Abstract
Heart rhythm complexity analysis has been shown to have good prognostic power in patients with cardiovascular disease. The aim of this study was to analyze serial changes in heart rhythm complexity from the acute to chronic phase of acute myocardial infarction (MI). We prospectively enrolled 27 patients with anterior wall ST segment elevation myocardial infarction (STEMI) and 42 control subjects. In detrended fluctuation analysis (DFA), the patients had significantly lower DFAα2 in the acute stage (within 72 hours) and lower DFAα1 at 3 months and 12 months after MI. In multiscale entropy (MSE) analysis, the patients had a lower slope 5 in the acute stage, which then gradually increased during the follow-up period. The areas under the MSE curves for scale 1 to 5 (area 1–5) and 6 to 20 (area 6–20) were lower throughout the chronic stage. Area 6–20 had the greatest discriminatory power to differentiate the post-MI patients (at 1 year) from the controls. In both the net reclassification improvement and integrated discrimination improvement models, MSE parameters significantly improved the discriminatory power of the linear parameters to differentiate the post-MI patients from the controls. In conclusion, the patients with STEMI had serial changes in cardiac complexity.
Similar content being viewed by others
Introduction
Acute myocardial infarction (AMI) is one of the leading causes of mortality and morbidity worldwide. After the acute phase of myocardial infarction (MI), neurohormonal and mechanical stimuli lead to progressive left ventricular (LV) remodeling1. Thus, post-MI LV remodeling is an arrhythmic complication and it is associated with post-MI heart failure and the long-term prognosis of MI2,3.
Analysis of variations in heart rate oscillation, also known as heart rate variability (HRV), is commonly used to assess the autonomic nervous system due to its simple and noninvasive approach4. HRV has also been used to predict the outcomes of patients with cardiovascular disease5. In recent years, newer methods of heart rhythm complexity analysis have been developed based on non-linear signal modeling6. The principle of heart rhythm complexity analysis is based on the assumption that a healthy system exerts complex control over time to maintain operation in an ever-changing environment7,8. In a living system, many physiological signals (e.g. heart rate) exhibit irregular fluctuations which are neither random nor rigidly regular under normal conditions9. In contrast, these fluctuations exert a complex and dynamic pattern over multiple time scales. The complexity of physiological signal fluctuations is thought to be due to system adaptability in response to internal and external changes, and is one of the most important characteristics of a healthy living system6.
In heart rate dynamics, several non-linear methods have been proposed to quantify complexity. Peng and coworkers introduced detrended fluctuation analysis (DFA), which can be used to estimate whether fluctuations in small time scales are statistically equivalent to those in large time scales (called self-similarity). When heart rhythm is observed over longer periods of time, fluctuations in stride intervals exhibit long-range, fractal like correlations10 which may be due to the influence of multiple control systems. DFA is derived from fractal analysis and is suitable for scale-invariant signals11. Compared to conventional methods (e.g. spectral analysis), DFA not only allows for the detection of long-range power-law correlations embedded in a seemingly non-stationary time series, but also avoids the false detection of apparent long-range correlations that are an artifact of non-stationarity10. The DFA method has been successfully applied in heart rhythm research12,13. In addition, complexity in heart rate dynamics can be also estimated by entropy. Approximate entropy and sample entropy were first used to quantify changing complexity by calculating the rate of new information generation14,15. Later, taking into account the multiple time scales in physical systems, Costa et al. introduced the multiscale entropy (MSE) approach to represent the complexity in a system8. MSE quantifies whether the richness of information content (certain degree of unpredictability) can be maintained over different scales. Since its introduction, MSE has become an important tool to quantify signal complexity16. MSE has been widely applied in various diseases such as sepsis, heart failure, stroke, primary aldosteronism and critical illnesses which cause the “breakdown” of complexity in heart rate dynamics to capture the picture of changes in complexity17,18,19,20,21. In recent years, several refinements and adjustments of entropy or MSE have been introduced. For instance, fuzzy entropy uses the soft and continuous boundaries of fuzzy functions to measure physiological signals which include unpredictable and irregular properties22. Valencia et al. proposed the refined MSE that can improve the complexity evaluation23. The refined MSE approach uses a coarse graining method to reduce the signal of variance and apply a finite-impulse response filter to remove the fast temporal scale. Furthermore, in order to measure the complexity of data over a short period of time, short-term MSE and distribution entropy have shown advantages in small data series24,25. However, in general, DFA and MSE are the most commonly used non-linear methods to quantify complexity in heart rate dynamics in clinical research17,21,26.
Compared to traditional HRV parameters, heart rhythm complexity analysis has been shown to have a better prognostic power in patients with cardiovascular disease21,27. In patients with heart failure, both DFA and MSE have been shown to be useful in predicting survival21,27,28. In addition, DFAα1 was shown to be an important predictor of prognosis in two large clinical studies27,28. In the DIAMOND-CHF trial, after adjusting for clinical parameters, DFAα1 but not linear parameters remained an independent predictor of mortality27. Furthermore, in our previous study, we showed that MSE had a better prognostic power than other linear and non-linear parameters including DFAα121.
