Abstract
Alien predators have on average twice the impact on native prey populations than do native predators, and are a severe threat to wildlife globally. Manipulation experiments can be used to quantify the impact of an alien predator on its prey population/s, but unless the results are compared to benchmarks, it is unclear whether this impact is indeed greater than that of a native predator. Here we use the Australian garden skink Lampropholis delicata and alien black rat Rattus rattus to test if black rats are an additive source of predation for the skink, and to judge whether the effect size of rat-impact on the skink represents that of an alien or native predator. We used replicated experiments to exclude black rats at local and landscape scales to test how rats affect skink activity and trapping frequency. Both manipulations had positive effects on skinks, however, the population-level effect size was lower than that described for alien predators but similar to that expected for native predators. We suggest that Australian skinks may respond appropriately to predatory alien rats because they coevolved with endemic Rattus species. This adds novel insights into the varying levels of impact that alien predators have on native prey.
Similar content being viewed by others
Introduction
Established alien species can pose severe on-going threats to local and global biodiversity and ecosystem function1. The impact of alien predators, in particular, is on average twice that of native predators2, and often reduces prey to low densities that put them at risk from other extinction forces. Much of the concern about alien predators is thus driven by the abnormally high direct predation pressure from aliens rather than predation per se which is, by nature, a limiting factor for prey populations. However, alien impacts are predicted to ultimately change with time, as prey overcome their naïveté and develop responses to better deal with the tactics of alien predators3,4. Alien predator impacts are also predicted to be lower on prey that have native predators of the same archetype as the alien5. Hence proper assessment of the level of impact of an alien predator on native species is key to assessing the functional ‘alien-ness’ of an alien predator. This assessment is important before attempts are made to control or remove the predator, otherwise removal may yield little conservation benefit or even have unwanted side-effects via removal of important ecosystem processes. So how do we determine whether the impacts of an alien predator species are likely to cause conservation concern?
Effect sizes—the ratio of treatment to control responses—provide an effective technique to quantify the impact that a predator has upon a prey population. Salo et al.’s2,6 meta-analyses provide benchmarks for the effect sizes (with confidence intervals) of the impacts of alien and native predators. We suggest that these benchmarks can also be used to categorize whether predator impacts align with those of an alien predator or a native predator, and thus allow assessment of exactly where the impact of a particular alien predator lies along the native-alien continuum.
In this paper, we examine the impacts of long-established introduced rats on native reptile populations. Alien rodents are among the world’s worst invasive predators and have caused global population declines of native birds7,8 and invertebrates9,10,11, as well as extinctions of mammals12. Owing to their size, small reptiles should also be at risk from alien rodents, however, current understanding of their responses to any alien predators is limited.
Alien rodents are often implicated in declines of reptiles, especially on islands13,14,15,16, prompting rodent-eradication programs17. However, evidence for rodent-impact is tenuous. There are anecdotal accounts of reptile recovery or range expansion after removal of alien rodents (e.g. refs 13, 18, 19, 20, 21), but the precise role of rodents in these cases is often confounded by removal of other alien species, e.g. ref. 19, or lack of replication, e.g. ref. 22. These uncertainties about rodent-impacts have led to our concern that the actual impacts of alien rodents on reptiles, even on islands, are unknown.
We describe local (single tree) and landscape (1-ha grid) scale experiments that examine the effects of the alien black rat Rattus rattus on the activity and trapping frequency of an endemic Australian skink, Lampropholis delicata. Importantly, we compare the size of skink responses with benchmark effect sizes from Salo et al.2 to establish how ‘alien’ are the impacts of black rats in the study system. Black rats arrived in Australia at or after European settlement in 1788 and are now widespread in coastal areas23. They are commensal with humans and, in some areas, have also moved into peri-urban bushland remnants24. In our study system, black rats have replaced other native small mammals such as the brown antechinus Antechinus stuartii and endemic bush rat Rattus fuscipes and are likely to threaten some native wildlife species25. Lampropholis delicata (De Vis, 1888) occurs commonly in forest, woodland and suburban gardens in eastern Australia, and has a generalist diet of flies, isopods, beetles, cockroaches and other small invertebrates26. It is largely diurnal, active on the ground and the lower trunks of trees, oviparous, and lives for around 2 years in the wild27. It falls prey occasionally to black rats, occurring in ~2% of rat scats by frequency of occurrence in our study region (N. Baczocha and C. R. Dickman, unpublished data).
