Abstract
Complex interactions exist between cytokines and the interleukin family plays a fundamental role in inflammation. Particularly circulating IL-1β, IL-6 and IL-8 are unregulated in systemic and chronic inflammatory conditions. Hypercoagulability is an important hallmark of inflammation and these cytokines are critically involved in abnormal clot formation, erythrocyte pathology and platelet hyper-activation and these three cytokines have known receptors on platelets. Although these cytokines are always unregulated in inflammation, we do not know how the individual cytokines act upon the structure of erythrocytes and platelets and which of the viscoelastic clot parameters are changed. Here we study the effects of IL-1β, IL-6 and IL-8 at low physiological levels, representative of chronic inflammation, by using scanning electron microscopy and thromboelastography. All three interleukins caused the viscoelastic properties to display an increased hypercoagulability of whole blood and pathology of both erythrocytes and platelets. The most pronounced changes were noted where all three cytokines caused platelet hyper-activation and spreading. Erythrocyte structure was notably affected in the presence of IL-8, where the morphological changes resembled that typically seen in eryptosis (programmed cell death). We suggest that erythrocytes and platelets are particularly sensitive to cytokine presence and that they are excellent health indicators.
Similar content being viewed by others
Introduction
Complex interactions exist between cytokines and inflammation and specifically the interleukin family plays a fundamental role in systemic inflammation. Particularly IL-1β, IL-6 and IL-8 are present in whole blood and measurable (in pg.mL−1) in most systemic inflammatory conditions. An important hallmark of systemic inflammation is a pathological coagulation potential and hypercoagulation is also found in (most) inflammatory conditions (discussed extensively in refs 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12).
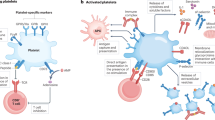
Proinflammatory cytokines are capable of activating the coagulation system and also play an important role in the down-regulation of important physiological anticoagulant pathways13. Also, plasma levels of several inflammation markers have been found to be associated with future cardiovascular risk in a variety of clinical settings14. The coagulation system is primarily triggered in response to damage to the endothelium, which allows the exposure of blood clotting factors to extravascular tissue. In healthy individuals, hemostasis is closely regulated by several anticoagulant mechanisms that balance the procoagulant forces and thus preventing untimely vascular clotting15. Pro-inflammatory cytokines and chemokines can affect all coagulation pathways16. Therefore the intricate relationship between the presence of cytokines resulting in inflammation and hyper-coagulation, are particularly relevant in the pathogenesis of vascular disease. See a high-level overview (Fig. 1) that illustrates how coagulation is affected by inflammation and a changed cytokine profile; adapted from refs 17, 18, 19, 20, 21.
The intricate relationship between inflammation and hyper-coagulation.
This diagram focuses on the bidirectional relationship between inflammation and coagulation and the role that increased pro-inflammatory cytokines in both acute and chronic systemic inflammation plays in the activation of the coagulation system.
In this paper, we are particularly interested in the effects of circulating IL-1β, IL-6 and IL-8 on both erythrocytes (RBCs) and platelets and how exposure to these interleukins may affect platelet and RBC structure during inflammation.
Interleukin 1 Receptor 1 (IL1R1) and its ligand, IL1β, are unregulated in cardiovascular disease and infection22. IL-1β is also known to be present in autoimmune conditions and contributes to several chronic diseases, including atherosclerosis and type 2 diabetes23,24,25. IL-1α and IL-1β have a natural antagonist, IL-1Ra and both bind to the same receptor molecule, IL-1 receptor 1; see refs 26,27 for extensive discussion on the role of particularly IL-1β in inflammation. Bursts of IL-1β are involved in acute attacks of systemic or local inflammation and also in myocardial infarction or stroke23. IL-1β also plays a significant role in the inflammation induced by Helicobacter pylori28. RBCs do not have an IL-1β receptor binding site, but platelets express the IL1R1 receptor and respond to IL-1β; and in e.g. maturing thrombi platelets can accumulate IL-1β29,30.
