Abstract
It is unclear whether the association between dietary acrylamide intake and endometrial cancer risk as observed in some epidemiological studies reflects a causal relationship. We aimed at clarifying the causality by analyzing acrylamide-gene interactions for endometrial cancer risk. The prospective Netherlands Cohort Study on diet and cancer includes 62,573 women, aged 55–69 years. At baseline, a random subcohort of 2589 women was selected for a case cohort analysis approach. Acrylamide intake of subcohort members and endometrial cancer cases (n = 315) was assessed with a food frequency questionnaire. Single nucleotide polymorphisms (SNPs) in genes in acrylamide metabolism, sex steroid systems, oxidative stress and DNA repair were assessed through a MassARRAY iPLEX Platform. Interaction between acrylamide and SNPs was assessed with Cox proportional hazards analysis, based on 11.3 years of follow-up. Among the results for 57 SNPs and 2 gene deletions, there were no statistically significant interactions after adjustment for multiple testing. However, there were nominally statistically significant interactions for SNPs in acrylamide-metabolizing enzymes: CYP2E1 (rs915906 and rs2480258) and the deletions of GSTM1 and GSTT1. Although in need of confirmation, the interactions between acrylamide intake and CYP2E1 SNPs contribute to the evidence for a causal relationship between acrylamide and endometrial cancer risk.
Similar content being viewed by others
Introduction
Acrylamide is a probable human carcinogen (IARC class 2A; based on rodent studies) that was discovered in 2002 in various heat-treated carbohydrate-rich foods, such as cookies, potato chips, French fries and coffee. Since then, epidemiological studies have been performed in order to investigate the impact of dietary acrylamide intake on human cancer risks. The results of these studies are inconsistent: for some cancers (endometrial, ovarian, breast and kidney cancer) increased risks have been observed in some studies but not all. A recent meta-analysis on the association between acrylamide intake and endometrial cancer risk shows a pooled relative risk for high vs. low intake of 1.39 (95% CI 1.09–1.77) in never-smokers1. In the most recent risk assessment of acrylamide, by the European Food Safety Authority (EFSA)2, the epidemiological findings on acrylamide and cancer risk are discussed but not incorporated in the actual risk assessment. The most important reason for this is that the causality of the observed associations is still unclear, mainly due to the inconsistent associations across studies.
It is important to get more clarity on the causality of the observed epidemiological associations. They indicate that risks may be present at current dietary intake levels and they are still an underestimation of the true risk because of random measurement error of the acrylamide intake. In addition, the observed risks are considerably higher than predicted from rodent studies3. Moreover, virtually everyone is exposed to acrylamide through diet.
It is generally thought that acrylamide may cause cancer through the genotoxic action of acrylamide’s metabolite glycidamide (generated by the action of cytochrome P4502E1 (CYP2E1)) but other mechanisms, such as effects on sex hormones, are hypothesized as well2.
In the present study, we investigated whether genetic make-up modifies the association between dietary acrylamide intake as assessed through a validated food frequency questionnaire and endometrial cancer risk, thereby contributing to evidence on acrylamide’s mechanism of action and the causality of the observed associations between acrylamide and endometrial cancer risk. We selected SNPs in candidate genes involved in acrylamide metabolism (CYP2E1, glutathione-s-transferases, and epoxide hydrolase) and in mechanisms through which acrylamide is hypothesized to cause cancer: mechanisms involving DNA damage, sex hormones, and oxidative stress4. We specifically also look at never-smokers because cigarette smoke is an important source of acrylamide exposure1.
Results
Table 1 shows the characteristics of the subcohort and endometrial cancer cases at baseline. Cases were more often never-smokers, smoked fewer cigarettes per day and for a shorter duration. They more often ever used postmenopausal hormone treatment and considerably less often ever used oral contraceptives. Cases had a lower age at menarche and a later age at menopause and they had fewer children. In addition, cases had a considerably higher BMI and more often a family history of endometrial cancer.
Main effect of acrylamide
There was no main effect of acrylamide with 20.3 years of follow-up (HR of highest versus the lowest quintile of intake: 1.03 (95% CI 0.71–1.51) and 0.98 (0.88–1.10) per 10 μg/day increment of intake (Table 2). A similar null association was observed for never-smokers. We decided to further focus on acrylamide-gene interactions for the first 11.3 years of follow-up period, for which we did see an association between acrylamide intake and endometrial cancer risk5.
