Abstract
To stabilise ferroelectric-tetragonal phase of BaTiO3, the double-doping of Bi and Mn up to 0.5 mol% was studied. Upon increasing the Bi content in BaTiO3:Mn:Bi, the tetragonal crystal-lattice-constants a and c shrank and elongated, respectively, resulting in an enhancement of tetragonal anisotropy, and the temperature-range of the ferroelectric tetragonal phase expanded. X-ray absorption fine structure measurements confirmed that Bi and Mn were located at the A(Ba)-site and B(Ti)-site, respectively, and Bi was markedly displaced from the centrosymmetric position in the BiO12 cluster. This A-site substitution of Bi also caused fluctuations of B-site atoms. Magnetic susceptibility measurements revealed a change in the Mn valence from +4 to +3 upon addition of the same molar amount of Bi as Mn, probably resulting from a compensating behaviour of the Mn at Ti4+ sites for donor doping of Bi3+ into the Ba2+ site. Because addition of La3+ instead of Bi3+ showed neither the enhancement of the tetragonal anisotropy nor the stabilisation of the tetragonal phase, these phenomena in BaTiO3:Mn:Bi were not caused by the Jahn-Teller effect of Mn3+ in the MnO6 octahedron, but caused by the Bi-displacement, probably resulting from the effect of the 6 s lone-pair electrons in Bi3+.
Similar content being viewed by others
Introduction
Barium titanate (BaTiO3) is one of the most well-known ferroelectric materials and has been used in electrical components such as multilayered ceramic capacitors1 and positive temperature coefficient thermistors2. BaTiO3 has outstanding ferroelectric and piezoelectric properties at room temperature, but shows a relatively low Curie temperature of about 130 °C and another phase transition (from tetragonal to orthorhombic) near room temperature. These phase transitions near room temperature and the narrow stable temperature range of the ferroelectric tetragonal phase are disadvantages for ferroelectric and piezoelectric applications.
To adjust the electrical characteristics of BaTiO3-based ceramic materials, especially to improve their insulating properties, some transition metal elements such as Mn, sometimes accompanied with non-transition metal doping elements, are often added to BaTiO31,3,4,5,6. However, adding a small amount of Mn to BaTiO3 lowers its ferroelectric transition temperature (Curie temperature; TC)7. This decrease in TC, while favourable for capacitor applications, is still a problem for ferroelectric and piezoelectric applications. For ferroelectric and piezoelectric device development, BaTiO3 is often alloyed with high-TC ferroelectric materials such as bismuth ferrite (BiFeO3). The resulting BaTiO3-BiFeO3 solid solution shows remarkable properties, including large field-induced strain, and unusual electro-structural behaviour such as ferroelectricity in a pseudo-cubic crystal lattice8,9. We expected that doping of Bi and transition metal elements into BaTiO3 would result in electrical properties superior to those of undoped one. Hence, we have investigated the effect of adding Bi to Mn-doped BaTiO3, which is one of the most common doped BaTiO3 ceramics. However, adding only 4% BiFeO3 to BaTiO3 made the ferroelectric phase transition diffuse, as in relaxor-ferroelectrics such as Pb(Mg1/3Nb1/2)O3-PbTiO310. Therefore, to retain normal ferroelectric behaviour in BaTiO3, including sharp first-order phase transitions, we have investigated how TC increases and how other characteristics improve with doping concentrations of less than 1%.
In the current work, we studied the effects of double-doping BaTiO3 with a very small amount of Bi/Mn on phase transitions, dielectric and ferroelectric properties, long-range (averaged) crystal lattice structures, short- and medium-range atomic correlations, and local structures around the doped atoms. In addition, the chemical states of the dopants were directly estimated. Finally, we discuss the microscopic mechanism of structural and electrical property evolution, and the variation of phase transition temperatures caused by low-level doping of Bi/Mn into BaTiO3.
Results
Electrical measurements
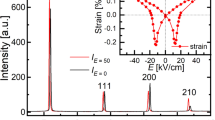
To characterise the effects of doping Bi/Mn into BaTiO3 on its electrical properties, the temperature dependence of the relative dielectric permittivity (εr) (Fig. 1a–c) and of the dielectric loss (tan δ) (Fig. 1d) for undoped, Mn-doped, Bi-doped, and Bi/Mn-doubly doped BaTiO3 were measured, with the results shown at 1 kHz. The doped compositions are abbreviated as BaTiO3:Mn, BaTiO3:Bi, and BaTiO3:Mn:Bi, respectively, and εr = ε/ε0, where ε is the permittivity of the material and ε0 is the vacuum permittivity. Dielectric anomalies were observed at around 120 and 0 °C, which correspond to the cubic (paraelectric)-tetragonal (ferroelectric) and the tetragonal-orthorhombic phase transitions, respectively. Compared with the dielectric properties of undoped BaTiO3, BaTiO3;Mn and BaTiO3:Mn:Bi samples showed relatively low εr values below the cubic-tetragonal phase transition temperature, which is equal to TC, regardless of Bi content, and also relatively low dielectric loss. However, BaTiO3:Bi without Mn exhibited a large dielectric permittivity and a huge dielectric loss (Fig. 1a and d). Mn is the dopant of choice to suppress the dielectric loss factor and leakage current for BaTiO3-based capacitors4,11, due to compensation for unintended impurities that create mobile carriers12,13. Therefore, the reduction of dielectric loss by Mn-doping in our experiment was attributed to the same mechanism. Presumably, the relatively large permittivity values for BaTiO3:Bi were due to charge carriers, which induced the huge dielectric loss, and Mn-doping caused the reduction of dielectric loss together with a decrease in permittivity. The increase in the dielectric loss, namely the increase in leakage current, by Bi-doping without Mn was probably caused by the creation of carrier electrons or holes in Ba2+Ti4+O3 by doping of aliovalent Bi3+ or Bi5+. However, it is worth noting that Bi/Mn-doubly doped BaTiO3 samples showed excellent dielectric behaviour with low dielectric loss regardless of Bi content, as did BaTiO3:Mn. This result suggests that Mn-doping compensated for the creation of charge carriers by Bi-doping.
(a) Temperature dependence of the relative permittivity, εr, for undoped and Bi/Mn-doped BaTiO3. (b) Expanded view of Fig. 1a around the Curie temperature, TC. (c) Expanded view of Fig. 1a around the orthorhombic-tetragonal phase transition temperature, Tot. (d) Temperature dependence of the dielectric loss (tan δ) for undoped and Bi/Mn-doped BaTiO3. (e) Dependence of TC and Tot on Bi concentration for Bi- and Bi/Mn-doped BaTiO3. (f) Polarisation-electric field (P-E) curves for undoped, Mn(0.3%)-doped, and Bi(0.2%,0.4%,0.5%)/Mn(0.5%)-doubly doped BaTiO3 with an AC field frequency of 1 Hz at room temperature. The thin straight lines are extrapolation lines of polarisations at high fields to zero field for evaluating spontaneous polarisation Ps.