IL-6 is a multifunctional cytokine that regulates the immune response, haemopoiesis, the acute phase response, inflammation31 and the central nervous system31,32. Its expression is high and transiently unregulated in nearly all pathophysiological inflammatory conditions and also in autoimmune diseases33,34. IL-6 trans-signaling is also critically involved in the maintenance of a disease state, by promoting transition from acute to chronic inflammation35. IL-6 exerts its biological activities through two molecules: IL-6R (IL-6 receptor) and by the membrane-bound β-receptor glycoprotein 130 (gp130)31,36. Transduction of the signal is mediated by gp130 and also by trans-signaling, where IL-6 binds to soluble forms of the IL-6R (sIL-6R). These agonistic IL-6/sIL-6R complexes can in principle activate all cells due to the uniform expression of gp130 (which is present on all cells)33,34,37,38. Resting platelets also express gp130 on their membranes and in the presence of IL-6 (produced by stressed endothelial cells), platelet-derived IL-6 trans-signaling happens and could be crucial in the development of inflammation within a damaged vessel39 and in platelet thrombogenicity40.
IL-8 is also a well-known circulating inflammatory cytokine41,42. Macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells produce IL-8. There are many receptors on the surface membrane capable of binding IL-8; the most frequently studied types are the G protein-coupled serpentine receptors CXCR1 and CXCR243,44. Platelets may have IL-8 receptors and it was found that IL-8–dependent activation of washed platelets may happen, leading to procoagulant activity45.
Plasma and serum levels of IL-1β, IL-6 and IL-8 are measured in pg.mL−1 and the mean concentrations of healthy individuals and those with inflammatory conditions are typically as follows:
-
IL-1β, ±0.7–1.1 pg.mL−1 in healthy individuals and46 and ±30 pg.mL−1 in unstable angina pectoralis14.
-
IL-6: ±4 pg.mL−1 in healthy individuals and levels in psoriasis ±14 pg.ml−1 47.
-
IL-8: ±14 pg.mL−1 in healthy individuals and in patients with active psoriasis: ±40 pg.mL−1 47 and unstable angina pectoralis14.
After taking all the evidence into consideration, we note that there is no research that we could find, that specifically looks at the individual effects of the 3 cytokines on RBCs and platelets, to determine their individual effects, using specifically ultrastructure. The question also arose: are all cytokines equal or is there 1 signal one among the 3 that has a more pronounced effect than the others. Our hypothesis therefore is that IL-1β, IL-6 and IL-8 individually cause changes to the coagulation profiles and to platelets due to their binding to platelets; but that IL-6 might possibly show the most effects on RBCs, due to the fact that it has an universal binding site on all cells, including RBCs. We tested this hypothesis by looking at clotting, platelet hyper-coagulation and eryptosis, adding these cytokines individually, at low concentrations and used thromboelastography and scanning electron microscopy to test our hypothesis. We also compared the results from this study with ultrastructural research previously done using blood from inflammatory conditions, with known cytokine upregulation.
Materials and Methods
Ethical statement
This study was approved by the Ethical Committee of the University of Pretoria (South Africa). A written form of informed consent was obtained from all healthy donors (available on request). The methods were carried out in accordance with the approved guidelines. Blood was collected and methods were carried out in accordance with the relevant guidelines of the ethics committee (ethics number: 506/2014 and 298/2016: E Pretorius and J Bester: principal investigators for use of control blood; ethics number for Alzheimer type study: 81/2013; ethics number for Parkinson’s disease study: 80/2013; ethics number for Type II study: 68/2014; and ethics number for rheumatoid arthritis study: 462/2013). We adhered strictly to the Declaration of Helsinki.
Concentration of interleukins used
We exposed healthy whole blood to a final exposure concentration of 20 pg.mL−1 IL-1β, 15 pg.mL−1 IL-6 and 40 pg.mL−1 IL-8, for 10 minutes at room temperature, as these concentrations are in line with concentrations of these cytokines found in plasma and serum of systemic chronic inflammation14,46,47. Concentrations of cytokines are mostly measured in either plasma or serum. We took exceptional care to determine from literature the concentrations in healthy and diseased individuals. We then decided on concentrations that are low, even for systemic inflammation (but higher than in healthy individuals); and not nearly as high as is found in acute inflammation. The interleukins were purchased from Sigma (catalogue numbers: IL-1β: I9401, IL-8: I1645, IL-6: I2786).