In patients with MI, DFA has also been shown to provide useful information on survival and to predict ventricular tachyarrhythmia in patients with AMI, and post-MI survival in those with depressed LV function29,30,31. However, data on MSE in patients with AMI are lacking. In addition, LV remodeling occurs after the acute phase of MI, which may then lead to heart failure1. It would therefore be interesting to know when changes in heart rhythm complexity occur and the associated factors. In addition, a study investigating serial changes in heart rhythm complexity may help to elucidate the mechanism by which heart rhythm complexity occurs. Furthermore, unrecognized MI or silent MI is not uncommon and can be hard to detect32. If heart rhythm complexity damage persists after an MI attack, it may serve as a tool to detect silent MI. Therefore, the aims of this study were to: (1) analyze a series of heart rhythm complexity changes from the acute to chronic phase of AMI; (2) investigate the factors potentially associated with changes in heart rhythm complexity; and (3) test the value of heart rhythm complexity as a tool to detect post-MI patients.
Results
Patient characteristics
A total of 27 patients with ST-segment elevation myocardial infarction (STEMI) and 42 control subjects were enrolled in this study (Table 1). The STEMI patients had significantly higher levels of triglycerides and fasting glucose than the controls, however there were no significant differences in age, gender, or levels of serum creatinine, total cholesterol, low-density lipoprotein and high-density lipoprotein. During the study period, none of the STEMI patients died or required un-scheduled admissions, and none developed heart failure symptoms.
Serial changes in echocardiographic data and plasma biomarkers in the STEMI patients
The patients had a higher LV end-systolic volume index and lower LV ejection fraction (LVEF) than the controls from day 1 to 1 year after MI, with a median LVEF of 55% at 1 year after MI (Table 2). The LV end-diastolic volume index of the patients was higher from 3 months to 1 year after MI. With regards to biomarkers, the patients had significantly higher levels of tissue inhibitor of metalloproteinase-1 (TIMP-1) and N-terminal pro-brain natriuretic peptide (NT-proBNP) on day 3 after MI than the controls. However, this difference became insignificant at 3 months, 6 months and 1 year after MI (Tables 2 and 3).
Linear analysis of the study subjects
Seven conventional HRV metrics were evaluated. The patients had a significantly lower mean NN, standard deviation of normal to normal R peak (SDNN), and low frequency (LF) than the controls in the acute stage. However, there were no significant differences between the patients and controls at 3 months, 6 months and 1 year after MI. There were no significant differences in the other four parameters between the controls and patients at any stage (Table 4).
Nonlinear analysis of the study subjects
With regards to the variables associated with DFA, the fractal correlation of the long-term (α2) exponent, expressed in the acute stage, was significantly lower in the patients compared to the controls, but not in the later chronic stage (3 months, 6 months and 1 year after MI). The fractal correlation of the short-term (α1) was significantly lower at 3 months and 1 year, but the short-term (α1) was not significantly different during the acute stage and 6 months after MI (TAble 4).
We then visually inspected all of the MSE curves, which represented the complexity in short and long time scales (Fig. 1). In the chronic stage (3 months, 6 months and 1 year after MI), all of the average MSE curves had a pattern of an initial decrease. In the acute stage, the patients had significantly lower slopes 1–5 than the controls, and this difference remained significant at 3 and 6 months after MI, becoming insignificant at 1 year after MI. The patients had significantly lower area 1–5 and area 6–20 than the controls at 3 months, 6, months and 1 year after MI.
Comparisons of all linear and non-linear parameters to differentiate the asymptomatic post-MI patients from the controls
To test the ability of the linear and non-linear parameters to detect the post-MI patients (1 year after MI) from the controls, we performed receiver operating characteristic (ROC) curve analysis, in which area 6–20 had the greatest discriminatory power for the two groups compared to all other linear and non-linear parameters. The areas under the curves (AUC) of mean NN, SDNN, percentage of absolute differences in normal RR intervals greater than 20 ms (pNN20), percentage of absolute differences in normal RR intervals greater than 50 ms (pNN50), LF, high frequency (HF), LF/HF, DFAα1, DFAα2, slope 1–5, area 1–5, and area 6–20, were 0.529, 0.546, 0.519, 0.586, 0.484, 0.589, 0.652, 0.701, 0.617, 0.660, 0.707, and 0.735, respectively. Figure 2 shows the ROC curves of parameters with an AUC larger than 0.6.
The advantage of adding non-linear parameters to linear parameters to discriminate the asymptomatic post-MI patients from the controls
In both net reclassification improvement (NRI) and integrated discrimination improvement (IDI) models, DFAα1, area 1–5, and area 6–20 significantly improved the discriminatory power of SDRR, pNN20, pNN50, LF, HF, and the ratio between LF and HF components (LF/HF). Slope 1–5 significantly improved the discriminatory power of pNN20 and LF in the NRI model (Table 5).