Main effect of SNPs
Table 3 lists the SNPs that showed a (borderline) statistically significant association with endometrial cancer risk (11.3 years of follow-up). Women with variant alleles of rs1056827 in CYP1B1, rs944722 in NOS2, and rs2228000 in XPC showed a decrease in risk (p-trend 0.04, 0.05, 0.06, respectively). Women with variant alleles of rs2472299 in CYP1A2, rs3219489 in MUTYH, rs660149 in PGR, and rs1042157 and rs6839 in SULT1A1 showed a positive trend over the number of variant alleles (p-trend 0.05, 0.09, 0.05, 0.07 and 0.07, respectively). A decreased risk of endometrial cancer was observed in women with a homozygous deletion of the GSTM1 gene when both SNP selected to represent the deletion were not called: HR: 0.80 (0.58–1.11). The association was similar when the deletion was based on missing calls in rs10857795 alone and there was a statistically significant decrease in endometrial cancer risk when the deletion was based on rs200184852 alone (HR 0.71 (0.52–0.96)). However, none of the SNPs was statistically significantly associated with endometrial cancer risk after adjustment for multiple comparisons; none of the Benjamini-Hochberg FDR values were lower than the chosen 0.20 threshold.
Interactions between acrylamide intake and SNPs
None of the SNPs showed a statistically significant multiplicative interaction with acrylamide for the 11.3 year follow-up period when adjusted for multiple comparisons. In Table 4, we show interactions with SNPs in genes involved in acrylamide metabolism that are interesting because they have a higher a priori probability of modifying the association between acrylamide and cancer risk than the other selected SNPs because they determine internal exposure to acrylamide and glycidamide.
We observed nominally (borderline) statistically significant multiplicative interaction for 2 SNPs in CYP2E1: rs915906 for all women (p-interaction = 0.02) and for never-smoking women (p-interaction = 0.07), and rs2480258 for all women (p-interaction = 0.03). There only was an association between acrylamide and endometrial cancer risk in homozygous wild types for both SNPs.
We observed nominally statistically significant multiplicative interaction for the deletion of GSTT1 for all women (p-interaction < 0.001) and never-smoking women (p-interaction = 0.02), although based on few cases with a homozygous deletion of GSTT1 (12 among all women and 7 among never-smoking women). Acrylamide was only positively associated with endometrial cancer risk in women with at least one copy of the GSTT1 allele. For the GSTM1 gene deletion, the same pattern of associations was observed: the acrylamide-associated risk of endometrial cancer was only increased in women with at least one copy of the GSTM1 gene but there was no statistically significant interaction with acrylamide intake. There were no interactions between acrylamide and SNPs in other acrylamide-metabolizing genes (GSTA5, GSTP1 and EPHX1) (results not shown).
There were some (borderline) nominally statistically significant interactions between acrylamide and other SNPs (Supplemental Table 3): rs11252859 in AKR1C1 (among never-smokers), rs1042157 and rs6839 in SULT1A1, rs3736599 in SULT1E1 (among never-smokers), rs10432782 in SOD1 (among never-smokers), rs3448 in GPX1 (among never-smokers), rs1800566 in NQO1 (among never-smokers), and rs2472299 in CYP1A2 (among never-smokers). In addition, differences in the acrylamide dose-response relationship between the genotypes were observed for rs5275 in PTGS2, rs1280350 in MGC12965, rs1056836 in CYP1B1, rs2228000 in XPC, rs4986938 in ESR2, rs6428830 in HSD3B1/B2 (among never-smokers) and rs64759180 in RRM2 (Supplemental Table 3).
Discussion
The current study is the first to analyze acrylamide-gene interactions for (endometrial) cancer risk. We followed a candidate gene approach for identifying SNPs in genes involved in acrylamide metabolism and genes involved in the mechanisms by which acrylamide might cause cancer: a sex hormonal effect, oxidative stress and DNA damage.
The positive association between acrylamide intake and endometrial cancer risk that we observed previously after 11.3 years of follow-up5 was not present after 20.3 years of follow-up. A possible explanation for this is that the positive association observed in the first follow-up period was a spurious finding, making the current acrylamide-gene interaction analysis all the more relevant. Another possible explanation is that the baseline assessment of dietary acrylamide in 1986 is insufficiently representative of the dietary acrylamide intake in the etiologically relevant exposure period for endometrial cancers occurring in the latter half of the follow-up period. For this reason, we focused on the interaction between acrylamide and SNPs in the first 11.3 years of follow-up.
Although there were several SNPs showing a statistically significant interaction with acrylamide intake, none withstood the adjustment for multiple comparisons. However, we observed some nominally statistically significant interactions with SNPs in genes involved in acrylamide metabolism, thus having a higher a priori probability of modifying the association between acrylamide and cancer risk than the other selected SNPs.
