain (LexADBD) in the pTLex vector (kindly provided by Suhn-Kee Chae, Paichai University, Daejeon, Korea) to yield pLexADBD-AslAF, pLexADBD-AslAN160, pLexADBD-AslAC166, pLexADBD-AslAM110 and pLeADBDx-AslAC112. The resulting plasmids were individually introduced into Saccharomyces cerevisiae L4053. For X-gal plate assay for visualization of β-galactosidase expression mediated by the LexADBD fusion proteins, two-fold serial dilutions of each transformants were spotted on synthetic complete dextrose (SCD) medium lacking uracil (SCD-U)54 supplemented with 40 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal; Sigma-Aldrich), and color of the spots was observed after 2-day culture at 30 °C. For quantitative analysis β-galactosidase expression, the yeast transformants were tested for β-galactosidase activity using a yeast β-galactosidase assay kit that contained the substrate o-nitrophenyl-β-D-galactopyranoside (ONPG; Sigma-Aldrich). For His3 reporter assay, we dilution-spotted the cells of each transformant on SCD lacking uracil and histidine (SCD-UH) supplemented with 5 mM 3-amino-1,2,4-triazole (3-AT; Sigma-Aldrich) and evaluated for their growth after 2-day incubation at 30 °C.
Additional Information
How to cite this article: Kim, Y. J. et al. Differential control of asexual development and sterigmatocystin biosynthesis by a novel regulator in Aspergillus nidulans. Sci. Rep. 7, 46340; doi: 10.1038/srep46340 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
26 May 2017
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has been fixed in the paper.
26 May 2017
Scientific Reports 7: Article number: 46340; published online: 19 April 2017; updated: 26 May 2017 The original version of this Article contained a typographical error in the spelling of the author Yeong Man Yu, which was incorrectly given as Yu Yeong Man. This has now been corrected in both the PDFand HTML versions of the Article.
References
Timberlake . Molecular genetics of Aspergillus development. Annu. Rev. Genet. 24, 5–36 (1990).
Timberlake, W. E. & Clutterbuck, A. J. Genetic regulation of conidiation. Prog. Ind. Microbiol. 29, 383–427 (1994).
Adams, T. H., Wieser, J. K. & Yu, J. H. Asexual sporulation in Aspergillus nidulans . Microbiol. Mol. Biol. Rev. 62, 35–54 (1998).
Skromne, I., Sanchez, O. & Aguirre, J. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology 141, 21–8 (1995).
Boylan, M. T., Mirabito, P. M., Willett, C. E., Zimmerman, C. R. & Timberlake, W. E. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans . Mol. Cell. Biol. 7, 3113–8 (1987).
Adams, T. H., Boylan, M. T. & Timberlake, W. E. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans . Cell 54, 353–62 (1988).
Aguirre, J. Spatial and temporal controls of the Aspergillus brlA developmental regulatory gene. Mol. Microbiol. 8, 211–8 (1993).
Sewall, T. C., Mims, C. W. & Timberlake, W. E. abaA controls phialide differentiation in Aspergillus nidulans . Plant Cell 2, 731–9 (1990).
Aramayo, R. & Timberlake, W. E. The Aspergillus nidulans yA gene is regulated by abaA . EMBO J. 12, 2039–48 (1993).
Sewall, T. C., Mims, C. W. & Timberlake, W. E. Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev. Biol. 138, 499–508 (1990).
Marshall, M. A. & Timberlake, W. E. Aspergillus nidulans wetA activates spore-specific gene expression. Mol. Cell. Biol. 11, 55–62 (1991).
Lee, B. N. & Adams, T. H. fluG and flbA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlAb activation. EMBO J. 15, 299–309 (1996).
D’Souza, C. A., Lee, B. N. & Adams, T. H. Characterization of the role of the FluG protein in asexual development of Aspergillus nidulans . Genetics 158, 1027–36 (2001).
Seo, J. A., Guan, Y. & Yu, J. H. Suppressor mutations bypass the requirement of fluG for asexual sporulation and sterigmatocystin production in Aspergillus nidulans . Genetics 165, 1083–93 (2003).
Seo, J. A., Guan, Y. & Yu, J. H. FluG-dependent asexual development in Aspergillus nidulans occurs via derepression. Genetics 172, 1535–44 (2006).