Bi/Mn-doping also affected the phase transition temperatures. TC decreased with increasing amounts of Mn in BaTiO3:Mn, and this lowered TC by Mn-doping increased with increasing amounts of Bi in BaTiO3:Mn:Bi, and then roughly recovered by 0.5 mol% of Bi (Fig. 1b). In contrast, the tetragonal-orthorhombic phase transition temperature (Tot) was not affected by doping Mn into BaTiO3, and decreased with increasing amounts of Bi in BaTiO3:Mn:Bi (Fig. 1c). Doping of Bi into BaTiO3 without Mn caused both phase transitions to broaden and the transition temperatures to decrease. Adding Bi to BaTiO3:Mn increased TC and decreased Tot; that is, the addition of Bi stabilised the tetragonal phase by expanding its temperature range (Fig. 1e).
Figure 1f shows the polarisation-electric field (P-E) curves for undoped, Mn(0.3%)-doped, and Bi(0.2–0.5%)/Mn(0.5%)-doubly doped BaTiO3. The P-E curves indicate undoped BaTiO3 yielded a larger spontaneous polarisation (Ps), which can be evaluated from extrapolation of saturated polarisation at high fields to zero field14 as indicated with thin straight lines in the figure, than did the other doped ones. Mn-doping into BaTiO3 decreased Ps, and Bi-doping into BaTiO3:Mn decreased Ps further. Increasing the amount of Bi doped into BaTiO3:Mn increased the coercive field (Ec), probably by impeding domain switching. This effect of Bi-doping was observed only in Bi/Mn-doubly doped BaTiO3 because BaTiO3:Bi showed a leakage current that was too large to carry out the P-E measurement, owing to the charge carriers created by Bi-doping.
Rietveld analysis from X-ray powder diffraction
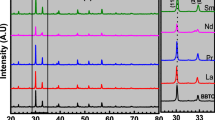
To determine the detailed lattice structures of Bi/Mn-doped BaTiO3, powder diffraction measurements at room temperature with a synchrotron X-ray source was carried out. All BaTiO3-based samples had a PbTiO3-type tetragonal crystal structure (space group: P4mm).
Figure 2a shows the tetragonal lattice constants a and c estimated by the first Rietveld analysis, which is only for determining the lattice constants using diffraction data from a sample mixed with a standard (details are shown in Supplementary Information). The lattice parameter ratio, c/a, and unit cell volume (=a2c) are also shown in Fig. 2b. The effect of doping Mn into BaTiO3 on the lattice can be seen in the figure by comparing the values for BaTiO3:Mn (triangle) and undopd BaTiO3 (circle) at a Bi content of zero. When doped with Mn, the BaTiO3 lattice elongated in the a direction but slightly shrank in the c direction, resulting in a decrease in the c/a ratio and an increase in the unit cell volume. Bi/Mn-doubly doped BaTiO3 exhibited a slight decrease in a and increase in c with increasing Bi content, resulting in an increase in the c/a ratio and an almost unchanged unit cell volume. These results indicate that doping BaTiO3 with Mn suppressed the anisotropy of the tetragonal crystal lattice and expanded the lattice, and then further doping of BaTiO3:Mn with Bi increased the anisotropy with no effect on volume. In contrast, doping BaTiO3 with Bi and without Mn yielded increases in both a and c, resulting in a simple lattice expansion without a change in the c/a ratio, preserving the anisotropy of the lattice. These results suggest that doping Bi into BaTiO3 enhances its tetragonal anisotropy only in the presence of Mn.
(a) Lattice constants a and c of Bi-doped and Bi/Mn-doubly doped BaTiO3 as a function of Bi content. (b) Ratio of lattice constants c/a and lattice volume of Bi-doped and Bi/Mn-doubly doped BaTiO3 as a function of Bi content. (c) Schematic view of the ferroelectric BaTiO3 crystal lattice, with c-axis atomic positions of Ti and O, z(Ti), z(O1), and z(O2), related to spontaneous polarization Ps. (d) Ps values calculated from the results of Rietveld analysis for Bi-doped and Bi/Mn-doubly doped BaTiO3 as a function of Bi content. Plotted points for the samples indicated in Fig. 1f are highlighted as larger closed symbols. (e) Atomic displacement factors of Ba and Ti (denoted as B(Ba) with left axis and B(Ti) with right axis, respectively) in Bi-doped and Bi/Mn-doubly doped BaTiO3 as a function of Bi content.
Atomic positions in the tetragonal lattice with space group P4mm were estimated by applying the second Rietveld analysis using the lattice constants obtained from the first analysis (details are shown in Supplementary Information). In this symmetry shown in Fig. 2c, the positions of the Ti, O1 and O2 atoms along the c-axis, denoted as z(Ti), z(O1), and z(O2), respectively, are the variable parameters if the Ba position is fixed, and the deviation of these positions from the centrosymmetrical positions, at z(Ti) =z (O2) = 0 and z(O1) = 1/2, should be related to spontaneous polarisation, Ps15, which is expressed with a classical point charge model14 as

Here V is the unit-cell volume, and Zi and Δi are the effective charges and atomic displacement vectors, respectively, for the ith ions. Ps values calculated from the results of Rietveld analysis with the assumption of the point charge model equation (1) are shown in Fig. 2d. The magnitude of Ps for each sample was calculated to be about 18 μC/cm2, and the Ps values of Mn-doped and Bi/Mn-doubly doped BaTiO3 were slightly smaller than that of undoped BaTiO3, consistent with the P-E results shown in Fig. 1f.
The atomic displacement factors of the Ba and Ti sites estimated by the second Rietveld analysis, denoted as B(Ba) and B(Ti), respectively, are shown in Fig. 2e. Both B(Ba) and B(Ti) increased with increasing Bi content. As described in the next subsection, doped Bi was located at the Ba site (“A site”) and Mn at the Ti site (“B site”). Doping with Bi was expected to cause B(Ba) to increase because the randomness of A-site atoms would be increased by the substitution of Bi for Ba. However, the increase of B(Ti) upon substitution of the A site with a small amount of Bi is unusual, and may have resulted from some indirect effect of A-site-substituted Bi on the B-site atoms. The parameters obtained by the Rietveld analyses are provided in Supplementary Information.
X-ray absorption fine structure (XAFS)
Figure 3a shows the Bi-L3 X-ray absorption near edge structure (XANES) spectra of BaTiO3:Bi and BaTiO3:Mn:Bi samples, and of BaBiO3, BiFeO3, and 0.67BiFeO3-0.33BaTiO3 solid solution standards. The Bi-L3 edge energies for BaTiO3:Bi and BaTiO3:Mn:Bi were similar to those of BiFeO3 and 0.67BiFeO3-0.33BaTiO3, in which the Bi valence is +3, although the edge energies were different from that of BaBiO3 with a mixed Bi3+/Bi5+ valence state. In addition, the oscillation structures of the Bi-L3 XANES spectra of the BaTiO3-based materials were similar to those of the BiFeO3-based substances, especially that of pseudo-cubic 0.67BiFeO3-0.33BaTiO3, but different from that of BaBiO3. Bi atoms are located at the A sites of perovskite-type structures in distorted rhombohedral BiFeO316 and pseudo-cubic 0.67BiFeO3-0.33BaTiO317, whereas they are located at B sites in BaBiO318. Therefore, Bi in the BaTiO3:Bi and BaTiO3:Mn:Bi samples had a valence state of +3 and were located at the A site in the perovskite-type structure, and thus at the Ba site in tetragonal BaTiO3 with a c/a ratio close to 1.