We predicted that if rat predation is additive in this system, there would be more sightings of L. delicata on trees in the absence than in the presence of rats, and populations of L. delicata would increase in response to rat-removal at a landscape scale. We expected that rat-removal effects would be more obvious on smooth-barked than on rough-barked trees owing to the higher exposure of skinks on smooth-barked trees. We then assessed the level of impact of black rats by calculating the experimental effect size and comparing it to benchmark effect sizes of alien predators and native predators from Salo et al.2. Because L. delicata has co-occurred with native R. fuscipes for many thousands of years and thus could be expected to have developed strategies to reduce deleterious impacts from this species cf.5, we hypothesized that the impacts of alien R. rattus on L. delicata would approximate those of a native rather than an alien predator. There are no appropriate effect size benchmarks for behavioral response experiments, so our effect size assessment is based only on the results of our landscape-scale experiment.
Results
Experiment 1: local-scale rat exclusion
We observed fewer L. delicata on control (rat-present) trees than on trees with rat-exclusion guards (Fig. 1) across our 3 sampling times: at sample time 1 we observed 68.28 ± 18.18% (mean ± SE) fewer skinks on trees with rats present compared with those on rat-excluded trees; sample times 2 and 3 revealed similar declines in skinks observed on trees with rat-access compared with those on trees with rat-exclusion guards (61.76 ± 13.13%, and 57.14 ± 27.08% reductions, respectively). The rat removal treatment significantly increased skink sightings at all sample times, but bark type and the interaction between bark type and treatment had no effect (Table 1, Fig. 2).
Experiment 2: landscape-scale rat exclusion
On average, we captured 50.4 ± 26.8% fewer skinks per site per day on unmanipulated (rat-present) sites compared to rat-removal sites, but the effect of treatment was not quite significant (F(1,6) = 5.34, p = 0.06, Fig. 3). The effect size for this experiment was 0.92.
Discussion
In this paper we tackle the question of exactly how alien a predator is in terms of its impact on native prey species. Resolution of this question is important both to inform the debate about when alien species can be considered to become native, and to assist managers in deciding whether to take action against alien species. All predators have some measurable impact on their prey populations, and alien predators appear to have, on average, twice the impact of native predators2. Yet some alien species have little more impact than their native counterparts, raising the question of whether they should continue to be considered as alien.
Here we quantified the impacts of an alien rodent species on a native reptile population and found a moderate impact. Salo et al.2 found that the typical effect size for prey response to landscape-scale predator-removals was 0.400–0.910 for native predators, and 1.224–3.046 for alien predators; see Table 12. Thus, the effect size we report here (0.92) is just outside the confidence intervals of those reported in analogous removal experiments on other alien predators, and best approximates the high-end effect size limits for a native predator. This result supports our initial hypothesis, and suggests that L. delicata responded to black rats as if they were native predators.
It is possible that the impact of R. rattus on L. delicata via the observed shifts in activity and trapping frequency could have arisen via another process. For example, if rats competitively dominate skinks or act as vectors of disease, their removal could facilitate moderate increases in skink numbers. Alternatively, the increase in skink abundance after rodent removal could equally be associated with some unexplained behavioural interaction that was not detected in this study.
Disease transfer has the potential to negatively affect immunologically naïve native wildlife, possibly even leading to extinction28, but this does not explain the shift in activity of L. delicata or the short timeframe over which we documented changes in trapping frequency. If competition occurs, it could be for food resources such as invertebrates26,29. But given that invertebrates comprise a relatively small part of the diet of black rats29, and the prey they would encounter during nocturnal foraging probably overlap little with the prey pursued by day-active skinks, this explanation seems unlikely.