Lee, B. N. & Adams, T. H. The Aspergillus nidulans fluG gene is required for production of an extracellular developmental signal and is related to prokaryotic glutamine synthetase I. Genes Dev. 8, 641–51 (1994).
Lee, B. N. & Adams, T. H. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 14, 323–34 (1994).
Yu, J. H., Wieser, J. & Adams, T. H. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 15, 5184–90 (1996).
Kwon, N. J., Garzia, A., Espeso, E. A., Ugalde, U. & Yu, J. H. FlbC is a putative nuclear C2H2 transcription factor regulating development in Aspergillus nidulans . Mol. Microbiol. 77, 1203–19 (2010).
Etxebeste, O. et al. Basic-zipper-type transcription factor FlbB controls asexual development in Aspergillus nidulans . Eukaryot. Cell 7, 38–48 (2008).
Garzia, A., Etxebeste, O., Herrero-Garcia, E., Ugalde, U. & Espeso, E. A. The concerted action of bZip and cMyb transcription factors FlbB and FlbD induces brlA expression and asexual development in Aspergillus nidulans . Mol. Microbiol. 75, 1314–24 (2010).
Garzia, A. et al. Aspergillus nidulans FlbE is an upstream developmental activator of conidiation functionally associated with the putative transcription factor FlbB. Mol. Microbiol. 71, 172–84 (2009).
Kwon, N. J., Shin, K. S. & Yu, J. H. Characterization of the developmental regulator FlbE in Aspergillus fumigatus and Aspergillus nidulans . Fungal Genet. Biol. 47, 981–93 (2010).
Lee, M. K. et al. NsdD is a key repressor of asexual development in Aspergillus nidulans . Genetics 197, 159–73 (2014).
Kim, H. et al. The veA gene activates sexual development in Aspergillus nidulans . Fungal Genet. Biol. 37, 72–80 (2002).
Calvo, A. M., Wilson, R. A., Bok, J. W. & Keller, N. P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66, 447–59 (2002).
Kato, N., Brooks, W. & Calvo, A. M. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell 2, 1178–86 (2003).
Bayram, O. et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320, 1504–6 (2008).
Bok, J. W. & Keller, N. P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 3, 527–35 (2004).
Bok, J. W. et al. Genomic mining for Aspergillus natural products. Chem. Biol. 13, 31–7 (2006).
Bok, J. W., Noordermeer, D., Kale, S. P. & Keller, N. P. Secondary metabolic gene cluster silencing in Aspergillus nidulans . Mol. Microbiol. 61, 1636–45 (2006).
Brown, D. W. et al. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans . Proc. Natl. Acad. Sci. USA 93, 1418–22 (1996).
Fernandes, M., Keller, N. P. & Adams, T. H. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 28, 1355–65 (1998).
Ehrlich, K. C., Cary, J. W. & Montalbano, B. G. Characterization of the promoter for the gene encoding the aflatoxin biosynthetic pathway regulatory protein AFLR. Biochim. Biophys. Acta 1444, 412–7 (1999).
Bouhired, S., Weber, M., Kempf-Sontag, A., Keller, N. P. & Hoffmeister, D. Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA. Fungal Genet. Biol. 44, 1134–45 (2007).
Park, D. S., Yu, Y. M., Kim, Y. J. & Maeng, P. J. Negative regulation of the vacuole-mediated resistance to K+ stress by a novel C2H2 zinc finger transcription factor encoded by aslA in Aspergillus nidulans . J. Microbiol. 53, 100–10 (2015).
Ni, M. & Yu, J. H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans . PLoS One 2, e970 (2007).
Etxebeste, O. et al. The bZIP-type transcription factor FlbB regulates distinct morphogenetic stages of colony formation in Aspergillus nidulans . Mol. Microbiol. 73, 775–89 (2009).
Lind, A. L. et al. Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus Aspergillus . PLoS Genet. 11, e1005096 (2015).
Calvo, A. M. & Cary, J. W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 6, 62 (2015).
Ramamoorthy, V. et al. The putative C2H2 transcription factor MtfA is a novel regulator of secondary metabolism and morphogenesis in Aspergillus nidulans . PLoS One 8, e74122 (2013).
Sarikaya Bayram, O. et al. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS Genet. 6, e1001226 (2010).