Discussion
There are five major findings of this study. First, in linear analysis, the STEMI patients had a significantly lower SDNN in the acute stage but not in the chronic stage. Second, in DFA, the patients had a significantly lower DFAα2 in the acute stage and lower DFAα1 at 3 months and 12 months after MI. Third, in MSE, the patients had smaller 1–5 and 6–20 areas throughout the chronic stage but not in the acute stage. In addition, the patients had a lower slope 1–5 in the acute stage which then gradually increased over the follow-up period. Fourth, area 6–20 had the greatest discriminatory power to detect the post-MI patients. Fifth, the cardiac complexity parameters improved the discriminatory power of the linear parameters to differentiate the post-MI patients from the controls.
In this study, we investigated serial changes in heart rhythm complexity in patients with MI using MSE and DFA. MSE was specifically developed to analyze heterogeneous complexity, and it has been shown to be able to extend the traditional entropy algorithm to quantify the richness of information over multiple time scales in physiological systems in recent years33. However, such complex structures have been shown to “break down” in patients with heart failure and other critical illnesses, and may be further affected in those with a poor prognosis19,21. In our previous studies, we found that MSE had the best prognostic power in patients with heart failure21 and critical illnesses requiring extracorporeal life support19. Furthermore, the usefulness of MSE is not limited to cardiovascular diseases, as it has also been shown to be able to predict the outcomes of patients with severe trauma requiring treatment in an intensive care unit34, the neurological outcomes of stroke patients17, and the clinical outcomes of patients with sepsis18. However, no previous study has reported the results of MSE analysis in patients with AMI, and to the best of our knowledge, this is the first study to use MSE to evaluate heart rhythm complexity in patients with AMI. Our results showed that the patients had significantly decreased MSE parameters including area 1–5 and area 6–20 from 3 months to 1 year after AMI. In addition, in ROC curve analysis, area 6–20 had the greatest discriminatory power to differentiate the post-MI patients from the controls. Moreover, the MSE parameters (area 1–5 and area 6–20) significantly improved the prognostic power of SDRR, pNN20, and pNN50 in both the NRI and IDI models. This demonstrates the additive effect of MSE parameters on traditional linear parameters to differentiate post-MI patients from normal subjects.
After the acute phase of MI, LV remodeling stress occurs under a series of neurohormonal and mechanical stimuli35, and post-MI LV stress and remodeling are the major determinants of post-MI heart failure and long-term prognosis2,3. In the present study, LV remodeling was demonstrated by increased LV chambers. In serial echocardiography, the LV end-diastolic volume index increased significantly from 3 months after MI and then throughout the follow-up period. In LV function analysis, the patients had a significantly lower LVEF than the controls from the acute to chronic stage. However, the median LVEF at 1 year post-MI was 55%, which was only mildly decreased. In addition, none of the patients developed heart failure symptoms during the follow-up period, and the NT-proBNP levels were comparable to the controls from 3 months after MI. Furthermore, the patients had comparable LF and LF/HF ratio to the controls in the chronic stage. These findings suggest that our patients recovered well after MI without serious complications in the clinical course, autonomic system modulation or LV function in the chronic stage. However, in MSE analysis, areas 1–5 and 6–20 significantly decreased from 3 months to 1 year post-MI, implying the close relationship between LV remodeling and changes in MSE parameters. This supports our hypothesis that MSE parameters may serve as a screening tool in clinical practice to detect post-MI patients without heart failure.
In the present study, we found different patterns of changes in DFA in the short- and long-term exponents. The patients had a significantly lower DFAα2 in the acute stage and lower DFAα1 at 3 months and 12 months after MI. In the patients, DFAα1 in the acute stage provided useful information on survival. In a sub-study of the DIAMOND MI trial, Huikuri reported that DFAα1 < 0.75 (recorded 5 to 10 days after MI) was the most powerful predictor of all-cause mortality after adjusting for other clinical parameters including age, LVEF, NYHA class, and medications29. In another study, Tapanainen et al. reported that DFAα1 < 0.65 (recorded during the first 7 days after AMI) was the most powerful predictor of mortality among AMI survivors36. In addition, Perkiomaki et al. reported that DFAα1 < 1.025 (recorded during the first 7 days after AMI) was a significant predictor of non-fatal acute coronary syndrome in patients with AMI37. Although a low DFAα1 in the acute stage of AMI is considered to be a poor prognostic factor, no study has compared DFA findings in such patients to normal subjects. Interestingly, in the present study, the patients had a significantly lower DFAα2 but comparable DFAα1 to the normal controls in the acute stage with a median DFAα1 of 0.99, which is similar to a previous study by Carvalho et al.38 and also similar to AMI patients who remained alive at the end of follow-up in the DIAMOND MI study29. In the present study, the median peak creatine kinase and creatine kinase-MB levels were 2452 and 208 U/L, respectively. In addition, none of the patients died or developed heart failure during 1 year of follow-up. These findings suggest that most patients had a small to medium sized MI, and that all of the patients recovered uneventfully. This may explain the relatively normal DFAα1 value during the acute stage in the STEMI patients.