Glycidamide (formed by epoxidation of acrylamide through CYP2E1) is often thought to be the compound responsible for acrylamide-induced carcinogenesis due to its genotoxicity. Therefore, studying the modifying effect of SNPs in CYP2E1 on the association between acrylamide and cancer risk contributes important information on the causality of the association. We observed nominally statistically significant multiplicative interaction for 2 SNPs in CYP2E1: rs915906 and rs2480258. These 2 CYP2E1 SNPs are in the intronic region of the gene and thus do not affect the protein code, but they may be in linkage disequilibrium with variants that are causative. It was shown that the allelic variants of both genes and specifically their combination were associated with an increase in micronuclei (MN) count in binucleated lymphocytes, a marker of DNA damage and an established risk marker for carcinogenesis6. CYP2E1 metabolizes several other compounds than acrylamide, e.g., ethanol, benzene, nitrosamines, and acetaminophen7, and the enzyme bioactivates these compounds and thus increases their MN-forming potential. The observed increase in MN count observed with the variant alleles thus suggests increased CYP2E1 activity of the variant alleles or alleles in linkage disequilibrium with these alleles. This then would suggest that acrylamide itself is the causative compound in endometrial carcinogenesis, because the strongest association between acrylamide and endometrial cancer risk was observed in homozygous wild types.
We also studied another SNP in CYP2E1 (rs6413432), which did not show a statistically significant interaction with acrylamide intake but among never-smoking women, the risk of endometrial cancer was considerably higher in the homozygous wild types than in women with variant alleles: HRs per 10 μg/day increment in acrylamide intake were 1.25 (95% CI: 1.04–1.50, n = 95) and 1.06 (95% CI: 0.61–1.82, n = 25) for homozygous wild types versus women with variant alleles, respectively. This result points in the same hypothesized direction of acrylamide itself being the more relevant compound as the result for the other 2 SNPs because the variant allele of rs6413432 leads to increased CYP2E1 gene expression8 and the association between acrylamide and endometrial cancer risk was strongest in women homozygous for the wild type allele.
The observed interaction with these CYP2E1 SNPs contributes to the evidence for a causal association between acrylamide and endometrial cancer risk. Acrylamide has a high affinity for binding to thiol groups in proteins. Its effect on the nervous system is hypothesized to occur through binding to and disruption of proteins involved in neurotransmission9. For neurotoxicity, it is hypothesized that acrylamide itself is mainly responsible because acrylamide has a higher affinity for binding to proteins than glycidamide10. Despite the fact that a lot of attention is given to the genotoxicity of acrylamide’s metabolite glycidamide as the mechanism of action, it is also hypothesized that acrylamide causes cancer through other mechanisms, such as effects on sex hormones. Those mechanisms may involve disruption of key proteins, in which acrylamide itself could be the causative compound.
We observed that women with at least one copy of GSTM1 and GSTT1 were at an increased acrylamide-associated risk of endometrial cancer, which was contrary to what we expected. Both acrylamide and glycidamide are detoxified by conjugation to glutathione and urinary excretion of the mercapturic acid complexes11. However, it is unclear if glutathione conjugation of acrylamide occurs non-enzymatically or through catalyzation by GSTs12. Interestingly, regardless of acrylamide intake, women with a double deletion of GSTM1 were at a decreased risk of endometrial cancer in our study, which has been observed before13, and also for some other cancers14,15. A possible explanation is that GSTs catalyze the conjugation of reduced glutathione (GSH) to compounds that protect against endometrial cancer or that they bioactivate compounds involved in endometrial carcinogenesis, for instance catechol estrogens16. In addition, conjugation of acrylamide with GSH can result in depletion of cellular GSH stores, leading to an altered redox status of the cell. This can affect gene expression directly or through regulating various redox-dependent transcription factors4. Considering the fact that acrylamide induces GST activity17,18, it would be expected that the positive association between acrylamide and endometrial cancer is only present among women in whom the activity of GST can be induced; i.e. women with at least one copy of the genes.
An interesting observation in this context is that in a study on 85 persons of whom 51 were occupationally exposed to acrylamide, persons with the GSTM1 null genotype had lower urinary levels of the mercapturic acid metabolite of acrylamide in combination with a higher ratio of the glycidamide mercapturic acid metabolite to the acrylamide mercapturic acid metabolite than GSTM1 positive persons19. The authors speculate that this indicates that in persons with the GSTM1 null genotype a higher percentage of acrylamide is converted to glycidamide. In combination with the fact that we only observed an association between acrylamide and endometrial cancer risk in women with at least one copy of GSTM1, this could, in line with the results for the CYP2E1 SNPS, suggest that acrylamide itself is the causative compound in endometrial carcinogenesis. Whatever the biological explanation behind the observed interactions with GSTs, it is remarkable that GSTM1 and GSTT1 show a similar interaction pattern.
There were some (borderline) nominally statistically significant interactions between acrylamide and other SNPs: rs11252859 in AKR1C1, rs1042157 and rs6839 in SULT1A1, rs3736599 in SULT1E1, rs10432782 in SOD1, rs3448 in GPX1, rs1800566 in NQO1, and rs2472299 in CYP1A2. In addition, differences in the acrylamide dose-response relationship between the genotypes were observed for rs5275 in PTGS2, rs1280350 in MGC12965, rs1056836 in CYP1B1, rs2228000 in XPC, rs4986938 in ESR2, rs6428830 in HSD3B1/B2 and rs64759180 in RRM2. For all these SNPs it is even more important that the interaction between acrylamide intake and these SNPs is first corroborated or refuted in other studies in order to be able to judge whether our findings were chance findings or not. Therefore it is premature to elaborately discuss their possible role in acrylamide-induced endometrial carcinogenesis here.