Landschulz, W. H., Johnson, P. F. & McKnight, S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240, 1759–64 (1988).
Escher, D., Bodmer-Glavas, M., Barberis, A. & Schaffner, W. Conservation of glutamine-rich transactivation function between yeast and humans. Mol. Cell. Biol. 20, 2774–82 (2000).
Yuan, G. F., Fu, Y. H. & Marzluf, G. A. nit-4, a pathway-specific regulatory gene of Neurospora crassa, encodes a protein with a putative binuclear zinc DNA-binding domain. Mol. Cell. Biol. 11, 5735–45 (1991).
Pontecorvo, G., Roper, J. A., Hemmons, L. M., Macdonald, K. D. & Bufton, A. W. The genetics of Aspergillus nidulans . Adv. Genet. 5, 141–238 (1953).
Yelton, M. M., Hamer, J. E. & Timberlake, W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc. Natl. Acad. Sci. USA 81, 1470–4 (1984).
Sambrook, J & Russell, D. W. Molecular cloning: A laboratory manual. 3 edn (Cold Spring Harbor Laboratory Press, 2001).
Hervas-Aguilar, A., Rodriguez, J. M., Tilburn, J., Arst, H. N. Jr. & Penalva, M. A. Evidence for the direct involvement of the proteasome in the proteolytic processing of the Aspergillus nidulans zinc finger transcription factor PacC. J. Biol. Chem. 282, 34735–47 (2007).
Yu, J. H. et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–81 (2004).
Park, H. S., Nam, T. Y., Han, K. H., Kim, S. C. & Yu, J. H. VelC positively controls sexual development in Aspergillus nidulans . PLoS One 9, e89883 (2014).
Bat-Ochir, C. et al. The signal peptide peptidase SppA is involved in sterol regulatory element-binding protein cleavage and hypoxia adaptation in Aspergillus nidulans . Mol. Microbiol. 100, 635–55 (2016).
Ito, H., Fukuda, Y., Murata, K. & Kimura, A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163–8 (1983).
Lee, Y. J., Kim, K. J., Kang, H. Y., Kim, H. R. & Maeng, P. J. Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells. J. Biol. Chem. 287, 44221–33 (2012).
Kwon, N. J., Park, H. S., Jung, S., Kim, S. C. & Yu, J. H. The putative guanine nucleotide exchange factor RicA mediates upstream signaling for growth and development in Aspergillus . Eukaryot. Cell 11, 1399–412 (2012).
Alkahyyat, F., Ni, M., Kim, S. C. & Yu, J. H. The WOPR domain protein OsaA orchestrates development in Aspergillus nidulans . PLoS One 10, e0137554 (2015).
Acknowledgements
This work was supported by a grant from the NRF of Korea (NRF-2014R1A2A1A11053776) and a grant from Chungnam National University (2015).
Author information
Authors and Affiliations
Contributions
Y.J.K. and Y.M.Y. performed the experiments. Y.J.K. and P.J.M. designed the experiments, analyzed the data and wrote the main manuscript text. Y.J.K. prepared all Figures and Tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kim, Y., Yu, Y. & Maeng, P. Differential Control of Asexual Development and Sterigmatocystin Biosynthesis by a Novel Regulator in Aspergillus nidulans. Sci Rep 7, 46340 (2017). https://doi.org/10.1038/srep46340
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46340
This article is cited by
-
Mannitol-1-phosphate dehydrogenase, MpdA, is required for mannitol production in vegetative cells and involved in hyphal branching, heat resistance of conidia and sexual development in Aspergillus nidulans
Current Genetics (2021)
-
Survival factor SvfA plays multiple roles in differentiation and is essential for completion of sexual development in Aspergillus nidulans
Scientific Reports (2020)
-
Aspergillus nidulans in the post-genomic era: a top-model filamentous fungus for the study of signaling and homeostasis mechanisms
International Microbiology (2020)
-
The putative C2H2 transcription factor RocA is a novel regulator of development and secondary metabolism in Aspergillus nidulans
Journal of Microbiology (2020)
-
Morphological, transcriptional, and metabolic analyses of osmotic-adapted mechanisms of the halophilic Aspergillus montevidensis ZYD4 under hypersaline conditions
Applied Microbiology and Biotechnology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.