(a) Bi-L3 XANES spectra of Bi-doped and Bi/Mn-doubly doped BaTiO3, and of standards BaBiO3, BiFeO3, and 0.67BiFeO3-0.33BaTiO3 solid solution. (b) Bi-L3 EXAFS spectra k3χ(k) of Bi-doped and Bi/Mn-doubly doped BaTiO3. (c) RSFs for Bi-doped and Bi/Mn-doubly doped BaTiO3 from Bi-L3 EXAFS spectra, and analytical results of the Bi RSF of BaTiO3:Mn:Bi(0.4%). (d) Schematic view of Bi and O displacements with respect to Ti atom coordination. (e) RSFs for undoped, Mn-doped, Bi-doped, and Bi/Mn-doubly doped BaTiO3 from Ba-K EXAFS spectra. (f) Mn-K XANES spectra of Mn-doped and Bi/Mn-doubly doped BaTiO3, and LaMnO3 and SrMnO3 standards. (g) Mn EXAFS spectra k2χ(k) of Mn-doped and Bi/Mn-doubly doped BaTiO3. (h) RSFs for Mn-doped and Bi/Mn-doubly doped BaTiO3 from Mn-K EXAFS spectra, and analytical results of the Mn RSF of BaTiO3:Mn:Bi(0.2%).
Bi-L3 extended X-ray absorption fine structure (EXAFS) spectra χ(k) for BaTiO3:Bi and BaTiO3:Mn:Bi samples are shown in Fig. 3b with a k-weighted form of k3χ(k) as a function of wavenumber k. All the samples yielded spectra with very similar profiles between 2 and 10 Å−1, regardless of the amount of Bi doping and whether Mn was present. The Bi radial structure function (RSF) of each sample was derived by the Fourier transform of k3χ(k), and all the RSFs are shown in Fig. 3c. As expected from the Bi-L3 EXAFS spectra of Fig. 3b, the RSFs were similar to each other, suggesting similar local structures around the Bi atom in all the samples. To determine the local structure around the Bi atom, curve-fitting analysis of the RSF was carried out by using the single-scattering EXAFS equation19,20,21

where rj is the distance from the central absorbing atom to the jth shell atoms, Nj is the coordination number, σ2j is the Debye-Waller factor, Fj(k) is the complex backscattering amplitude, φj(k) is the phase shift of the atoms in the jth shell, S0 is the overall amplitude reduction factor due to the many-body effect (so-called intrinsic loss factor), and λ(k) is the mean-free path of the photoelectron (e−2r/λ is so-called extrinsic loss factor related to inelastic scattering). In this analysis, we employed rj, σ2j, and ΔE0 which is the correction of the absorption edge energy, as the variables to refine. S0 was fixed as 1.09, which was derived from EXAFS analysis of the BiFeO3 standard sample, imposing constraints based on the symmetry of the crystal structure22. Fj(k), λj(k), and λ(k) were calculated by using FEFF in ARTEMIS software and then these parameters were applied to the EXAFS analysis automatically. These RSFs did not fit well with a structural model of Bi at the Ba site based on the Ba local structure in the BaTiO3 crystal derived from the Rietveld analysis. The obtained reliability factor, R, which indicates quality of fit, from a fit with this model was on the order of 10−2. However, fitting was drastically improved (R reduced to ~10−4) by using a model with off-centre Bi compared with the ideal Ba position in the BaTiO3 crystal lattice. The fitting results were not sensitive to the direction of Bi displacement, although they were sensitive to the difference in r between shorter and longer Bi-O bonds, and also the difference of r for different Bi-Ti bonds. Therefore, we assumed that Bi was displaced mainly along a slightly elongated c-axis in the tetragonal lattice, like the model used to analyse the local Pb structure in highly distorted tetragonal PbTiO323. Representative fitting results for the RSFs of BaTiO3:Bi and BaTiO3:Mn:Bi(0.4%) shown in Fig. 3c are listed in Table 1, and a comparison of the fitted curve to an experimentally obtained RSF of BaTiO3:Mn:Bi(0.4%) is shown at a the bottom of Fig. 3c. The calculated curve fits the RSF well, and the R-factors are very small, meaning that the off-centre Bi structural model reproduced the RSFs of BaTiO3:Bi and BaTiO3:Mn:Bi. Figure 3d illustrates the local structure around Bi with the parameters of BaTiO3:Mn:Bi(0.4%) in Table 1. The large displacement of Bi from the centrosymmetric position and a distorted BiO12 cluster are visible. The average length of Bi-O bonds is much shorter than the Ba-O bonds calculated from the Rietveld analysis. This difference is caused by the substitution of the higher-valence Bi3+ for Ba2+, explained by the electrostatic force between the cations and O2−; in contrast, the average length of the Bi-Ti bonds is almost unchanged from that of Ba-Ti. Doping the BaTiO3-based crystal with a very small amount of Bi yielded a local Bi displacement and shrinkage of Bi-O bonds, which appeared to have affected the entire crystal, especially the length of the c-axis, according to the results of Rietveld analysis (Fig. 2a).
Figure 3e shows Ba RSFs for the undoped and doped BaTiO3 samples from Ba-K EXAFS spectra k3χ(k). All of these RSFs were very similar, indicating that the local structure around the Ba atom in Mn-doped, Bi-doped, and Bi/Mn-doubly doped BaTiO3 was almost unchanged from that of undoped BaTiO3. However, there was a slight decrease in RSF intensity, especially for the Ba-Ba peak, which suggests a small increase in the Debye-Waller factor of Ba, and which is consistent with the result of the Rietveld analysis shown in Fig. 2e.
Mn-K XANES spectra of BaTiO3:Mn, and BaTiO3:Mn:Bi, and the LaMn3+O3 and SrMn4+O3 standards are shown in Fig. 3f. The Mn-K absorption edge and the white-line peak beside the edge shifted slightly from higher energy to lower energy as the amount of doped Bi was increased. Similarly, the edge of the AMnO3 perovskite-type materials (A = Sr or La) shifted from higher energy for SrMn4+O3, which has a relatively high Mn valence, to lower energy for LaMn3+O3 with its lower Mn valence. This result suggests that Mn valence in BaTiO3:Mn:Bi may decrease with increasing Bi content.