This study has some limitations. Acrylamide intake was only assessed once, at baseline. The association between acrylamide and endometrial cancer risk was only present in the first half of the 20.3 year follow-up period, possibly due to the fact that the single dietary intake measurement was not representative of the relevant exposure of the later cases. Using the full 20.3 year follow-up period for analysis (results not shown), there were some similar nominally statistically significant interactions as with the 11.3 year follow-up period, namely with rs6839 (SULT1A1), rs2472299 (CYP1A2), and rsrs3448 (GPX1). However, the differences between the genotypes were not as clear as with the 11.3 year follow-up period. The other statistically significant interactions that were observed with 11.3 years of follow-up were not statistically significant with 20.3 years of follow-up. With 20.3 years of follow-up, there were some statistically significant interactions that were not observed with 11.3 years of follow-up: rs11252887 (AKR1C1) (only women with 1 or 2 variant alleles showed a clear increase in acrylamide-associated endometrial cancer risk), rs28362491 (NFKB1) (increased acrylamide-associated risk only in homozygous wild types), rs2228000 (XPC) (increased acrylamide-associated risk only in never-smoking homozygous wild types) and rs5275 (PTGS2) (increased acrylamide-associated risk only in homozygous wild types). None of the interactions were statistically significant after adjustment for multiple testing.
Rs2480258 in CYP2E1 that statistically significantly modified the association between acrylamide intake and endometrial cancer risk was not in Hardy-Weinberg equilibrium, although the deviation was minor (p = 0.03) and not statistically significant after adjustment for multiple testing. This may indicate that the genotypes for this SNP were measured with some error. However, there is no reason to assume that this error is different for cases and subcohort members or for different categories of acrylamide intake. Therefore, this potential genotyping error would rather have led to missing a true interaction (if any) than detecting an interaction20.
Some of the interactions that we discussed may be chance findings, considering that none of the interactions withstood the adjustment for multiple comparisons. However, finding interactions for multiple SNPs in the same gene for CYP2E1 decreases the likelihood that they are chance findings, especially when there are clear differences in the dose-response pattern of acrylamide between the genotypes.
Both the homozygous deletion of GSTM1 and that of GSTT1 in our population (based on the combination of SNPs selected for these genes) were low (31% and 8%, respectively) compared to the reported prevalence in Caucasian populations (40–60% for GSTM1 and 10–20% for GSTT1). In a PCR study (not shown), we tested some of the samples (n = 33) that showed a discrepancy in the iPLEX assay between rs10857795 and rs200184852 to represent the GSTM1 deletion and rs4630 and rs1040309 to represent the GSTT1 deletion (n = 37). All the samples that had no call for rs200184852 (but did have a call for rs10857795) in the iPLEX assay showed absence of a PCR product in the PCR study (results not shown). Only 51% of the samples that had no call for rs4630 (but did have a call for rs1040309) in the iPLEX assay showed absence of a PCR product in the PCR study. Thus, it can be assumed that the percentage of study participants with a deletion of GSTM1 is closer to 42% (as reflected by rs200184852) than to the 31% reflected by both GSTM1 SNPs. For GSTT1, it cannot be concluded which SNP best represents absence of the deletion but the true percentage is probably somewhere between 11% (rs104003609) and 15% (rs4630). In conclusion, the percentages of the GST deletions in this study are within the ranges of published percentages for Caucasian populations.
Strengths of this study are the prospective nature, the complete follow-up, and the fact that we observed a main effect of acrylamide, which may mean that it was assessed reasonably well in this study.
In conclusion, when we adjusted for multiple comparisons, there was no statistically significant interaction between SNPs and acrylamide intake for endometrial cancer risk. However, the nominally statistically significant interaction between acrylamide and SNPs in CYP2E1 contributes to the evidence of a causal association between acrylamide intake and endometrial cancer risk but confirmation is needed. Based on this study, we recommend prospective cohort studies on acrylamide-gene interactions and for some genes in particular: CYP2E1 and GSTs. These studies are preferably larger than the present study.
Methods
Study Cohort, Cases and Follow-up
The Netherlands Cohort Study on diet and cancer began in September 1986 with the inclusion of 62,573 women aged 55–69 years. Data on dietary habits and other risk factors were collected through a self-administered questionnaire at baseline in 1986. In addition, 75% of the participants sent in toenail clippings. Participants gave informed consent by returning the completed questionnaire. The NLCS, using toenail DNA for genotyping, and associated protocols were approved by the review boards of TNO Nutrition and Food Research (Zeist, the Netherlands) and Maastricht University (Maastricht, the Netherlands). All methods were applied according to the approved guidelines.