For these reasons, and the occasional inclusion of small skinks in the diet of R. rattus, we are confident that predation - or some yet to be determined behavioural interaction - provides the most parsimonious explanation for our results.
Our work is the first to quantify the impacts of alien rats on reptiles using a multi-scale, manipulative, controlled and replicated experimental design, and shows that black rats affect the trapping frequency and activity of L. delicata on the ground and in trees, respectively. Despite the short duration of the study, we observed strong and consistent changes in activity levels of L. delicata when rats were excluded from trees, suggesting that black rats do indeed suppress skink activity. Bark type, however, did not appear to influence activity of skinks at the local scale, with no difference in reptile sightings on rough-barked compared with smooth-barked trees for all 3 sampling times. Moreover, predation pressure from visual predators such as pied currawongs Strepera graculina or kookaburras Dacelo novaeguineae is not sufficiently strong that skinks benefit from using rougher types of bark as better camouflage, with smooth-barked and rough-barked species being equally favorable.
While there was higher skink activity on trees in the absence than in the presence of rats, the more muted demographic response of L. delicata to rat-removal challenges the notion that the black rat always acts as an alien predator in Australia. Our interpretation is based on the demographic response of L. delicata to black rats; we did not calculate an effect size for the behavioral experiment because there are, at present, no meaningful behavioral effect-size benchmarks with which to compare. We suggest that it would be beneficial for researchers to develop behavioral effect size benchmarks for future comparisons.
The striking result that alien black rats appear to function as native predators is consistent with the hypothesis that native prey species will resist the impacts of an alien predator if they have coevolved with similar predator archetypes4,5,30, whereas evolutionarily naïve prey suffer severe impacts due to lack of effective antipredator responses, e.g. refs 31, 32, 33. Our result is also consistent with the fact that L. delicata is commonly seen in gardens and urban remnants around Sydney; L. delicata presumably maintains its numbers in the presence of alien rats by either modifying reproductive output, avoiding predation and/or making use of resources in both gardens and urban remnants.
Endemic Rattus species have been part of Australia’s native fauna for over a million years34, but additional, closely related Rattus species have invaded in the last 200+ years. For example, the native bush rat Rattus fuscipes is physically very similar to the recently-arrived black rat; the 2 species interact competitively with each other35. Unlike other regions that are evolutionarily rat-free, such as many islands, native wildlife in Australia may respond differently to the arrival of alien Rattus species: thus, we could reasonably expect the impact of alien Rattus species to more closely match the impacts of native predators rather than alien ones, and that native prey species would respond as if the predator is native because the prey have experience with similar native-predator archetypes.
Prey naïveté is thought to be a key mechanism allowing alien predators to suppress native prey populations to lower levels than do native predators4,5,36,37. Predator recognition abilities can be lost in just 130 years of isolation from predators38, or regained within one generation when historic predators are reintroduced39. One study even found that diurnal skinks avoided alien black rats on an island that had a long-standing colony (ca. 3000 years) of alien Pacific rats Rattus exulans; the authors suggested that skinks were ‘prepared’ for the invasion of alien rat-like predators due to their prior exposure to Pacific rats40. The apparently adaptive nature of anti-predator behavior suggests that native prey may in fact recognize alien predators that are functionally similar to local predators, and respond in a manner that is not totally naïve5,38. Cox and Lima5 argued that native wildlife species in freshwater ecosystems are more sensitive to alien predators than is wildlife in terrestrial or marine ecosystems due to many historical biotic interchanges in the latter environments that have exposed prey to more diverse predator experiences than in freshwater systems. The same line of argument may be used on a more local scale and applied to the results of our study.