The DFAα1 value decreased to 0.85 at 1 year post-MI, which is close to the value in patients with heart failure39. To date, only one study (OAT-EP) has investigated changes in DFA between the subacute stage (3–28 days, median 12 days) and 1 year after MI. In that study, no significant changes in DFAα1 were noted between the subacute stage and 1 year in both medication and intervention groups. In the present study, DFAα1 during the acute stage was not significantly lower than that in the controls. In contrast, DFAα1 after 1 year in the patients was significantly lower than that in the controls, and the difference in DFAα1 between the acute stage and 1 year was borderline significant (p = 0.059, data not shown). The discrepancy between the two studies may be due to differences in Holter sampling time points (i.e. when the Holter signals were recorded), sampling duration, and reperfusion time and strategy. In the OAT-EP study, DFAα1 at 1 year was very close to 1 (mean 1.02 in the medical group and 1.06 in the intervention group), which is closer to normal subjects rather than patients with heart failure27,38. Serial changes in DFAα1 and their clinical implications should be confirmed by larger prospective studies with a longer follow-up period and multiple check-ups.
There are several limitations to this study. First, this is a small pilot study with multiple Holter sampling points, and the findings should be confirmed by a larger clinical study. Second, in order to avoid the effect of Bezold-Jarisch reflexes on heart rhythm complexity analysis40, we only enrolled STEMI patients with anterior wall infarction in which the infarct-related artery was ultimately the left anterior descending artery. Therefore, the results cannot be applied to STEMI patients with right coronary artery or left circumflex occlusion. Third, possible confounding factors such as different levels of physical activity during the recording period41, or different recording periods chosen for analysis among the study subjects may also have affected the results. Of note, the STEMI patients usually had strict restrictions on physical activity (they were mostly required to remain in bed) during the acute stage, whereas there were no such restrictions in physical activity in the patients with other stages of STEMI or in the control group. This is a potential confounding factor. Fourth, because of the limited number of participants in this small study, we only used some linear and non-linear parameters. In addition, several refinements and adjustments of entropy or MSE have been introduced in recent years, such as fuzzy approximate entropy, distribution entropy, refined composite MSE, modified MSE, multi-scale symbolic entropy, and generalized MSE16,19,22,23,25,42,43,44,45. A larger study may be needed to investigate and compare the usefulness of other linear parameters, non-linear parameters, and new MSE refinements. Fifth, we found a V-shape in shorter MSE scales in the chronic stage in STEMI patients. The linear slope (slope 1–5) may not be the best model for this curve.
In conclusion, the patients with STEMI had different changes in cardiac complexity in the acute and chronic stages. Cardiac complexity parameters are useful to detect post-MI patients without heart failure.
Methods
Patients
We enrolled patients with a first STEMI who were treated within 12 hours after the onset of symptoms and who received a primary percutaneous coronary intervention. Another group of patients who were admitted for coronary angiography with no signs of significant stenosis were enrolled as the controls. Patients with a history of chronic atrial fibrillation, sick sinus syndrome, high degree conduction block, cardiovascular diseases including coronary artery disease, myocardial infarction, heart failure, cerebrovascular events, or peripheral artery disease were excluded.
In the patients, echocardiographic studies were performed at day 1 (first 24 hours after symptom onset), 3 months post-MI (90 ± 14 days after MI), 6 months post-MI (180 ± 28 days after MI), and 1 year post-MI (365 ± 28 days after MI). Blood samples were taken on day 3 (48–72 hours after symptom onset), 3 months post-MI (90 ± 14 days after MI), 6 months post-MI (180 ± 28 days after MI) and 1 year post-MI (365 ± 28 days after MI). In the control group, blood samples were taken on the morning of the coronary angiogram check-up, and echocardiographic studies were performed during the admission.
All of the patients underwent 24-h ambulatory ECG Holter recording (MyECG E3-80, Microstar Company, Taipei) during the acute stage (within 72 hours after symptom onset), 3 months post-MI (90 ± 14 days after MI), 6 months (180 ± 28 days after MI), and 1 year (365 ± 28 days after MI). The control group underwent 24-h ambulatory ECG Holter recording on the day before angiography.
This study was approved by the Institutional Review Board of National Taiwan University Hospital, and all subjects provided written informed consent including for storage of their information in the hospital database and usage for research. The methods of this study were carried out in accordance with the approved guidelines.
Echocardiography
Standard transthoracic echocardiography (iE33 xMATRIX Echocardiography System, Philips, Amsterdam, Netherlands) was performed in each patient. The echocardiographic measurements included two-dimensional, M-mode and Doppler ultrasound recordings. LVEF measurements were based on an apical 4-chamber view measured using the area-length method or by M-mode via the parasternal long axis view (if there were no regional wall abnormalities) in accordance with the recommendations of the American Society of Echocardiography46.