Following the case-cohort approach, cases were enumerated for the entire cohort, while the accumulated person-time at risk for the full cohort was estimated from a random subcohort of 2589 women. Since baseline, the subcohort has been followed up regularly for vital status information. Incident cases in the full cohort were detected by annual computerized record linkages to the regional cancer registries and the Netherlands Pathology Registry. Further details on design and methods of follow-up are presented elsewhere21,22,23,24.
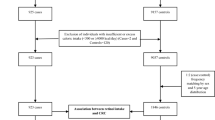
After 20.3 years of follow-up (Sept. 1986–Dec. 2006), there were 588 microscopically confirmed primary carcinomas of the endometrium ([ICD-O]-3:C54). Cohort members were excluded from analysis if their dietary data were incomplete or inconsistent, they had not sent in toenail clippings, they had no or inferior (call rate <95%) data on SNPs, or if they reported to have had a hysterectomy. Figure 1 shows the selection and exclusion steps that resulted in the numbers of cases and subcohort members available for analysis.
Acrylamide Intake Assessment
A food frequency questionnaire with questions on 150 food items was used for estimating dietary habits. The acrylamide intake was estimated from the mean acrylamide level of foods on the Dutch market, and the frequency of consumption and portion size of the foods, as described in detail elsewhere5.
Selection of genes and SNPs
The selection of genes focused on genes involved in 1) acrylamide metabolism (CYP2E1, GSTs and EPHX1) and 2) the hypothesized mechanisms of acrylamide-induced carcinogenesis:4 2a) a sex hormonal effect (involving sex hormone synthesis/metabolism or sex hormone nuclear receptors); 2b) oxidative stress; 2c) genotoxicity (DNA repair); or 2d) genes, not belonging to 1 or 2a–c, that were shown to be significant in an acrylamide-related polymorphism study19,25,26,27,28 or because they are in genes that were shown differentially expressed upon acrylamide exposure in acrylamide-related gene expression studies17,18,29,30,31,32,33,34,35,36,37,38,39,40.
Genes and SNPs of interest were identified from the literature (HugeNavigator and PubMed) and from a personal communication (for SNP rs1280350 in MGC12965) with Jos Kleinjans (Dept. of Toxicogenomics, Maastricht University). This latter SNP was shown to be associated with the level of acrylamide-hemoglobin adducts in cord blood of newborns in the Newborns and Genotoxic exposure risks (NewGeneris) project.
Preferably SNPs shown to be associated with a cancer involving sex hormones (endometrial, ovarian, breast and prostate cancer) were selected. However, we also selected some SNPs with no literature on their relation with the cancers of interest but that were shown to be have an association or effect in the above-mentioned acrylamide-related polymorphism study19,25,26,27,28 or gene expression studies17,18,29,30,31,32,33,34,35,36,37,38,39,40. It is unsure if in vitro or in vivo animal gene expression studies can be extrapolated to humans but at least these studies give indications that acrylamide exposure may involve effects on or interfere with these genes/enzymes.
Only validated SNPs with a minor allele frequency ≥10% in dbSNP (Caucasians) were selected. The functionality of the SNPs (as based on the F-value in F-SNP)41 and the region of the SNP in the gene were no selection criteria per se but they were used to choose between SNPs when there were many interesting SNPs per gene.
There were too many potentially interesting genes (see Supplemental Table 1), so we prioritized SNPs in acrylamide-metabolizing genes and (SNPs in) genes that showed an association or effect in acrylamide studies on gene polymorphisms and gene expression changes.
GSTM1 and GSTT1 are genes that are completely deleted in a large proportion of the population. The beginning and ending of the deleted sequences of GSTM1 and GSTT1 are not precisely known. Thus, it was impossible to design 1 assay (based on single base extension) for the deletion, as is done for SNPs. Therefore, we chose 3 SNPs in GSTM1 and GSTT1 each to represent the deletions (see Supplemental Table 1); when all 3 SNPs were not called, we assumed deletion of the gene.
66 SNPs were designed to fit together onto the 2 multiplexes that we budgeted: 6 SNPs to determine the GST deletions and 60 SNPs in other genes, see Supplemental Table 2.
DNA isolation and genotyping
DNA was isolated from 15 mg of toenail clippings, following a protocol described elsewhere42. Genotyping was performed by Agena, on the MassARRAY platform using the iPLEX TM assay43. This method has been used before to successfully genotype DNA from toenails42,44,45.