The various BaTiO3:Mn and BaTiO3:Mn:Bi samples yielded very similar Mn-K EXAFS spectra k2χ(k) (Fig. 3g), suggesting that the doped Mn atoms were in the same location and had similar local structures in these samples, regardless of the amount of Bi. Because the Mn-fluorescent XAFS signals were weak, the k2χ(k) profiles in high k region were noisy, so that only a k range of 2 to 7 Å−1 could be used for the RSF calculations. The calculated Mn RSFs in Fig. 3h are similar, as expected from the k2χ(k) profiles, but with small differences in the details, probably due to the noisy k2χ(k) spectra. Therefore, the quantitative description provided above for the Bi-L3 EXAFS analysis appears to not be appropriate for the EXAFS analysis with these Mn RSFs, but these RSFs can nevertheless be used to qualitatively check substitution sites in the perovskite-type lattice and the local structure around Mn. Results of the analysis for BaTiO3:Mn:Bi(0.2%) as a typical example are shown at the bottom of Fig. 3h. This Mn RSF was easily fitted in the r-range of 1 to 3 Å with an RSF curve calculated from a model of Mn at the Ti site in BaTiO3 that includes appropriate parameters, whereas a model of Mn at the Ba site could not simulate the RSF. Therefore, we confirmed by XAFS analysis that a small amount of Mn occupied the Ti site in BaTiO3 in our sample. It has been expected that doped Mn atoms can be substituted for Ti based on indirect data, although there has been hardly any direct evidence7,24,25. Our results provide compelling evidence for this substitution. The analysis suggests the local structures around Mn and Ti are similar, with the analysis of all the RSFs in Fig. 3h indicating that the Mn-O and Mn-Ba bond lengths are slightly shorter than those of Ti-O and Ti-Ba. This difference in bond lengths may explain the local distortion and the reduction of the anisotropy of the tetragonal crystal lattice indicated by the c/a ratio shown in Fig. 2b. However, as mentioned above, more convincing and precise data are needed to provide a quantitatively significant description.
Atomic pair-distribution function (PDF) analysis from high-energy X-ray diffraction
An X-ray diffraction (scattering) pattern at high wavenumbers includes short-range (local) and medium-range structural information. To extract the short-range structural information, we calculated PDFs of undoped, Mn-doped, Bi-doped, and Bi/Mn-doubly doped BaTiO3 by Fourier transformation of the corresponding high-energy X-ray diffraction patterns. Figure 4a shows the PDF profiles. These profiles indicate similar structures above a bond length r of ~2 Å, and only the shortest Ti-O bonds yielded different shapes for the different samples (Fig. 4b). Although the PDF profile around 2 Å for the undoped BaTiO3 can be fitted with a calculation based on a tetragonal structure model, the data for BaTiO3:Mn:Bi(0.4%) fits to rather a rhombohedral structure model calculation. As shown in refs 26 and 27, PDF spectrum around 2 Å corresponding to Ti-O bonds in BaTiO3 with the tetragonal structure shows a broad single peak and it transforms to a doubly split peak accompanied by a phase transition to the orthorhombic structure and finally the rhombohedral one. The PDF spectra of the undoped BaTiO3 and BaTiO3:Mn:Bi(0.4%) exhibit the features of tetragonal and rhombohedral structures, respectively. This result means that not only B-site-substituted Mn but also A-site-substituted Bi affected the Ti-O bond, perhaps by affecting the local structure around the B-site atoms with rhombohedral distortion. This may result in the increase of the atomic displacement factor B(Ti) by the Rietveld analysis with the tetragonal average structure, as mentioned in the Rietveld analysis subsection and Fig. 2e.
(a) Atomic pair-distribution functions of undoped, Bi-doped, and Bi/Mn-doubly doped BaTiO3 derived from high-energy X-ray diffraction data. (b) Expanded view of Fig. 4a around the peaks related to Ti-O bonds. Calculation results for undoped BaTiO3 based on a tetragonal structure model and BaTiO3:Mn:Bi(0.4%) based on a rhombohedral structure model are also represented using thin lines in Fig. 4b.
Estimation of Mn valence from the temperature dependence of magnetic susceptibility
Although a change in Mn valence resulting from the doping of Bi into BaTiO3:Mn and BaTiO3:Mn:Bi was confirmed by XANES as shown in Fig. 3f, it is difficult to estimate Mn valence quantitatively from the Mn-absorption edge energy compared with reference samples in which the Mn valence is known, such as LaMnO3 and SrMnO3, because the shapes of the XANES spectra are too different to compare their edge-energies. Many researchers have through the years attempted to estimate the valence of Mn slightly doped in BaTiO3. Electron spin resonance (ESR) has been most often used for this purpose25,28,29, because it is very sensitive for the Mn2+ ion, but it cannot detect Mn3+ and is not very sensitive for Mn4+. Therefore, ESR was not suitable for determining the slight change in the Mn valence expected between Mn4+ and Mn3+ or between Mn3+ and Mn2+ as a result of Bi-doping. Hagemann et al. determined valence states of 3d-transition metal elements doped in BaTiO3 from the spin states of the dopants from magnetic susceptibility data measured above 77 K30. However, this method provided only a rough estimation because it used very small signals detected at high temperatures above 77 K, and used highly doped (up to 2 mol%) materials in which Mn may not have completely dissolved in BaTiO3 resulting in some segregation of a secondary phase. Owing to the difficulty of precisely determining the valence of Mn in BaTiO3 experimentally, several first-principles studies have been carried out31,32. Because these calculations may be applicable to a doubly doped BaTiO3 system, it would be desirable to use the method to estimate the valence states of Mn in BaTiO3:Mn:Bi. Here we attempted to obtain the Mn valence more accurately and precisely not by a first-principles calculation but by an experimental approach, estimating the spin-state of Mn from magnetic susceptibility data acquired more sensitively than in a prior study30.
Because the amount of doped Mn is so dilute and the base BaTiO3 and the other dopant, Bi, are non-magnetic (diamagnetic), the doped materials should not show any magnetically ordered phase transition even at low temperatures, and the magnetic susceptibility χ should obey the Curie-Weiss law. We assumed that the Mn valence is unchanged at temperatures ranging from room temperature to low temperature, because BaTiO3:Mn and BaTiO3:Mn:Bi showed no significant increase in the dielectric loss relating to electrical conductivity (Fig. 1d), owing to the lack of charge carriers that would have been associated with a valence change. Therefore, magnetic susceptibility was measured down to a low temperature of 2 K; these measurements can be accurate and precise because susceptibility at low temperatures is much larger than at higher temperatures.
Figure 5a shows, as an example, the temperature dependence of the magnetic susceptibility (χ-T curve) of BaTiO3:Mn(0.3%). No anomalous magnetic phase transition of the main phase and any secondary phase33 was found down to 2 K, which suggests that the susceptibility data can be used in the analysis for estimating the state of Mn. This χ-T curve accurately obeys the Curie-Weiss law with an additional assumption of a coexisting component of temperature-independent susceptibility, such as diamagnetic susceptibility34, expressed as
(a) Temperature dependence of magnetic susceptibility (χ-T curve) for BaTiO3:Mn(0.3%). Solid line indicates the fit to the Curie-Weiss law with the parameters indicated in Table 2. Inverse susceptibility with subtraction of the constant term (χ−χc)−1 is also indicated. Inset shows the χ-T curve with a fitted curve in the temperature range of 2 to 300 K. (b) Dependence of the Mn valence estimated from the Curie-Weiss analysis of the χ−T curves on the Bi/Mn content ratio in Mn-doped and Bi/Mn-doubly doped BaTiO3.

where C, θp, and χc denote the Curie constant, paramagnetic Curie temperature, and temperature-independent susceptibility (constant term), respectively. The relationship between temperature T and the inverse of susceptibility minus the constant term, (χ−χ c)−1, is linear when equation (3) holds. Such linear relationship was found for BaTiO3:Mn(0.3%) as seen in Fig. 5a, suggesting that the material containing Mn obeys the Curie-Weiss law very well. Because the magnetic susceptibility obeyed the Curie-Weiss law (3) even at high temperatures up to 300 K, as shown in the inset, the Mn spin state and valence state presumably remained unchanged in the temperature range from room temperature down to 2 K.