Data pre-processing
All of the patients underwent 24-h ECG monitoring with a sampling rate of 250 Hz, and the data were stored on a secure digital memory card for offline analysis on a computer. Each digitized ECG data point was annotated using an automatic algorithm, and then carefully inspected and corrected by technicians to obtain the RR intervals, and ectopic beats were interpolated by the adjacent RR intervals. Four hours of ECG data within the daytime were selected for analysis in order to avoid the confounding effects that may occur due to sleep or diurnal rhythm47. To avoid the interference of physical activity on the analysis, the 4-hour period of RR intervals we selected in this study was based on the following criteria: (1) between 9 AM–6 PM; and (2) without sudden increases in heart rate of more than 40 bpm within 1 minute. In linear analysis, the ECG data were calculated from 5-minute segments averaged over the entire 4-hour period. In addition, two nonlinear methods, MSE and DFA, were used to analyze the sequence of NN intervals over the entire 4-hour period of ECG data.
Linear analysis
HRV was calculated from the 4-hour ECG data in the time and frequency domains according to the guidelines developed by the Task Force of the European Society of Cardiology48. In this study, we computed four time domain metrics: (1) the mean of normal to normal R peak, reflecting the mean value of RR interval time series; (2) SDNN, reflecting the overall magnitude of variability; (3) pNN20, the percentage of absolute differences in normal RR intervals greater than 20 ms; (4) pNN50, the percentage of absolute differences in normal RR intervals greater than 50 ms. pNN20 and pNN50 were used to evaluate the function of the autonomic system49. The frequency domain metrics were included in band-HF (0.15–0.4 Hz), LF (0.04–0.15 Hz), and LF/HF, which were calculated using the average power spectrum.
Nonlinear methods
Two non-linear methods, MSE and DFA, were used to probe the fundamental characteristics of the ECG data. Both MSE and DFA can be used to quantify heartbeat complexity and variability in heartbeat time series.
MSE
MSE analysis can be used to estimate the physiological signals in different time scales and evaluate the predictability of a time series33. The MSE analysis consisted of two steps: (1) coarse-graining of the signals into different time scales; (2) quantifying the degree of irregularity in each coarse-grained time series using sample entropy50. In order to construct a coarse-grained series to analyze time series data, the data were first divided into N/τ non-overlapping windows of length τ. Finally, the data points within each window were averaged. For a given time series {1 ≤ j ≤ N}, the coarse-grained series was calculated as follows:

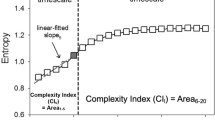
where τ is the scale factor and 1 ≤ j ≤ N/τ. In addition, the complex pattern of a time series was divided into long- and short-term curves. In this study, the different scales were useful for clinical categorization and the MSE curve up to a scale factor of 20. This scale factor was then divided into two types of parameters: (1) the first five scales were defined as the short-fitted slope (slope 1–5); and (2) the first five scales were defined as a short-fitted area (areas 1–5), and the final 15 scales were defined as the long-fitted area (area 6–20, Fig. 3). Although MSE analysis can be applied to physiological signals, the entropy values are sensitive to non-stationary artifacts, and especially trends. Hence, detrending can attenuate the spurious influence prior to MSE analysis51. In this study, we used empirical mode decomposition as an adaptive filter and eliminated the intrinsic mode functions slower than predefined time durations by matching their temporal frequency distribution (e.g. 2 hours−1) in the original R-R interval signals. The remaining intrinsic mode functions were summed up to reconstruct the signals without the trend or component slower than the predefined time duration52.
The common profile of entropy gradually increased as the time scale increased and reached a plateau where information richness could be accumulated rapidly if the system responded well. Three parameters of the MSE were assessed: (1) the linear-fitted slope between scales 1–5 (slope 1–5); (2) the area under the curve between scales 1–5 (area 1–5), which was used to represent complexity between short scales; (3) the area under curve between scales 6–20 (area 6–20), which was used to represent complexity between long scales.
The underlying mechanism for short-term scales of RR intervals can be mainly attributed to respiratory sinus arrhythmia (RSA) modulated by the parasympathetic system. Therefore, slope 1–5 and area 1–5 of MSE were quantitative estimations of information richness on that particular mechanism. Area 1–5 probes the complexity structure of heart rate dynamics entrained by RSA, and a decreased area 1–5 of RR intervals has been reported in the elderly as well as in heart failure patients compared to young individuals in previous studies21,33. Slope 1–5 outlines the structure of heart rate dynamics, and a negative slope has been observed in heart failure and critically ill patients, indicating highly irregular but less information richness structure (i.e. uncorrelated fluctuations with the loss of feedback interactions)8,19,21. Similarly, the information richness structure of scales longer than RSA can also be quantified by the summation of specific scales. However, the underlying mechanisms accounting for the indexes of long-term scales are mostly unclear, since several physiological mechanisms exist beneath the time scales such as baroreflex and the hormonal system (e.g. aldosterone)20. It is still difficult to establish a standard to simply target one specific mechanism over a range of time-scales. Therefore, we used area 6–20 to estimate the overall long-term complexity.