The notion that co-occurrence of prey with predators that are ecologically similar to alien predators can engender inbuilt ‘resistance’ to the aliens (the ‘predator resistance hypothesis’) has been refined4,30 but subjected to limited testing. For example, several studies show that the magnitude of a prey’s anti-predator response is related to the degree of similarity between the novel and the native predator41,42, but there has been limited exploration of this concept in terms of net impact. Our test system of Rattus species in Australia addresses this gap, although the notion could apply equally to the invasion of functionally similar predator species elsewhere. The replacement of native Galaxias depressiceps, G. eldoni and G. anomalus by brown trout Salmo trutta in New Zealand43 and the replacement of the Santiago rice rat Nesoryzomys swarthi by the black rat on Santiago Island, Galápagos Islands, Ecuador44 provide good examples.
In our system, the moderate population-level response of skinks to alien predator removal suggests that L. delicata may have behavioral strategies such as avoidance that reduce the intensity of rat predation compared to skinks elsewhere. Native rats that co-occur with L. delicata, such as Rattus fuscipes, opportunistically eat small vertebrates45 and may select for anti-predator responses that assist skinks in reducing depredation by alien rats and hence in resisting the impacts of the invaders. By contrast, in regions such as New Zealand where reptiles have evolved without mammalian predators, skinks may be naïve to rodent predation and appear to be affected more strongly when Rattus species invade. For example, Oligosoma smithi, a similar sized skink to L. delicata, showed a 30-fold increase in numbers after rat control46.
One anomaly in our results concerns the arboreality of L. delicata. Arboreal nesting birds are susceptible to nest predation by black rats most likely because black rats climb and are an additive novel source of nest predation in Australian habitats47. Lampropholis delicata also makes use of the arboreal zone (experiment 1), but appeared to respond appropriately to black rats. This suggests that Australian natives display differing degrees of naïveté to alien rodents37. The fact that skinks used both ground and arboreal habitats may help to account for why they have appropriate responses to alien rats; time spent on the ground would increase encounters with more terrestrial native species of rats and hence select for predator-avoidance strategies.
Quantifying and comparing alien predator effect sizes with known benchmarks is a novel approach to assessing the level of impact of an introduced predator on a native species and hence its ‘alien-ness’. Here we experimentally demonstrate that alien black rats substantially reduce the arboreal activity of L. delicata, but the population-level response of this skink to black rat removal is within the range expected for a native predator-prey relationship. This does not suggest that black rats are generally harmless to Australian lizards, but provides an example of a common species that continues to persist in remnant urban bushland regardless of high densities of black rats. Understanding the mechanisms by which such coexistence occurs is important for informing effective conservation strategies. Our work also adds a novel dimension to the native/alien debate and helps in understanding that alien predators have varying levels of impact on native prey. At present, much effort by management authorities focuses on minimizing the impacts of alien predators, even if the case for intervention is weak or circumstantial. Past experience has taught us that exotic predator removal can in fact make things worse e.g. refs 48, 49, 50. We suggest that appropriate experiments and effect-size benchmarks, as demonstrated here, provide a useful approach to determine both the relative impact of alien species compared with native ones and to help set effective conservation priorities.
Materials and Methods
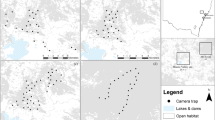
We tested the impacts of the black rat on L. delicata at local and landscape scales in Sydney, Australia in the Austral summer 2012. Local rat exclusion experiments, at the scale of individual trees, were conducted at 2 wooded urban sites in south-eastern Sydney (34°S, 151°E), and landscape-scale exclusions in Sydney Harbour National Park (34°S, 151°E). This work was conducted under Scientific License (SL100174), with Animal Care and Ethics approval from The University of Sydney (L04/6-2011/3/5549). All experimental protocols were carried out in accordance with the approved methods.