5% of the samples (n = 190) were duplicate samples to check the reproducibility of genotyping, which was >99%. Supplemental Table 2 shows the 60 SNPs that were analyzed. Three of the 60 SNPs that were genotyped had a call rate <80% and were excluded from the analyses. Six SNPs out of the remaining 57 SNPs did not adhere to Hardy-Weinberg equilibrium (p < 0.05). We excluded samples with a call rate <95% (18 cancer cases, 76 subcohort members). With regard to the SNPs selected to represent the GSTM1 deletion, rs10857795 was not called in 36%, rs200184852 in 42% and rs74837985 in only 2% of the subcohort. The latter value appears to be due to genotyping error. Therefore, we decided to base the assessment of the deletion of the GSTM1 gene only on rs10857795 and rs200184852. 31% had a missing value for both rs10857795 and rs200184852. With regard to GSTT1, rs2844008 was not called in 58%, rs4630 in 15%, and rs140309 in 11% of the subcohort. 8% had a missing value for all 3 GSTT1 SNPs.
Statistical Analysis
Hazard rate ratios (HRs) and 95% confidence intervals were obtained through Cox proportional hazards regression with STATA software (package 13), with standard errors estimated using the robust Huber-White sandwich estimator to account for additional variance introduced by sampling from the cohort. The proportional hazards assumption was tested using scaled Schoenfeld residuals.
Covariables, selected from the literature, for the models of the main effect of acrylamide and acrylamide-gene interactions were: age, body mass index, age at menarche, age at menopause, ever use of oral contraceptives, parity, ever use of postmenopausal hormone, family history of endometrial cancer, and energy intake. Smoking status, the duration of smoking and the number of cigarettes per day were included in the model, because cigarette smoke is an important source of acrylamide. Smokers have been shown to have on average four times higher exposure to acrylamide than non-smokers46. Moreover, smoking is inversely associated with endometrial cancer risk47. Therefore, subgroup analyses for never-smokers were performed. The main associations between SNPs and endometrial cancer risk were adjusted for age only.
In a previous analysis, we observed a positive main effect of acrylamide intake on endometrial cancer risk after 11.3 years of follow-up5. In the present study, our first step was to investigate whether this main effect was also present with 20.3 years of follow-up.
Multiplicative interaction between acrylamide intake and SNPs was tested using product terms of the continuous acrylamide intake variable and genotype. For statistical power reasons, we used a dominant genetic model (i.e., 1 or 2 variant alleles versus homozygous wild type). Tests for acrylamide dose-response trends in genotype strata were performed by fitting the mean acrylamide intake in the tertiles as a continuous variable.
We applied the False Discovery Rate method developed by Benjamini-Hochberg to adjust for multiple testing48 with the expected proportion of false positives set at 20%, which is applied regularly in candidate gene studies49,50. We performed separate adjustment for multiple testing for all women and for never-smoking women.
Two-sided p values are reported throughout this paper.
Additional Information
How to cite this article: Hogervorst, J. G. F. et al. The influence of single nucleotide polymorphisms on the association between dietary acrylamide intake and endometrial cancer risk. Sci. Rep. 6, 34902; doi: 10.1038/srep34902 (2016).
References
Je, Y. Dietary acrylamide intake and risk of endometrial cancer in prospective cohort studies. Arch. Gynecol. Obstet. 291, 1395–1401 (2015).
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on acrylamide in food. EFSA Journal 13, 4104, 321pp (2015).
Mucci, L. A. & Adami, H. O. The role of epidemiology in understanding the relationship between dietary acrylamide and cancer risk in humans. Adv. Exp. Med. Biol. 561, 39–47 (2005).
Besaratinia, A. & Pfeifer, G. P. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28, 519–528 (2007).
Hogervorst, J. G., Schouten, L. J., Konings, E. J., Goldbohm, R. A. & van den Brandt, P. A. A Prospective Study of Dietary Acrylamide Intake and the Risk of Endometrial, Ovarian, and Breast Cancer. Cancer Epidemiol. Biomarkers Prev. 16, 2304–2313 (2007).
Merlo, D. F. et al. Micronuclei in Cord Blood Lymphocytes and Associations with Biomarkers of Exposure to Carcinogens and Hormonally Active Factors, Gene Polymorphisms, and Gene Expression: The NewGeneris Cohort. Environ. Health Perspect. 122, 193–200 (2014).
Cederbaum, A. I. CYP2E1–biochemical and toxicological aspects and role in alcohol-induced liver injury. Mt. Sinai J. Med. 73, 657–672 (2006).
Uematsu, F. et al. Restriction fragment length polymorphism of the human CYP2E1 (cytochrome P450IIE1) gene and susceptibility to lung cancer: possible relevance to low smoking exposure. Pharmacogenetics 4, 58–63 (1994).
LoPachin, R. M. & Gavin, T. Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ. Health Perspect. 120, 1650–1657 (2012).
Carere, A. Genotoxicity and carcinogenicity of acrylamide: a critical review. Ann. Ist. Super Sanita 42, 144–155 (2006).
Fennell, T. R. et al. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol. Sci. 85, 447–459 (2005).