Owing to the small signals with large errors at high temperatures and the appearance of a very small difference between field-cooled and zero-field-cooled magnetisations, the χ−T curves in the temperature range of 2 to 70 K corrected in field-cooled magnetisation were used for a fitting analysis with the Curie-Weiss law (3) to estimate C, θp, and χc. Estimated parameters for BaTiO3:Mn and BaTiO3:Mn:Bi are listed in Table 2 with the reliability factor, R2, indicating quality of fit, for which a value close to 1 shows that the fitting is reliable. The Curie constant, C, is related to Mn spin S according to ref. 34

where N, g, μB, and kB denote the number of Mn ions, the Lande g-factor, the Bohr magneton, and the Boltzmann constant, respectively. The Mn ion in BaTiO3 was assumed to have a high-spin state (spin S = 5/2, 2, and 3/2 for Mn2+, Mn3+, and Mn4+, respectively), and based on this assumption the obtained C was fitted with a linear combination of calculated values of C for Mn ions with different valences. Then, the mean valence was derived from the fraction Mn4+/Mn3+ or Mn3+/Mn2+, which are listed in Table 2 and plotted in Fig. 5b as a function of the Bi/Mn ratio. The Mn valence decreased monotonically as the Bi/Mn ratio increased. This suggests that doped Mn in BaTiO3 was originally Mn4+ substituted for Ti4+, and Mn4+ then received an electron created by the Bi3+ substitution for Ba2+ so that a corresponding amount of Mn4+ was converted to Mn3+. The solid red line in Fig. 5b indicates the ideal dependence of Mn valence on Bi/Mn ratio, and the Mn valence estimated from the magnetic susceptibility data almost obeyed this relationship. By this mechanism, Bi can be doped into BaTiO3:Mn with stable dielectric properties; in contrast, doping Bi into BaTiO3 without Mn degraded the properties with an increase in dielectric loss, caused by an increase in charge carrier density with Bi3+ doped at the Ba2+ site. The valence of Mn in BaTiO3:Mn was estimated to be +3.92, and it is difficult to judge whether the difference between this value and a valence value of +4 is meaningful or within the margin of error. If this difference is meaningful, some of the Mn4+ ions may act as acceptors to compensate for intrinsic donors in BaTiO3, such as oxygen vacancies, rather than for Bi3+ donors, and dielectric loss may have decreased as a result of doping Mn acceptors into BaTiO3 (Fig. 1d).
As shown in Table 2, θp had a negative and small value, in the order of −0.1 K, indicative of an antiferromagnetic and weak magnetic exchange interaction between Mn spins. This finding is consistent with Mn ions being dispersed thinly in the BaTiO3 crystal lattice. The absolute value of θp increased with an increasing Bi/Mn ratio, suggesting an increase in the antiferromagnetic exchange interaction between Mn spins due to an increase in the Mn magnetic moment accompanied by a change of the valence of the Mn ion from +4 to +3. The temperature-independent susceptibility χc values for BaTiO3 and BaTiO3:Bi were small negative values, which is typical for diamagnetic behaviour in nonmagnetic insulators, but the χc value for BaTiO3:Mn:Bi(0.5%) was positive. Furthermore, although the χc values for Mn-doped and Bi(0.1–0.4%)/Mn-doubly doped BaTiO3 remained negative, their absolute values were smaller than those for BaTiO3 and BaTiO3:Bi. These results support the existence of a positive component of temperature-independent magnetic susceptibility in this system, and that this positive component was enhanced by Mn doping and Bi/Mn double doping. For example, a magnetically ordered (especially ferromagnetic) impurity might have acted in this way, but the details of this component have not been clarified yet.
Discussion
As shown in Figs 1e and 2b, doping Bi into BaTiO3:Mn stabilised the tetragonal phase with an increase in TC and decrease in Tot, and also increased the c/a ratio, the anisotropy of the tetragonal crystal lattice. Two possible reasons for this behaviour should be considered: (i) the effect of the Bi 6 s2 electron lone pair, or (ii) the Jahn-Teller effect of Mn3+ in the MnO6 octahedron.
The XAFS analysis revealed the doped Bi atom at the Ba site to be considerably displaced from the centrosymmetric position and the BiO12 cluster to be distorted, as shown in Fig. 3d, probably due to the effects of the Bi 6 s2 electron lone pair. Lattice distortion attributed to the Bi 6 s2 lone pair has been often reported, for example, in BiMnO335 and BiCoO336, and Bi displacement from the centrosymmetric position at the A site due to the Bi 6 s2 lone pair has also been reported in a similar substance, the 0.85BaTiO3-0.15BiFeO3 solid solution10. The tetragonal distortion of PbTiO3 has also been attributed to 6 s2 lone-pair electrons of Pb2+ according to the results of a first-principles calculation37 and analysis of electron density derived from X-ray diffraction data38,39. However, Mn3+ at the centre of an MnO6 octahedron may distort the octahedron and crystal lattice tetragonally, due to the Jahn-Teller effect of the 3d4 high-spin state, such as in RMnO3 (R: rare earth)40,41 and ZnMn2O440,42. The enhancement of the anisotropy in the tetragonal crystal lattice and the tetragonal phase stabilisation of these samples were confirmed and the mechanism was determined by preparing BaTiO3:Mn:(Bi,La) and BaTiO3:Mn:La by La3+ substitution with no lone-pair electrons for a half or all of the Bi in BaTiO3:Mn(0.5%):Bi(0.5%), and then analysing them by X-ray powder diffraction and εr−T measurement to determine their lattice constants and transition temperatures, respectively.
Figure 6a and b show the lattice constants a and c, the c/a ratio, and unit cell volume for BaTiO3:Mn3+:Bi, BaTiO3:Mn3+:(Bi,La), and BaTiO3:Mn3+:La, as a function of Bi content, compared with those for BaTiO3:Mn:Bi, in which Mn valence depends on Bi content (detailed data are available in Supplementary Information). The dependence of the lattice parameters on Bi content for BaTiO3:Mn3+:(Bi,La) was the same as that for BaTiO3:Mn:Bi, even though the Mn valence was always +3 independently of the Bi content. The dependence of the transition temperatures TC and Tot on Bi content for BaTiO3:Mn3+:(Bi,La) were similar to those for BaTiO3:Mn:Bi, as shown in Fig. 6c. These results indicate that the tetragonality enhancement and the tetragonal phase stabilisation should be attributed not to the Mn3+ fraction but to the Bi content. Therefore, we conclude that local distortion by Bi 6 s2 lone pair electrons enhances the tetragonal anisotropy and stabilises the tetragonal phase in BaTiO3:Mn:Bi.
(a) Lattice constants a and c for BaTiO3:Mn3+:(Bi,La) as a function of Bi content, with those for BaTiO3:Mn:Bi shown in Fig. 2a for comparison. (b) Lattice volume and the lattice constant c/a ratio for BaTiO3:Mn3+:(Bi,La) as a function of Bi content, with those for BaTiO3:Mn:Bi shown in Fig. 2b for comparison. (c) TC and Tot for BaTiO3:Mn3+:(Bi,La) as a function of Bi content, with those for only BaTiO3:Mn:Bi shown in Fig. 1e for comparison.