Experiment 1: local-scale rat exclusion
We selected at random 24 mature trees (dbh: 40–70 cm: Eucalyptus botryoides, E. piperita, E. punctata, Angophora costata) with an even representation of smooth and rough barked trees in each of the 2 study sites. Twelve trees were then randomly selected per site for rat exclusion and 12 for controls (rat-present). Rat exclusion was achieved by attaching excluder rings (aluminum sheet, 20 cm wide, angled upward at 45°) 50 cm above the ground, with spacers providing a gap of 0.5–1.0 cm with the tree boles. Incomplete rings, with 10 cm gaps every 20–25 cm, were used on rat-present control trees. Observations at night showed that rats did not ascend trees with the excluders, but regularly used the trunks of rat-present control trees. Strips of fabric-based sticky tape hung on boles 1 m above the ground collected rat hair on 88% of the rat-present control trees but on none of the experimental trees, further confirming that rat-exclusion was effective. Skinks moved freely on all trees. Three to 5 months after establishing the experimental trees, observers sat 2–3 m from trees around midday and counted L. delicata above the ring-guards for one hour. We assigned 10 minute intervals between observations to minimize double-counts, repeating observations at all trees for 4–6 days in January (Austral summer). We also repeated the experiment at one site the following year, using different trees but the same protocols, to yield 3 sampling times.
Experiment 2: landscape-scale rat exclusion
We established 8 1-ha sites >1 km apart in woodland patches. Each site comprised 36 points spaced 20 m apart in a 6 × 6 trapping grid. Four sites (‘removal’ sites) were intensively live-trapped for 10 nights to remove black rats and then re-trapped 3 nights a month thereafter to remove reinvaders. In 4 other sites (‘unmanipulated’ controls) black rats were live-trapped for 3 nights every 2 months. Rat-removal sites maintained 1.73 ± 0.75 black rats/ha (mean ± standard error: all error values reported in this paper are standard errors); unmanipulated controls averaged 18.04 ± 4.06 black rats/ha. We assessed skink trapping frequency on all treatment sites after 18 months of rat-removal, allowing time for one full breeding season and the start of the second breeding season51. This protocol agrees with Salo et al.2, who set a minimum experimental requirement of one prey generation to ensure that a demographic response is possible. We set 4 pitfall traps (height 117 mm, base diameter 85 mm, diameter at top 98 mm) at every second grid point for 3 nights at each site for skinks, checking captures at first light. Skinks were weighed, marked with a unique identifier on the underside of the body using a permanent black marker, and classified as breeders or non-breeders following Joss and Minard51. Since other predators of reptiles in the system (e.g. birds, cats) are consistent between treatment sites, any difference in reptile capture rates reflects the relative difference between sites with high rat densities and low rat densities.
Statistical analyses
Analysis of Experiment 1: local-scale rat exclusion
We used JMP version 9.0.052 for all statistical analyses. We used a repeated measures multivariate analysis of variance (MANOVA) to test the effect of time (i.e. ‘day’) on the frequency of L. delicata sightings per tree and found that time was not significant. We therefore proceeded to analyze the local-scale exclusion data using a restricted maximum likelihood (REML) analysis of variance (ANOVA), where the average number of L. delicata sightings per tree per day was the dependent variable. We compared how the average number of L. delicata sightings per tree was affected by ‘treatment’ (rat-present or rat-excluded) and ‘bark’ (rough or smooth), and the interaction between ‘treatment’ and ‘bark’, as fixed independent variables. Individual ‘tree’ identity was also included as a random dependent variable in our models, since each tree was randomly allocated a treatment. We used a square root transformation to ensure the data met the assumptions of normally distributed residuals and homogeneity of variance. We checked these assumptions using the Shapiro-Wilk and Q-Q plot (both are needed as Shapiro-Wilk tests can fail on normally distributed data at high sample size) and Bartlett’s test, respectively.