Doroshyenko, O. et al. In vivo role of cytochrome P450 2E1 and glutathione-S-transferase activity for acrylamide toxicokinetics in humans. Cancer Epidemiol. Biomarkers Prev. 18, 433–443 (2009).
Ashton, K. A. et al. Polymorphisms in genes of the steroid hormone biosynthesis and metabolism pathways and endometrial cancer risk. Cancer Epidemiol. 34, 328–337 (2010).
Emeville, E. et al. Copy number variation of GSTT1 and GSTM1 and the risk of prostate cancer in a Caribbean population of African descent. Plos one 9, e107275 (2014).
Roodi, N., Dupont, W. D., Moore, J. H. & Parl, F. F. Association of homozygous wild-type glutathione S-transferase M1 genotype with increased breast cancer risk. Cancer Res. 64, 1233–1236 (2004).
Butterworth, M., Lau, S. S. & Monks, T. J. Formation of catechol estrogen glutathione conjugates and gamma-glutamyl transpeptidase-dependent nephrotoxicity of 17beta-estradiol in the golden Syrian hamster. Carcinogenesis 18, 561–567 (1997).
Lee, T. et al. Expression analysis of hepatic mitochondria-related genes in mice exposed to acrylamide and glycidamide. J. Toxicol. Environ. Health A 75, 324–339 (2012).
Sen, A., Ozgun, O., Arinc, E. & Arslan, S. Diverse action of acrylamide on cytochrome P450 and glutathione S-transferase isozyme activities, mRNA levels and protein levels in human hepatocarcinoma cells. Cell. Biol. Toxicol. 28, 175–186 (2012).
Huang, Y. F. et al. Association of CYP2E1, GST and mEH genetic polymorphisms with urinary acrylamide metabolites in workers exposed to acrylamide. Toxicol. Lett. 10, 118–126 (2011).
Fardo, D. W., Becker, K. D., Bertram, L., Tanzi, R. E. & Lange, C. Recovering unused information in genome-wide association studies: the benefit of analyzing SNPs out of Hardy-Weinberg equilibrium. Eur. J. Hum. Genet. 17, 1676–1682 (2009).
van den Brandt, P. A. et al. A large-scale prospective cohort study on diet and cancer in The Netherlands. J. Clin. Epidemiol. 43, 285–295 (1990).
van den Brandt, P. A., Schouten, L. J., Goldbohm, R. A., Dorant, E. & Hunen, P. M. Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int. J. Epidemiol. 19, 553–558 (1990).
Goldbohm, R. A. et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J.Clin. Nutr. 48, 253–265 (1994).
Goldbohm, R. A. et al. Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur. J. Clin. Nutr. 49, 420–429 (1995).
Pingarilho, M. et al. Genetic polymorphisms in detoxification and DNA repair genes and susceptibility to glycidamide-induced DNA damage. J. Toxicol. Environ. Health A 75, 920–933 (2012).
Duale, N. et al. Biomarkers of human exposure to acrylamide and relation to polymorphisms in metabolizing genes. Toxicol. Sci. 108, 90–99 (2009).
Pingarilho, M. et al. Induction of sister chromatid exchange by acrylamide and glycidamide in human lymphocytes: role of polymorphisms in detoxification and DNA-repair genes in the genotoxicity of glycidamide. Mutat. Res. 752, 1–7 (2013).
Kjuus, H. et al. Chromosome aberrations in tunnel workers exposed to acrylamide and N-methylolacrylamide. Scand. J Work Environ. Health 31, 300–306 (2005).
Clement, F. C., Dip, R. & Naegeli, H. Expression profile of human cells in culture exposed to glycidamide, a reactive metabolite of the heat-induced food carcinogen acrylamide. Toxicology 240, 111–124 (2007).
Mei, N. et al. Gene expression changes associated with xenobiotic metabolism pathways in mice exposed to acrylamide. Environ. Mol. Mutagen. 49, 741–745 (2008).
Camacho, L. et al. Effects of acrylamide exposure on serum hormones, gene expression, cell proliferation, and histopathology in male reproductive tissues of Fischer 344 rats. Toxicol. Lett. 211, 135–143 (2012).
Ehlers, A. et al. Dose dependent molecular effects of acrylamide and glycidamide in human cancer cell lines and human primary hepatocytes. Toxicol. Lett. 217, 111–120 (2013).
Hasegawa, K. et al. Acrylamide-responsive genes in the nematode Caenorhabditis elegans. Toxicol. Sci. 101, 215–225 (2008).
Hochstenbach, K. et al. Global gene expression analysis in cord blood reveals gender-specific differences in response to carcinogenic exposure in utero. Cancer Epidemiol. Biomarkers Prev. 21, 1756–1767 (2012).
Kim, K. Effect of subchronic acrylamide exposure on the expression of neuronal and inducible nitric oxide synthase in rat brain. J. Biochem. Mol. Toxicol. 19, 162–168 (2005).