Conclusions
In summary, enhancement of the anisotropy of the tetragonal crystal lattice and the tetragonal phase stabilisation of Bi/Mn-doubly doped BaTiO3 ceramics were investigated. XAFS measurements confirmed that Bi and Mn were located at the A site (Ba site) and B site (Ti site), respectively. Powder X-ray diffraction revealed the tetragonal crystal lattice constants a and c shrank and elongated, respectively, and hence the ratio c/a increased with increasing Bi content, although with no change in the unit cell volume. Moreover, TC lowered by Mn-doping was increased to recover and Tot was decreased by doping very small amounts of Bi (up to 0.5 mol%) into BaTiO3:Mn. Analysis of Bi-L3 EXAFS clarified the presence of a considerable deviation of the Bi position from the centrosymmetric position, and its resulting distortion of the BiO12 cluster in Bi-doped and Bi/Mn-doubly doped BaTiO3. PDF and Rietveld analyses also revealed that doped Bi at the A site caused local structure distortions around the B-site atoms. The Mn valence was evaluated from its spin state estimated by magnetic susceptibility measurements, revealing a change of the Mn valence from +4 to +3 upon addition of the same molar amount of Bi as Mn, probably due to the compensating behaviour of the Mn that originally has a valence state of +4 at Ti4+ sites for donor doping of Bi3+ into the Ba2+ site. Based on the Bi-L3 EXAFS results and the observation that adding La3+ instead of Bi3+ did not increase the c/a ratio or increase TC and decrease Tot, the enhancement of the anisotropy in the tetragonal crystal lattice and stabilisation of the tetragonal phase by adding Bi in BaTiO3:Mn appeared to be caused by the effect of the 6 s lone-pair electrons in Bi3+ at the Ba site in BaTiO3, not by the Jahn-Teller effect of Mn3+ in the MnO6 octahedron, similar to Pb2+ in tetragonally distorted PbTiO3.
Methods
Sample preparation
BaTiO3, BaTiO3-0.004BiO3/2, Ba(Ti0.995Mn0.005)O3-xBiO3/2 (x = 0.001, 0.002, 0.003, 0.004, and 0.005), Ba(Ti0.995Mn0.005)O3, Ba(Ti0.997Mn0.003)O3, Ba(Ti0.995Mn0.005)O3-0.005LaO3/2, and Ba(Ti0.995Mn0.005)O3-0.005(La0.5Bi0.5)O3/2 ceramic samples denoted, respectively, as undoped BaTiO3, BaTiO3:Bi, BaTiO3:Mn:Bi(0.1, 0.2, 0.3, 0.4, 0.5%), BaTiO3:Mn(0.3, 0.5%), BaTiO3:Mn:La, and BaTiO3:Mn:(La,Bi) were prepared by conventional solid state synthesis and sintering. Submicrometre-sized BaTiO3 powder (BT01, Sakai Chemical Industry) and stoichiometric amounts of micrometre-sized Bi2O3 (99.999%, Kojundo Chemical Laboratory), MnO2 (99.99%, Kojundo), BaCO3 (99.95%, Kojundo), and La2O3 (99.999%, Kojundo, dehydrated by heating at 900 °C before weighing) powders were weighed and mixed in a planetary ball mill with zirconia beads (1 and 3 mm in diameter) and ethanol at a rotation speed of 500 rpm for 12 h to homogenise. An ethanol solution of polyvinyl butyral was added to the mixed powders as a binder, they were mixed well and dried, and then the powders were pressed uniaxially into pellets 17 mm in diameter. The pellets were fired at 1350 °C for 4 h in a conventional box furnace with molybdenum disilicide heaters. The surfaces of the ceramic disks were ground slightly to remove surface layers that might have been contaminated during the firing process. The amounts of Bi and Mn additives in the ceramics were checked by using inductively coupled plasma mass spectroscopy and atomic emission spectroscopy, respectively (data are shown in Supplementary Information). All plots with respect to Bi content or Bi/Mn content ratio used the measured amounts of Bi/Mn, although the nominal values were used in the chemical formula. Homogeneity of the samples were checked by scanning electron microscopy and transmission electron microscopy, and discussed using the data of electrical characteristics, x-ray diffraction, XAFS, and magnetic measurements. The details are given in Supplementary Information.
Dielectric and ferroelectric characterisations
For electrical characterisation, the ceramic disks were polished to a thickness of about 0.5 mm. Gold films (300 nm thick) with titanium glue layers (30 nm) were deposited on both polished sides of each sample as electrodes by magnetron sputtering in an argon atmosphere. Then, the samples were cut into 2.5 × 10.0 mm pieces with a dicing saw. The temperature dependence of the dielectric permittivity and loss factor were measured with frequencies of 1, 10, and 100 kHz under ambient conditions by using an impedance analyser (IM3570, Hioki) and a temperature-controlled stage system (LTS350, Linkam). Polarisation-electric field (P–E) curves were characterised with a ferroelectric characteristics evaluation system (FCE-1, Toyo) at room temperature.
Synchrotron X-ray powder diffraction and Rietveld analysis
X-ray diffraction of BaTiO3:Mn(0.3%), BaTiO3:Bi, BaTiO3:Mn:Bi, BaTiO3:Mn:La, and BaTiO3:Mn:(La,Bi) powders was carried out at room temperature with a large-scale Debye-Scherrer camera43 at the BL19B2 bending-magnet beamline of SPring-844. The incident X-ray beam was monochromated to a wavelength λ = 0.39984 or 0.39987 Å. Pure sample powder and sample powder well mixed with CeO2 standard material (NIST 640a) were prepared for each substance for more accurate estimation of lattice constants. Data were recorded on an imaging plate serving as a two-dimensional detector with a step interval of 0.01°.
The Rietveld method was used to analyse the powder diffraction data with Rietan-FP45 software with the VESTA46 program for visualising and analysing crystal structures. First, a Rietveld analysis for determining lattice constants was performed with data taken from the mixed specimen with the standard. Then, other lattice parameters (atomic positions and displacement factors) were successively derived from data taken from the pure powder sample using the lattice constants derived from the Rietveld analysis with the data taken from the powder sample mixed with the standard (see Supplementary Information).