Analysis of Experiment 2: landscape-scale rat exclusion
At the landscape scale we likewise ran a repeated measures MANOVA to compare the total number of skinks caught per site over the 3 day trapping period and found that skink capture rates did not vary with time. We also found extremely low recapture rates, suggesting that our marking system may not have been effective. Thus, we opted to use the average number of skinks trapped per site per day as a measure of skink trapping frequency rather than the minimum number known to be alive (as calculated by the total number trapped in 3 days). This was because we could not be certain of the detection probability (for discussion see ref. 53), and we were primarily interested in the relative differences in catches between treatments. As above, we used a REML ANOVA to test the effect of ‘treatment’ (rat removal sites versus unmanipulated control sites) with ‘site’ as a random factor (since each site was randomly allocated a treatment) on the dependent variable, which was the average number of skinks caught per site per day.
In the absence of similar Rattus removal studies, we compared the results of our landscape experiment to other landscape-scale predator-removals using the mean effect size estimates of Salo et al.2 for prey responses to native and alien predators. We used METAWIN version 2.154 to calculate the standardized effect size as Hedges’ d, analogous to Salo et al.2, but only for our landscape-scale experiment, as Salo et al.2 reviewed only the demographic responses of prey to predator removal, and did not consider activity.
Additional Information
How to cite this article: Smith, H. M. et al. Using effect size benchmarks to assess when alien impacts are actually alien. Sci. Rep. 7, 38627; doi: 10.1038/srep38627 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Mack, R. N. et al. Biotic invasions: causes, epidemiology, global consequences and control. Ecol. Appl. 10, 689–710 (2000).
Salo, P., Korpimäki, E., Banks, P. B., Nordström, M. & Dickman, C. R. Alien predators are more dangerous than native predators to prey populations. Proceedings of the Royal Society of London . Series B: Biological Sciences 274, 1237–1243 (2007).
Carthey, A. J. R. & Banks, P. B. When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator. PloS one 7, e31804, doi: 10.1371/journal.pone.0031804 (2012).
Sih, A. et al. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. OIKOS 119, 610–621 (2010).
Cox, J. G. & Lima, S. L. Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends. Ecol. Evol. 21, 674–680 (2006).
Salo, P., Banks, P. B., Dickman, C. R. & Korpimäki, E. Predator manipulation experiments: impacts on populations of terrestrial vertebrate prey. Ecol. Monog. 80, 531–546 (2010).
Jones, H. P. et al. Severity of the effects of invasive rats on seabirds: A global review. Conserv. Biol. 22, 16–26, doi: 10.1111/j.1523-1739.2007.00859.x (2008).
Blackburn, T. M., Cassey, P., Duncan, R. P., Evans, K. L. & Gaston, K. J. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958, doi: 10.1126/science.1101617 (2004).
St Clair, J. J. H. The impacts of invasive rodents on island invertebrates. Biol. Conserv. 144, 68–81 (2011).
Ruscoe, W. A., Sweetapple, P. J., Perry, M. & Duncan, R. P. Effects of spatially extensive control of invasive rats on abundance of native Invertebrates in mainland New Zealand forests. Conserv. Biol. 27, 74–82 (2013).
Watts, C. H., Armstrong, D. P., Innes, J. & Thornburrow, D. Dramatic increases in weta (Orthoptera) following mammal eradication on Maungatautari—Evidence from pitfalls and tracking tunnels. New Zealand Journal of Ecology 35, 261–272 (2011).
Harris, D. B. Review of negative effects of introduced rodents on small mammals on islands. Biol. Invasions. 11, 1611–1630, doi: 10.1007/s10530-008-9393-0 (2009).
Towns, D. R., Parrish, R. & Westbrooke, I. Inferring vulnerability to introduced predators without experimental demonstration: case study of Suter’s skink in New Zealand. Conserv. Biol. 17, 1361–1371 (2003).
Whitaker, A. The effects of rodents on reptiles and amphibians. New Zealand Department of Lands and Survey information series 4, 75–86 (1978).
Case, T. J. & Bolger, D. T. The role of introduced species in shaping the distribution and abundance of island reptiles. Evolutionary Ecology 5, 272–290 (1991).