Lyn-Cook, L. E. Jr. et al. Food contaminant acrylamide increases expression of Cox-2 and nitric oxide synthase in breast epithelial cells. Toxicol. Ind. Health 27, 11–118 (2011).
Sadek, I. A. Short-term studies of the effect of acrylamide on the testes of the Egyptian toad. Folia Morphol. (Praha) 37, 427–430 (1989).
Shan, X. et al. Curcumin and (−)-epigallocatechin-3-gallate attenuate acrylamide-induced proliferation in HepG2 cells. Food Chem. Toxicol. 66, 194–202 (2014).
Song, J. et al. Protection of cyanidin-3-glucoside against oxidative stress induced by acrylamide in human MDA-MB-231 cells. Food Chem. Toxicol. 58, 306–310 (2013).
Yang, H. J. et al. Toxicological effects of acrylamide on rat testicular gene expression profile. Reprod. Toxicol. 19, 527–534 (2005).
Lee, P. H. & Shatkay, H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucl. Acids Res. 36 (suppl 1), D820–D824, Database accessed on Dec 1 2013) (2008).
Hogervorst, J. G. et al. DNA from nails for genetic analyses in large-scale epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 23, 2703–2712 (2014).
Gabriel, S., Ziaugra, L. & Tabbaa, D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet Chapter 2, Unit 2 12 (2009).
Geybels, M. S. et al. Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer. J. Natl. Cancer Inst. 106, dju003 (2014).
Deckers, I. A. et al. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: Interplay with hypertension and intakes of sodium, potassium and fluid. Int. J. Cancer 136, 1104–1116 (2015).
Schettgen, T. et al. Determination of haemoglobin adducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int. J. Hyg. Environ. Health 207, 531–539 (2004).
Al-Zoughool, M. et al. Risk of endometrial cancer in relationship to cigarette smoking: results from the EPIC study. Int. J. Cancer 121, 2741–2747 (2007).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. 57, 289–300 (1995).
Geybels, M. S., van den Brandt, P. A., van Schooten, F. J. & Verhage, B. A. Oxidative stress-related genetic variants, pro- and antioxidant intake and status, and advanced prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 24, 178–186 (2015).
Kim, C. et al. Genetic polymorphisms in oxidative stress pathway genes and modification of BMI and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomarkers Prev. 21, 866–868 (2012).
Acknowledgements
The authors thank the study participants, the Netherlands Cancer Registry, the Dutch Pathology Registry, and the Biobank of the Maastricht University Medical Center. We also thank Sacha van de Crommert, Jolanda Nelissen, Conny de Zwart, Ellen Dutman, Henny Brants, and Annemie Pisters for their assistance with data entry or data management, Harry van Montfort for programming assistance, and Stijn Lumeij, Kristien Lemmens, Joy Goessens, and Leonie Jonkers for technical assistance with DNA isolation and genotyping. Janneke Hogervorst is a postdoctoral research fellow from the Research Foundation - Flanders (FWO).
Author information
Authors and Affiliations
Contributions
The author contributions were as follows: J.G.F.H. conceived the analyses, coordinated the genotyping, conducted the analyses, interpreted the results and wrote the manuscript; P.A.v.d.B. conceived, coordinates and supervises the Netherlands Cohort Study, and critically reviewed the manuscript; R.W.L.G. supervised the genotyping and critically reviewed the manuscript; F.-J.v.S. provided laboratory facilities for genotyping and critically reviewed the manuscript; and L.J.S. co-conceived the analyses, coordinated and supervised the genotyping, coordinates and supervises the data management for the Netherlands Cohort Study, and critically reviewed the manuscript. This study was funded by the Dutch Cancer Society (KWF), grant number: UM 2011-5123.
Ethics declarations
Competing interests
Dr. Leo Schouten received compensation as a member of a scientific advisory panel on acrylamide risk assessment of the European Food Safety Authority. The other authors have no potential competing financial interests to declare.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hogervorst, J., van den Brandt, P., Godschalk, R. et al. The influence of single nucleotide polymorphisms on the association between dietary acrylamide intake and endometrial cancer risk. Sci Rep 6, 34902 (2016). https://doi.org/10.1038/srep34902
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34902
This article is cited by
-
Cord blood acrylamide levels and birth size, and interactions with genetic variants in acrylamide-metabolising genes
Environmental Health (2021)
-
Prediction of baking quality using machine learning based intelligent models
Heat and Mass Transfer (2020)
-
Interaction between dietary acrylamide intake and genetic variants for estrogen receptor-positive breast cancer risk
European Journal of Nutrition (2019)
-
The polymorphism rs2480258 within CYP2E1 is associated with different rates of acrylamide metabolism in vivo in humans
Archives of Toxicology (2018)
-
Genetic polymorphisms of 3′-untranslated region of SULT1A1 and their impact on tamoxifen metabolism and efficacy
Breast Cancer Research and Treatment (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.