Healthy volunteer details and blood collection
Blood samples were obtained from 10 healthy individuals of ages ranging from 18 to 60. Blood was collected in one 4.5 mL citrate tube. This collection was done by a medical doctor and all handling of samples were performed under very strict aseptically conditions, in order to prevent contamination of samples.
Inflammatory patient details and blood collection
In this paper, we include SEM micrographs taken as part of previous published papers, to support the in vitro results presented here48,49,50,51,52. The inflammatory blood sample preparation was done as discussed for the healthy volunteers and also in the various papers itself.
Data sharing
Raw data, extensive SOPs for TEG and SEM, including original images without color and micrographs can be accessed at: https://1drv.ms/f/s!AgoCOmY3bkKHbAm9Z0xJbqtthTA and on the corrresponding author’s researchgate profile, https://www.researchgate.net/profile/Etheresia_Pretorius, as raw data.
Thromboelastography
Coagulation parameters, using whole blood (WB) of healthy individuals, were done using thromboelastography (TEG). WB collected in citrate tubes were left for 30 minutes at room temperature before the experiment was started. 30 minutes after blood was drawn in citrated tubes, the WB was incubated for 10 minutes with each of the interleukins at the final exposure concentration mentioned above. 340 μl of the interleukin-incubated WB and naïve WB were placed in a disposable cup in a computer-controlled TEG hemostasis system (Model 5000, Hemoscope, Niles, IL), with addition of 20 μl CaCl2 as the last step to initiate clotting. Thrombelastographic data was collected until maximum amplitude (MA) is reached or 60 min had elapsed53,54,55,56,57,58. See Table 1 for the parameters that are obtained when whole blood-clotting profiles are studied using the TEG; this table was adapted from refs 59, 60, 61. TEG is typically used to determine clot formation and clot strength62. Statistical analysis was done with the program StatsDirect and p-values were obtained using non-parametric Mann-Whitey analysis.
Scanning electron microscopy
After the blood was collected (also left in citrate tubes fro 30 minutes) and incubated with the interleukins for 10 minutes, 10 μl of WB with added interleukins and naïve WB were placed directly on a glass cover slip, fixed, dehydrated, dried, mounted and coated with carbon according to previously described methods63. A high-resolution crossbeam 540 Zeiss scanning electron microscope was used to study the surface morphology of erythrocytes and platelets. Micrographs were taken at 1 kV. Due to the high quality of the SEM images, no processing was done except to add color using Adobe®Photoshop CS6® version 13.0 × 64. For a significant selection of our raw data see https://1drv.ms/f/s!AgoCOmY3bkKHbAm9Z0xJbqtthTA.
To relate our in vitro results to actual in vivo results as seen in whole blood of actual patients with known systemic inflammation, we add SEM micrographs from individuals with type II diabetes, rheumatoid arthritis, Parkinson’s disease and Alzheimer’s type dementia.
Results
Table 2 shows the TEG whole blood results of the samples. Addition of the three cytokines to whole blood, all showed an increased clotting potential, confirming their role in increased hypercoagulability, during inflammation. Noteworthy changes in the following parameters are that the R-time was shortened with addition of all three interleukins, with a decreased maximum velocity of clot growth (MA), as well as a decreased velocity of clot growth (MRTG) and a decrease in time before maximum velocity of clot growth (TMRTG) and clot strength (TTG). Significant changes in MRTG, TMRTG and TTG are indicative of specific modifications during fibrin formation from fibrinogen, while the other parameters are indicative of interactions of all cellular components involved in coagulation. From these results, IL-1β caused significant changes in the blood clotting profiles (see Table 2). The resulting clot is formed faster, is significantly less stable (MA), with a significantly decreased velocity (MRTG) to reach the maximum clot growth, resulting in a unstable and fragile clot. This is an indication of clot hypercoagulability due to IL-1β exposure. IL-6 also caused the clot to form faster, it is also significantly less stable, but it has a significantly decreased TMRTG and TTG, indicating that a unstable, fragile clot is formed faster. Il-6 therefore caused a more hypercoagulable clot than IL-1β, likely due to a significant change to fibrin(ogen). IL-8 caused significant changes in all TEG parameters, suggesting that it is the most potent procoagulant cytokine from the 3 studied. It caused the clot to form faster, reaching clot strengh significanlty slower, with a decreased thrombin burst, resulting in a slower fibirn cross-linking (indicated by the angle parameter). All of the above-mentioned parameters contributes to a significantly unstable clot (shown by a significantly decreased MA). IL-8 is therefore the cytokine that caused the most significant changes at all levels of coagulation, including fibrin(ogen), thrombin and cellular interactions.