XAFS
X-ray absorption spectra of Bi-L3, Ba-K, and Mn-K were collected at the BL14B2 bending magnet beamline of SPring-847. To control the wavelength (energy) of the incident X-ray beam, a double-crystal monochromator with Si(311) for Bi-L3 and Ba-K, and with Si(111) for Mn-K XAFS measurements was employed. The fluorescent method with an array of 19 elements of Ge solid-state detectors was used for Bi-L3 and Mn-K XAFS measurements for BaTiO3:Mn(0.5%), BaTiO3:Bi, and BaTiO3:Mn:Bi samples because Bi and Mn were dilute, whereas Ba-K XAFS spectra were taken by the transmission method with an ionisation chamber with samples diluted with boron nitride (BN) powder. The intensity of the incident X-ray beam was monitored with another ionisation chamber in both fluorescence and transmission modes. As standard samples, BaBiO3, BiFeO3, and 0.67BiFeO3-0.33BaTiO3 solid solution48 were prepared by a conventional solid-state reaction method for Bi-L3 XAFS, and commercially available LaMnO3 (99.9%, Toshima Manufacturing) and SrMnO3 (99.9%, Toshima Manufacturing) were used for Mn-K XAFS analysis. XAFS measurements of the standard samples were taken by using the transmission method with BN-diluted disk-samples. The collected data were analysed with ATHENA/ARTEMIS software programs49 in which FEFF6 code21 was used to calculate scattering paths. The Fourier transformation of the Bi-L3 EXAFS profile, k3χ(k), to calculate the Bi RSF was performed with k from 2.5 to 9.5 Å−1, and curve fitting of the Bi RSF with single scattering EXAFS equation (2) was performed in an r space of 1.1 to 4.3 Å, in which no multiple scattering path exists in the (Ba,Bi)TiO3 model. In addition, the Fourier transformation of the Ba-K EXAFS profile, k3χ(k), to derive the Ba RSF was performed with k from 3.0 to 12.0 Å−1, and that of the Mn-K EXAFS profile, k2χ(k), to derive the Mn RSF was performed with k from 2.0 to 7.0 Å−1. The fitted curve of the Mn RSF was calculated in an r space of 1.0 to 3.5 Å.
High-energy powder x-ray diffraction and PDF analysis
High-energy (60 keV) X-ray diffraction experiments for PDF analysis were carried out at the BL14B1 bending-magnet beamline at SPring-850. A kapton (polyimide) capillary tube was filled with the powder sample. A Ge point detector was scanned to collect the scattered X-rays from the sample up to a wavenumber Q = 17 Å−1. The data were corrected for background, absorption, multiple-scattering, and inelastic effects, and then were normalised to the incident flux and the total sample scattering cross section to yield the total scattering structure function, S(Q). The reduced PDF, G(r), was derived by Fourier transformation of S(Q). The obtained PDF profiles were analysed calculations using the program PDFgui51.
Magnetic susceptibility measurements
The temperature dependence of magnetisation was measured by using a superconducting quantum interference device DC magnetometer (MPMS-7/XL7, Quantum Design) with a temperature range of 2–300 K under a DC magnetic field of 100 Oe. A linear dependence of the magnetisation on the magnetic field up to 100 Oe was confirmed for every sample and in the entire temperature range, so magnetic susceptibility could be derived easily from the magnetisation values divided by the magnitude of the magnetic field. A gelatine capsule was filled with the accurately weighted powder sample (~200 mg) and mounted in the magnetometer with a plastic straw sample holder. DC mode was used for the measurements and the scan length was set to 5 cm. A Pd reference sample (Quantum Design) was also used to calibrate the magnetisation value of the machine each time. The temperature dependence of the magnetisation was determined under both zero-field cooling and field cooling conditions. To estimate the magnetic moment of Mn in the sample, susceptibility measured with field cooling at 2–70 K was extracted and fitted according to the Curie-Weiss law.
Additional Information
How to cite this article: Yabuta, H. et al. Enhancement of tetragonal anisotropy and stabilisation of the tetragonal phase by Bi/Mn-double-doping in BaTiO3 ferroelectric ceramics. Sci. Rep. 7, 45842; doi: 10.1038/srep45842 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kishi, H., Mizuno, Y. & Chazono, H. Base-metal electrode-multilayer ceramic capacitors: Past, present and future perspectives. Jpn. J. Appl. Phys. 42, 1–15 (2003).
Haertling, G. H. Ferroelectric ceramics: History and technology. J. Am. Ceram. Soc. 82, 797–818 (1999).
Chen, Y. L. & Yang, S. F. PTCR effect in donor doped barium titanate: Review of compositions, microstructures, processing and properties. Adv. Appl. Ceram. 110, 257–269 (2011).
Okazaki, K. Factors and mechanisms affecting the electrical properties of barium titanate ceramics. Memoirs of the Defence Academy Japan 5, 341–364 (1966).
Okazaki, K. & Igarashi, H. Processing-property relations in ceramic dielectric capacitors. Ferroelectrics 27, 263–268 (1980).
Kishi, H. et al. Occupational sites and dielectric properties of rare-earth and Mn substituted BaTiO3 . J. Eur. Ceram. Soc. 21, 1643–1647 (2001).
Desu, S. B. & Subbarao, E. C. Inhibition of reduction of BaTiO3 . J. Mater. Sci. 15, 2113–2115 (1980).
Leontsev, S. O. & Eitel, R. E. Dielectric and piezoelectric properties in Mn-modified (1−x)BiFeO3-xBaTiO3 ceramics. J. Am. Ceram. Soc. 92, 2957–2961 (2009).
Ozaki, T. et al. Ferroelectric properties and nano-scaled domain structures in (1−x)BiFeO3-xBaTiO3 (0.33<x<0.50). Ferroelectrics 385, 155–161 (2009).
Singh, A., Moriyoshi, C., Kuroiwa, Y. & Pandey, D. Evidence for diffuse ferroelectric phase transition and cooperative tricritical freezing of random-site dipoles due to off-centered Bi3+ ions in the average cubic lattice of (Ba1−x Bi x )(Ti1−x Fe x )O3 . Phys. Rev. B 85, 064116 (2012).
Matsubara, S. & Miyasaka, Y. Japan Patent JP,05-87166,B (1993).
Copel, M. et al. Compensation doping of Ba0.7Sr0.3TiO3 thin films. Appl. Phys. Lett. 73, 1832–1834 (1998).
Wang, X., Gu, M., Yang, B., Zhu, S. & Cao, W. Hall effect and dielectric properties of Mn-doped barium titanate. Microelectronic Engineering 66, 855–859 (2003).
Lines, M. E. & Glass, A. M. Structural crystallography and phase transitions In Principles and Applications of Ferroelectrics and Related Materials 174–208 (Oxford University Press, 1977).
Kittel, C. Dielectrics and ferroelectrics In Introduction to Solid State Physics 8th ed. 453–486 (John Wiley & Sons, 2005).
Moreau, J. M., Michel, C., Gerson, R. & James, W. J. Ferroelectric BiFeO3 x-ray and neutron diffraction study. J. Phys. Chem. Solids 32, 1315–1320 (1971).
Yoneda, Y., Yoshii, K., Kohara, S., Kitagawa, S. & Mori, S. Local structure of BiFeO3-BaTiO3 mixture. Jpn. J. Appl. Phys. 47, 7590–7594 (2008).
Cox, D. E. & Sleight, A. W. Crystal structure of Ba2Bi3+Bi5+O6 . Solid State Commun. 19, 969–973 (1976).
Bunker, G. Theory in Introduction to XAFS 106–133 (Cambridge University Press, 2010).
Stern, E. A., Sayers, D. E. & Lytle, F. W. Extended x-ray-absorption fine-structure technique. III. Determination of physical parameters. Phys. Rev. B 11, 4836–4846 (1975).
Zabinsky, S. I., Rehr, J. J., Ankudinov, A., Albers, R. C. & Eller, M. J. Multiple-scattering calculations of x-ray-absorption spectra. Phys. Rev. B 52, 2995–3009 (1995).
Ishimatsu, N. et al. α-ε transition pathway of iron under quasihydrostatic pressure conditions. Phys. Rev. B 90, 014422 (2014).