DFA
DFA can be used to evaluate the fractal correlation coefficient in a heart rate time series. In general, fluctuations in heart rate originate from interactions of regulatory mechanisms. To calculate the scaling exponents in DFA, a given time series x(i), 1 ≤ i ≤ N, should first be integrated as follows:

where xave is the mean of the time series x(i) and the integrated time series is divided into boxes of equal length n. Each box contains a least square line to fit the divided time series, and the time series can be detrended by removing the linear-fitted “local” trend. The root-mean-square fluctuation of the integrated time series can then be obtained as follows:

where the average fluctuations F(n) are represented as a function of the box size. The average fluctuations of the data sets are repeated in different time scales, and then a crossover phenomenon exists between the short- and long-term scales. The short-term (4–11 beats) and long-term (11–64 beats) fractal correlation exponents were calculated to obtain a clearer understanding of the fractal correlation property in a physiological system53. The short-term (α1) and long-term (α2) fractal correlations were calculated.
Laboratory analysis
Serum biomarker analysis was performed in 17 STEMI patients and 37 control patients. Blood was drawn into tubes containing sodium citrate. After centrifugation, plasma was collected and stored at −80 °C until analysis. Plasma TIMP-1 was measured using an enzyme immunoassay kit (DTM100, R & D Systems, Minneapolis, USA). The intra- and inter-assay variations were <5%, and the detection limit of this method was 0.08 ng/ml. Plasma NT-proBNP was measured using an enzyme immunoassay kit (SEA485Hu, USCN Life Science, Inc., Houston, TX, USA). The intra-assay variation was <10% and inter-assay variation was <12%, and the range of detection was 39.06–2500 pg/mL.
Statistical analysis
All of the data including the linear metrics, non-linear metrics and clinical data were expressed as median (25th and 75th percentiles). The ECG data and clinical characteristics of the control and STEMI groups were compared using the Mann-Whitney U test and Fisher’s exact test with relevant variables as indicated. In order to compare the ability of different Holter parameters to differentiate the STEMI patients (at 1 year after MI) from the controls, we used AUC analysis with logistic regression models. We used C-statistics to describe the discrimination of the models before and after adding non-linear parameters54,55,56.
NRI and IDI models were used to assess improvements in prediction using two different logistic regression models55. For each individual, we computed the case (STEMI patients at 1 year after MI) probability using the original model, Po, and the case probability using the updated model, Pu. The cases and controls were classified according to their Po and Pu values (Table 6).
Definitions:
p1 = Nss/Ns (increasing rate of successfully predicting cases)
p2 = Nnn/(N − Ns) (increasing rate of successfully predicting controls)
p3 = Nns/Ns (decreasing rate of successfully predicting cases) = 1 − p1
p4 = Nsn/(N − Ns) (decreasing rate of successfully predicting controls) = 1 − p2
Then NRI = p1 + p2 − p3 − p4 = 2 (p1 + p2 − 1). IDI was defined as the average improvement in case detection probability for all patients after adopting the updated model. That is,

where Poi and Pui were the case detecting probabilities using the original model and the updated model for the ith individual; i = 1, …, N.
All statistical analyses were performed using R 3.3.2 software (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p value of less than 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Chiu, H.-C. et al. Serial heart rhythm complexity changes in patients with anterior wall ST segment elevation myocardial infarction. Sci. Rep. 7, 43507; doi: 10.1038/srep43507 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wang, T. J. et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 108, 977–982, doi: 10.1161/01.cir.0000085166.44904.79 (2003).
Eschalier, R. et al. Extracellular matrix turnover biomarkers predict long-term left ventricular remodeling after myocardial infarction: insights from the REVE-2 study. Circulation. Heart failure 6, 1199–1205, doi: 10.1161/CIRCHEARTFAILURE.113.000403 (2013).
St John Sutton, M. et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 89, 68–75 (1994).
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996).
Tsuji, H. et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94, 2850–2855 (1996).
Seely, A. J. & Macklem, P. T. Complex systems and the technology of variability analysis. Crit Care 8, R367–384, doi: 10.1186/cc2948 (2004).
Yuan, H. K. et al. Acute increase of complexity in the neurocardiovascular dynamics following carotid stenting. Acta Neurol Scand 123, 187–192, doi: 10.1111/j.1600-0404.2010.01384.x (2011).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 89, 068102, doi: 10.1103/PhysRevLett.89.068102 (2002).
Lo, M. T. et al. Outlier-resilient complexity analysis of heartbeat dynamics. Sci Rep 5, 8836, doi: 10.1038/srep08836 (2015).
Peng, C. K., Havlin, S., Stanley, H. E. & Goldberger, A. L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82–87, doi: 10.1063/1.166141 (1995).
Sassi, R. et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 17, 1341–1353, doi: 10.1093/europace/euv015 (2015).
Stein, P. K. et al. Novel measures of heart rate variability predict cardiovascular mortality in older adults independent of traditional cardiovascular risk factors: the Cardiovascular Health Study (CHS). J Cardiovasc Electrophysiol 19, 1169–1174, doi: 10.1111/j.1540-8167.2008.01232.x (2008).