Towns, D. R. & Ferreira, S. M. Conservation of New Zealand lizards (Lacertilia: Scincidae) by translocation of small populations. Biol. Conserv. 98, 211–222 (2001).
Towns, D. R. Eradications as reverse invasions: lessons from Pacific rat (Rattus exulans) removals on New Zealand islands. Biol. Invasions. 11, 1719–1733, doi: 10.1007/s10530-008-9399-7 (2009).
Monks, J. M., Monks, A. & Towns, D. R. Correlated recovery of five lizard populations following eradication of invasive mammals. Biol. Invasions. 16, 167–175 (2014).
Tocher, M. D. Survival of grand and Otago skinks following predator control. J. Wildlife. Manag. 70, 31–42 (2006).
Towns, D. The role of ecological restoration in the conservation of Whitaker’s skink (Cyclodina whitakeri), a rare New Zealand lizard (Lacertilia: Scincidae). N. J. Z. Zool. 21, 457–471 (1994).
Towns, D. R. Changes in habitat use by lizards on a New Zealand island following removal of the introduced Pacific Rat (Rattus exulans). Pacific Conserv. Biol. 2, 286–292 (1996).
Towns, D. Response of lizard assemblages in the Mercury Islands, New Zealand, to removal of an introduced rodent: the kiore (Rattus exulans). J. R. Soc. NZ 21, 119–136 (1991).
Banks, P. B. & Smith, H. M. The ecological impacts of commensal species: black rats, Rattus rattus, at the urban–bushland interface. Wildlife Res. 42, 86–97 (2015).
Banks, P. B., Cleary, G. P. & Dickman, C. R. Sydney’s bubonic plague outbreak 1900–1910: A disaster for foreshore wildlife? Australian Zoologist 35, 1033–1039 (2011).
Banks, P. B. & Hughes, N. K. A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildlife Res. 39, 78–88 (2012).
Lunney, D., Ashby, E., Grigg, J. & O’Connell, M. Diets of scincid lizards Lampropholis guichenoti (Dumeril and Bibron) and Lampropholis delicata (De Vis) in Mumbulla State Forest on the south coast of New South Wales. Wildlife Res. 16, 307–312 (1989).
Hutchinson, M., Swain, R. & Driessen, M. Snakes and lizards of Tasmania (Nature Conservation Branch, Department of Primary Industries, Water and Environment, 2001).
Wyatt, K. B. et al. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PloS one 3, 1–9, doi: 10.1371/Journal.Pone.0003602 (2008).
Clark, D. A. Foraging behavior of a vertebrate omnivore (Rattus rattus): meal structure, sampling, and diet breadth. Ecology 67, 763–772 (1982).
Sih, A., Ferrari, M. C. & Harris, D. J. Evolution and behavioural responses to human‐induced rapid environmental change. Evolutionary Applications 4, 367–387 (2011).
Gamradt, S. C. & Kats, L. B. Effect of introduced crayfish and mosquitofish on California newts. Conserv. Biol. 10, 1155–1162 (1996).
Ricciardi, A. & Atkinson, S. K. Distinctiveness magnifies the impact of biological invaders in aquatic ecosystems. Ecology Letters 7, 781–784 (2004).
Snyder, W. E. & Evans, E. W. Ecological effects of invasive arthropod generalist predators. Annual Review of Ecology, Evolution, and Systematics 37, 95–122 (2006).
Breed, B. & Ford, F. Native mice and rats. (CSIRO Publishing, 2007).
Stokes, V. L., Banks, P. B. & Pech, R. P. Influence of residency and social odors in interactions between competing native and alien rodents. Behavioral Ecology and Sociobiology 66, 329–338, doi: 10.1007/s00265-011-1280-5 (2012).
Carthey, A. J. R. & Banks, P. B. Naïveté in novel ecological interactions: lessons from theory and experimental evidence. Biological Reviews 89, 932–949 (2014).