Figure 2A,B shows representative RBCs and a close-up of the membrane (120 000x machine magnification) of a healthy individual is shown in Fig. 2C. Figure 2D shows a typical platelet, with slight pseudopodia formation due to contact activation. Figures 3, 4, 5 shows similar micrographs where the three interleukins were added to whole blood respectively.
(A) A low magnification to show overall view with RBCs and a platelet with slight pseudopodia formation due to contact activation. (B) Representative RBC from a healthy individual; (C) high magnification of RBC membrane; (D) platelet showing slight pseudopodia formation due to contact activation. Micrographs were taken at 1 kV using a crossbeam 540 Zeiss scanning electron microscope. No changes were done on actual figures and color enhancement was done using Adobe®Photoshop CS6® version 13.0 × 64.
(A,B) Representative RBCs and platelet clumps from a healthy individual, after whole blood was exposed to IL-1β; (C) high magnification of RBC membrane; (D) Platelet that shows spreading and hyper-activation. Micrographs were taken at 1 kV using a crossbeam 540 Zeiss scanning electron microscope. No changes were done on actual figures and color enhancement was done using Adobe®Photoshop CS6® version 13.0 × 64.
(A,B) Representative RBCs and platelet clumps from a healthy individual, after whole blood was exposed to IL-6; (C) high magnification of RBC membrane; (D) Platelet that shows spreading and hyper-activation. Micrographs were taken at 1 kV using a crossbeam 540 Zeiss scanning electron microscope. No changes were done on actual figures and color enhancement was done using Adobe®Photoshop CS6® version 13.0 × 64.
(A,B) Representative RBCs and platelet clumps from a healthy individual, after whole blood was exposed to IL-8; eryptotic cells indicated with arrows. (C) High magnification of RBC membrane, showing ultrastructural changes; (D) Platelet that shows hyper-activation. Micrographs were taken at 1 kV using a crossbeam 540 Zeiss scanning electron microscope. No changes were done on actual figures and color enhancement was done using Adobe®Photoshop CS6® version 13.0 × 64.
It is also well-known that a general hypercoagulable state is present in various inflammatory conditions and we and others have shown changes to RBC (eryptosis) and platelets hyper-activation in various conditions, including type 2 diabetes, Parkinson’s disease, Alzheimer’s disease and rheumatoid arthritis48,49,50,51,52. In all of these diseases, the 3 cytokines of interest in this paper, feature prominently as major mechanistic role players in the inflammatory profiles of the diseases. See Fig. 6 to show micrographs that were taken as part of the previously mentioned studies, to support the evidence presented here. Obviously in these in vivo situations, all 3 cytokines are upregulated simultaneously and in this paper the idea is to show the effects of the individual cytokines.
Micrographs from inflammatory diseases where IL-1β, IL-6 and IL-8 upregulation plays a fundamental role in the pathogenesis and hypercoagulability of the diseases.
(A) Alzheimer’s disease; (B) Parkinson’s disease; (C) type 2 diabetes; (D) rheumathoid arthritis. Micrographs were taken at 1 kV using a crossbeam 540 Zeiss scanning electron microscope. No changes were done on actual figures and color enhancement was done using Adobe®Photoshop CS6® version 13.0 × 64.
As seen with the TEG results, the SEM confirms that all 3 cytokines act upon the cellular component of WB and causes platelet hyper-activation and specifically IL-8 causes changes that we relate to the initiation of RBC eryptosis and visible structural changes to RBC membranes were noted (see Fig. 5).