Ho, K. K. et al. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation 96, 842–848 (1997).
Pincus, S. M. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 88, 2297–2301 (1991).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278, H2039–2049 (2000).
Humeau-Heurtier, A. The multiscale entropy algorithm and its variants: A review. Entropy 17, 3110–3123 (2015).
Tang, S. C. et al. Complexity of heart rate variability predicts outcome in intensive care unit admitted patients with acute stroke. J Neurol Neurosurg Psychiatry 86, 95–100, doi: 10.1136/jnnp-2014-308389 (2015).
Ahmad, S. et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 4, e6642, doi: 10.1371/journal.pone.0006642 (2009).
Lin, Y. H. et al. Multi-scale symbolic entropy analysis provides prognostic prediction in patients receiving extracorporeal life support. Crit Care 18, 548, doi: 10.1186/s13054-014-0548-3 (2014).
Lin, Y. H. et al. Reversible heart rhythm complexity impairment in patients with primary aldosteronism. Sci Rep 5, 11249, doi: 10.1038/srep11249 (2015).
Ho, Y. L., Lin, C., Lin, Y. H. & Lo, M. T. The prognostic value of non-linear analysis of heart rate variability in patients with congestive heart failure--a pilot study of multiscale entropy. PLoS One 6, e18699, doi: 10.1371/journal.pone.0018699 (2011).
Chen, W., Zhuang, J., Yu, W. & Wang, Z. Measuring complexity using FuzzyEn, ApEn, and SampEn. Med Eng Phys 31, 61–68, doi: 10.1016/j.medengphy.2008.04.005 (2009).
Valencia, J. F. et al. Refined multiscale entropy: application to 24-h Holter recordings of heart period variability in healthy and aortic stenosis subjects. IEEE Trans Biomed Eng 56, 2202–2213, doi: 10.1109/TBME.2009.2021986 (2009).
Chang, Y.-C. et al. Application of a Modified Entropy Computational Method in Assessing the Complexity of Pulse Wave Velocity Signals in Healthy and Diabetic Subjects. Entropy 16, 4032 (2014).
Li, P. et al. Assessing the complexity of short-term heartbeat interval series by distribution entropy. Med Biol Eng Comput 53, 77–87, doi: 10.1007/s11517-014-1216-0 (2015).
Lin, Y. H. et al. Heart rhythm complexity impairment in patients undergoing peritoneal dialysis. Sci Rep 6, 28202, doi: 10.1038/srep28202 (2016).
Makikallio, T. H. et al. Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. Am J Cardiol 87, 178–182 (2001).
La Rovere, M. T. et al. Autonomic markers and cardiovascular and arrhythmic events in heart failure patients: still a place in prognostication? Data from the GISSI-HF trial. European journal of heart failure 14, 1410–1419, doi: 10.1093/eurjhf/hfs126 (2012).
Huikuri, H. V. et al. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 101, 47–53 (2000).
Tapanainen, J. M. et al. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. The American journal of cardiology 90, 347–352 (2002).
Perkiomaki, J. S. et al. Risk factors of self-terminating and perpetuating ventricular tachyarrhythmias in post-infarction patients with moderately depressed left ventricular function, a CARISMA sub-analysis. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 13, 1604–1611, doi: 10.1093/europace/eur166 (2011).
Leening, M. J. et al. Unrecognised myocardial infarction and long-term risk of heart failure in the elderly: the Rotterdam Study. Heart 96, 1458–1462, doi: 10.1136/hrt.2009.191742 (2010).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of biological signals. Physical review. E, Statistical, nonlinear, and soft matter physics 71, 021906, doi: 10.1103/PhysRevE.71.021906 (2005).
Riordan, W. P. Jr., Norris, P. R., Jenkins, J. M. & Morris, J. A. Jr. Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. The Journal of surgical research 156, 283–289, doi: 10.1016/j.jss.2009.03.086 (2009).
Weir, R. A. et al. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circulation. Heart failure 6, 492–498, doi: 10.1161/CIRCHEARTFAILURE.112.000146 (2013).
Tapanainen, J. M. et al. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. American Journal of Cardiology 90, 347–352, doi: Pii S0002-9149(02)02488-810.1016/S0002-9149(02)02488-8 (2002).
Perkiomaki, J. S., Jokinen, V., Tapanainen, J., Airaksinen, K. E. J. & Huikuri, H. V. Autonomic markers as predictors of nonfatal acute coronary events after myocardial infarction. Ann Noninvas Electro 13, 120–129, doi: 10.1111/j.1542-474X.2008.00211.x (2008).
Carvalho, T. D. et al. Fractal correlation property of heart rate variability in chronic obstructive pulmonary disease. Int J Chronic Obstr 6, 23–28, doi: 10.2147/Copd.S15099 (2011).
Makikallio, T. H. et al. Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. American Journal of Cardiology 87, 178–182, doi: 10.1016/S0002-9149(00)01312-6 (2001).
Wei, J. Y., Markis, J. E., Malagold, M. & Braunwald, E. Cardiovascular reflexes stimulated by reperfusion of ischemic myocardium in acute myocardial infarction. Circulation 67, 796–801 (1983).