Banks, P. B. & Dickman, C. R. Alien predation and the effects of multiple levels of prey naiveté. Trends. Ecol. Evol. 22, 229–230 (2007).
Blumstein, D. T., Daniel, J. C. & Springett, B. P. A test of the multi‐predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology 110, 919–934 (2004).
Berger, J., Swenson, J. E. & Persson, I.-L. Recolonizing carnivores and naive prey: conservation lessons from Pleistocene extinctions. Science 291, 1036–1039 (2001).
Gérard, A., Jourdan, H., Cugnière, C., Millon, A. & Vidal, E. Is naïveté forever? Alien predator and aggressor recognition by two endemic island reptiles. Naturwissenschaften 101, 921–927 (2014).
Ferrari, M. C., Gonzalo, A., Messier, F. & Chivers, D. P. Generalization of learned predator recognition: an experimental test and framework for future studies. Proceedings of the Royal Society B: Biological Sciences 274, 1853–1859 (2007).
Stankowich, T. & Coss, R. G. The re-emergence of felid camouflage with the decay of predator recognition in deer under relaxed selection. Proceedings of the Royal Society B: Biological Sciences 274, 175–182 (2007).
Townsend, C. R. Individual, population, community, and ecosystem consequences of a fish invader in New Zealand streams. Conserv. Biol. 17, 38–47 (2003).
Harris, D. B. & Macdonald, D. W. Interference competition between introduced black rats and endemic Galapagos rice rats. Ecology 88, 2330–2344 (2007).
Warneke, R. M. Field study of the bush rat (Rattus fuscipes). Wildlife Constributions Victoria 14, 1–115 (1971).
Towns, D. R. & Daugherty, C. H. Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. N. J. Z. Zool. 21, 325–339 (1994).
Smith, H. M. When Commensals Go Wild: The Ecological Consequences Of Exotic Black Rats (Rattus rattus) Invading Beyond The Urban Boundary. (The University of Sydney, 2015).
Caughley, G. & Gunn, A. Conservation biology in theory and practice (1996).
Courchamp, F., Langlais, M. & Sugihara, G. Cats protecting birds: modelling the mesopredator release effect. Journal of Animal Ecology 68, 282–292, doi: 10.1046/j.1365-2656.1999.00285.x (1999).
Dickman, C. In Pest or Guest: the zoology of overabundance (eds D. Lunney, P. Eby, P. Hutchings, & S. Burgin ) 208–215 (Royal Zoological Society of New South Wales, 2007).
Joss, J. M. P. & Minard, J. A. On the reproductive cycles of Lampropholis guichenoti and L. delicata (Squamata: Scincidae) in the Sydney Region. Aust. J. Zool. 33, 699–704 (1985).
JMP version 9.0.0 (SAS Institute Inc., Cary, NC, USA, 2014).
White, G. C. Correcting wildlife counts using detection probabilities. Wildlife Res. 32, 211–216 (2005).
Rosenberg, M. S., Adams, D. C. & Gurevitch, J. METAWIN: Statistical software for meta-analysis (Version 2). (Sinauer Associates, Inc., 2000).
Acknowledgements
This work was funded by an ARC grant (LP100100600). Thanks to P. Harvey, A. Saul and many students for field assistance. Also thanks to the Banks Lab at Sydney University for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
H.M.S., C.R.D. & P.B.B. designed the experiments. H.M.S. & P.B.B. collected data. H.M.S. prepared tables and figures. H.M.S., C.R.D. & P.B.B. wrote and reviewed the manuscript. C.R.D. & P.B.B. provided funding via an Australian Research Council grant.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Smith, H., Dickman, C. & Banks, P. Using effect size benchmarks to assess when alien impacts are actually alien. Sci Rep 7, 38627 (2017). https://doi.org/10.1038/srep38627
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38627
This article is cited by
-
Exotic black rats increase invertebrate Ordinal richness in urban habitat remnants
Biological Invasions (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.