Miyanaga, T., Diop, D., Ikeda, S. & Kon, H. Study of the local structure changes in PbTiO3 by Pb L III EXAFS. Ferroelectrics 274, 41–53 (2002).
Burn, I. Mn-doped polycrystalline BaTiO3 . J. Mater. Sci. 14, 2453–2458 (1979).
Kirianov, A., Ozaki, N., Ohsato, H., Kohzu, N. & Kishi, H. Studies on the solid solution of Mn in BaTiO3 . Jpn. J. Appl. Phys. 40, 5619–5623 (2001).
Kwei, G. H., Billinge, S. J. L., Cheong, S.-W. & Saxton, J. G. Pair-distribution functions of ferroelectric perovskites: Direct observation of structural ground states. Ferroelectrics 164, 57–73 (1995).
Page, K., Kolodiazhnyi, T., Proffen, T., Cheetham, A. K. & Seshadri, R. Local structural origins of the distinct electronic properties of Nb-substituted SrTiO3 and BaTiO3 . Phys. Rev. Lett. 101, 205502 (2008).
Desu S. B. & Subbarao, E. C. Effect of oxidation states of Mn on the phase stability of Mn-doped BaTiO3 . Ferroelectrics 31, 665–668 (1981).
Muller, K. A., Berlinger, W. & W. Blazey, K. Electron paramagnetic resonance of Mn4+ in BaTiO3 . Solid State Commun. 61, 21–25 (1987).
Hagemann, H.-J. & Ihrig, H. Valence change and phase stability of 3d-doped BaTiO3 annealed in oxygen and hydrogen. Phys. Rev. B 20, 3871–3878 (1979).
Moriwake, H., Fisher, C. A. J. & Kuwabata, A. First-principles calculations of electronic structure and solution energies of Mn-doped BaTiO3 . Jpn. J. Appl. Phys. 49, 09MC01 (2010).
Nossa, J. F., Noumov, I. I. & Cohen, R. E. Effects of manganese addition on the electronic structure of BaTiO3 . Phys. Rev. B 91, 214105 (2015).
Valant, M. et al. The origin of magnetism in Mn-doped SrTiO3 . Adv. Funct. Mater. 22, 2114–2122 (2012).
Cox, P. A. Insulating oxides in Transition Metal Oxides: An Introduction to their Electronic Structure and Properties 100–156 (Oxford University Press, 1998).
Seshadri, R. & Hill, N. Visualizing the role of Bi 6s “lone pairs” in the off-center distortion in ferromagnetic BiMnO3 . Chem. Mater. 13, 2892–2899 (2001).
Kanungo, S. & Saha-Dasgupta, T. Pressure-driven changes in electronic structure of BiCoO3 . Phys. Rev. B 83, 104104 (2011).
Cohen, R. E. Origin of ferroelectricity in perovskite oxides. Nature 358, 136–138 (1992).
Kuroiwa, Y. et al. Evidence for Pb-O covalency in tetragonal PbTiO3 . Phys. Rev. Lett. 87, 217601 (2001).
Tanaka, H., Kuroiwa, Y. & Takata, M. Electrostatic potential of ferroelectric PbTiO3: Visualized electron polarization of Pb ion. Phys. Rev. B 74, 172105 (2006).
Dunitz, J. D. & Orgel, L. E. Electronic properties of transition-metal oxides-I: Distortions from cubic symmetry. J. Phys. Chem. Solids 3, 20–29 (1957).
Rodríguez-Carvajal, J. et al. Neutron-diffraction study of the Jahn-Teller transition in stoichiometric LaMnO3 . Phys. Rev. B 57, R3189–R3192 (1998).
Åsbrink, S., Waśkowska, A., Gerward, L., Olsen, J. S. & Talik, E. High-pressure phase transition and properties of spinel ZnMn2O4 . Phys. Rev. B 60, 12651–12656 (1999).
Nishibori, E. et al. The large Debye-Scherrer camera installed at SPring-8 BL02B2 for charge density studies. Nucl. Instrum. Methods Phys. Res. A 467–468, 1045–1048 (2001).
Osaka, K. et al. The advanced automation for powder diffraction toward industrial application. AIP Conf. Proc. 1234, 9–12 (2010).
Izumi, F. & Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 130, 15–20 (2007).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658 (2008).
Honma, T. et al. Full-automatic XAFS measurement system of the Engineering Science Research II beamline BL14B2 at SPring-8. AIP Conf. Proc. 1234, 13–16 (2010).
Yabuta, H. et al. Microstructure of BaTiO3-Bi(Mg1/2Ti1/2)O3-BiFeO3 piezoelectric ceramics. Jpn. J. Appl. Phys. 51, 09LD04 (2012).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for x-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Yoneda, Y., Matsumoto, N., Furukawa, Y. & Ishikawa, T. Fixed-height exit bender of synchrotron x-rays above 40 keV. J. Synchrotron Rad. 8, 18–21 (2001).
Farrow, C. L. et al. PDFfitt2 and PDFgui: computer programs for studying nanostructure in crystals. J. Phys.: Condens. Matter 19, 335219 (2007).
Acknowledgements
XAFS measurement and X-ray powder diffraction for Rietveld analysis were performed at BL14B2 (proposal Nos 2013B1877 and 2014A1537) and BL19B2 (proposal Nos 2013B1811 and 2014A1874), respectively, in SPring-8. High-energy X-ray diffraction for PDF analysis was carried out at JAEA beamline BL14B1 in SPring-8 (proposal No. 2014B3625) under the Shared Use Program of JAEA Facilities (proposal No. 2014B-E16), and magnetisation measurements using SQUIDs were conducted in IMS/NINS and JAIST, supported by Nanotechnology Platform Program (Advanced characterization) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We would like to thank Dr. Tetsuo Homma, Dr. Hironobu Ofuchi for XAFS measurement, Dr. Keiichi Osaka for X-ray powder diffraction measurement, Prof. Hideo Iwasaki, and Dr. Motoyasu Fujiwara for use of SQUID magnetometers. We also thank Masatoshi Watanabe and Tatsuya Suzuki for electron microscope observations.
Author information
Authors and Affiliations
Contributions
H.Y. performed most of the experiments and characterisation of materials. H.T. and T.F. processed samples for electrical measurement and measured some electrical properties. T.W. and M.K. supported the XAFS and powder X-ray diffraction experiments. Y.Y. conducted high-energy X-ray diffraction experiments and processed the diffraction data to PDF data. H.Y. wrote the manuscript, and T.I. discussed and directed this work. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yabuta, H., Tanaka, H., Furuta, T. et al. Enhancement of tetragonal anisotropy and stabilisation of the tetragonal phase by Bi/Mn-double-doping in BaTiO3 ferroelectric ceramics. Sci Rep 7, 45842 (2017). https://doi.org/10.1038/srep45842
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45842
This article is cited by
-
Synthesis and physical properties of the ferroelectromagnetic composites (1 − x)PbMn1/3Ta2/3O3–xPbTiO3
Applied Physics A (2021)
-
Direct correlation between the band gap and dielectric loss in Hf doped BaTiO3
Journal of Materials Science: Materials in Electronics (2019)
-
Direct evidence of correlation between the second harmonic generation anisotropy patterns and the polarization orientation of perovskite ferroelectric
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.