McNarry, M. A. & Lewis, M. J. Interaction between age and aerobic fitness in determining heart rate dynamics. Physiol Meas 33, 901–914, doi: 10.1088/0967-3334/33/6/901 (2012).
Wu, S.-D., Wu, C.-W., Lin, S.-G., Lee, K.-Y. & Peng, C.-K. Analysis of complex time series using refined composite multiscale entropy. Physics Letters A 378, 1369–1374 (2014).
Costa, M. D. & Goldberger, A. L. Generalized multiscale entropy analysis: application to quantifying the complex volatility of human heartbeat time series. Entropy 17, 1197–1203 (2015).
Xiong, G.-l., Zhang, L., Liu, H.-s., Zou, H.-j. & Guo, W.-z. A comparative study on ApEn, SampEn and their fuzzy counterparts in a multiscale framework for feature extraction. Journal of Zhejiang University SCIENCE A 11, 270–279 (2010).
Zhang, L., Xiong, G., Liu, H., Zou, H. & Guo, W. Applying improved multi-scale entropy and support vector machines for bearing health condition identification. Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science 224, 1315–1325 (2010).
Li, S.-W. et al. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. European Journal of Pharmacology 738, 125–132, doi: 10.1016/j.ejphar.2014.05.028 (2014).
Vanoli, E. et al. Heart-Rate-Variability During Specific Sleep Stages - a Comparison of Healthy-Subjects with Patients after Myocardial-Infarction. Circulation 91, 1918–1922 (1995).
Camm, A. J. et al. Heart rate variability - Standards of measurement, physiological interpretation, and clinical use. Circulation 93, 1043–1065 (1996).
Mietus, J. E., Peng, C. K., Henry, I., Goldsmith, R. L. & Goldberger, A. L. The pNNx files: re-examining a widely used heart rate variability measure. Heart 88, 378–380 (2002).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. American Journal of Physiology-Heart and Circulatory Physiology 278, H2039–H2049 (2000).
Costa, M. et al. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Epl 77, doi: 10.1209/0295-5075/77/68008 (2007).
Wu, Z., Huang, N. E., Long, S. R. & Peng, C. K. On the trend, detrending, and variability of nonlinear and nonstationary time series. Proc Natl Acad Sci USA 104, 14889–14894, doi: 10.1073/pnas.0701020104 (2007).
Peng, C. K., Havlin, S., Stanley, H. E. & Goldberger, A. L. Quantification of Scaling Exponents and Crossover Phenomena in Nonstationary Heartbeat Time-Series. Chaos 5, 82–87, doi: 10.1063/1.166141 (1995).
Pencina, M. J., D’Agostino, R. B. Sr., D’Agostino, R. B. Jr. & Vasan, R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27, 157–172, discussion 207–112, doi: 10.1002/sim.2929 (2008).
Steyerberg, E. W. et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21, 128–138, doi: 10.1097/EDE.0b013e3181c30fb2 (2010).
Pencina, M. J. & D’Agostino, R. B. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 23, 2109–2123, doi: 10.1002/sim.1802 (2004).
Acknowledgements
The authors thank Dr. Albert C. Yang and Mr. Brian J. Gow for their suggestions in preparing the manuscript. This study was supported by Delta Environmental and Educational Foundation, National Taiwan University Hospital (NTUH 104-S2696, UN103-065), Ministry of Science and Technology (MOST 103-2220-E-002 -011, MOST 103-2221-E-008-006-MY3), Ministry of Science and Technology support for the Center for Dynamical Biomarkers and Translational Medicine, National Central University, Taiwan (NSC 102-2911- I-008-001), and the joint foundations (grant Nos 103CGH-NCU-A1, VGHUST103-G1-3-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.H.L., H.C.C., Y.L.H. and H.P.M. conceived and designed the experiments. H.C.C., C.L., C.K.W., J.Y.C., J.K.L., L.Y.D.L., C.S.H. and T.D.W. analyzed the data. H.C.C. and Y.H.L. wrote the paper. L.Y. L., M.T.L. and C.K.P. made scientific comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chiu, HC., Ma, HP., Lin, C. et al. Serial heart rhythm complexity changes in patients with anterior wall ST segment elevation myocardial infarction. Sci Rep 7, 43507 (2017). https://doi.org/10.1038/srep43507
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43507
This article is cited by
-
Heart rhythm complexity analysis in patients with inferior ST-elevation myocardial infarction
Scientific Reports (2023)
-
Usefulness of heart rhythm complexity in heart failure detection and diagnosis
Scientific Reports (2020)
-
Heart Rhythm Complexity Impairment in Patients with Pulmonary Hypertension
Scientific Reports (2019)
-
Investigation of Ensemble Empirical Mode Decomposition Applied for Composite Multiscale Cross-Sample Entropy Analysis
Multiscale Science and Engineering (2019)
-
The association between heart rhythm complexity and the severity of abdominal aorta calcification in peritoneal dialysis patients
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.