Discussion
Increased levels of IL-1β, IL-6 and IL-8 are known to play an important role in both acute and chronic inflammation, with resulting pathological clotting. However, we know little about the effects of these interleukins on the ultrastructure of RBCs and platelets or how they change the individual viscoelastic properties involved in clot formation. As shown in Fig. 1, the interactions of the 3 cytokines are complex and IL-1β, IL-6 as well as IL-8 and their mechanism of action are typically implicated in pro-inflammatory induced coagulation17,18,19,20,21. IL-6 is primarily involved in the up-regulation of tissue factor that brings about initiation of coagulation. IL-1β down-regulates thrombomodulin and consequently causes defects in anticoagulant proteins, specifically impairing activation of protein C. The activation of protein C (in a healthy individual) is an important step in the anti-coagulant pathway. During IL-1β presence, as seen in inflammation, it acts as a procoagulant to impair protein C activity. In a healthy individual, thrombomodulin has an anti-inflammatory activity and down-regulation due to IL-1β, impairs this protective effect. IL-8 promotes procoagulant activity, by triggering platelet activation45. In various papers, others and we have shown the effect of an upregulated inflammatory profile due to conditions like Alzheimer’s disease, Parkinson’s disease, T2D and rheumathoid arthritis in blood of these patients. Various research papers that support the effects of these upregulated cytokines resulting in a dysregulated immune system, specifically resulting in eryptosis, is therefore important to note64,65,66,67,68,69,70,71,72.
In this paper we used TEG to track clot formations over time and we used the standard procedure where we measured clotting parameters, from clot initiation until maximum clot strength or stiffness of clot were reached (MA), after addition of CaCl2 to reverse the anti-clotting action of citrate. TEG therefore provides descriptive clotting parameters over time. SEM ultrastructure, displays a single snapshot after 10 minutes exposure of WB to the 3 interleukins, to visually show how the interleukins affect cell structure.
Our hypothesis stated that IL-1β, IL-6 and IL-8 individually will cause changes to the coagulation profiles and to platelets due to their binding to platelets; but that IL-6 might possibly show the most effects on RBCs, due to the fact that it has an universal binding site on all cells, including RBCs. All 3 cytokines did indeed change clotting profiles and caused platelet hyper-activation. Here we confirmed a changed clotting profile in the presence of IL-1β, IL-6 and IL-8, using TEG. IL-8 showed the most pronounced hypercoagulability in TEG, where all the clotting parameters where significantly different to the naïve whole blood parameters (Table 2). The very sensitive SEM analysis showed changes in RBCs and platelets after exposure to all three interleukins. SEM analysis showed that, with the addition of IL-1β, IL-6 and IL-8 to whole blood (Figs 3, 4, 5), platelets were hyper-activated, showing pronounced spreading and clumped together. They were also closely associated and attached their pseudopodia onto RBC membranes. Platelets have receptors for all three interleukins, however, IL-6, is the only one of the three that actually has a receptor on RBCs; as in principle it activates all cells due to the uniform expression of gp130. To our surprise and against our hypothesis (where we believed that possibly only IL-6 might cause eryptosis), the addition of IL-8 not only showed the most pronounced effects on TEG parameters, but also on SEM and it caused some RBCs to become eryptotic (see Fig. 5A,B). The other 2 cytokines did not significantly affect RBC structure. Most RBC membranes, in the presence of IL-8, showed pathological membrane changes (see Fig. 5C). Eryptosis is RBC programmed cell death and has been discussed numerous times by73,74,75,76,77,78,79,80,81,82. It is characterized by cell shrinkage, cell membrane blebbing and cell membrane phospholipid scrambling83; and it involves COX, PGE2, ceramide and activation of calpain. All of these biochemical changes inside the cell lead to the morphological pathology, which is characteristic of eryptosis. These pathologies are visible as cell shrinkage, membrane scrambling and membrane blebbing52,82. We have previously reported eryptotic RBCs in inflammatory conditions like Parkinson’s disease52 and Alzheimer’s disease84, where IL-8 is also known to be unregulated. We show micrographs from such individuals in Fig. 6. Although our results showed that IL-8 causes the most pronounced changes in WB, we could not find literature evidence that IL-8 has receptors on RBC membranes. We could also not find evidence that previous researchers have found that IL-8 is associated with the induction of eryptosis, however, it is a well-known activator of apoptosis (which is similar to eryptosis vs. programmed cell death)85,86. It is also well known that IL-8 is closely involved in the traditional apoptotic pathways where e.g. COX and PGE2 play important roles in apoptosis initiation. Although we do not have evidence that IL-8 indeed binds to an RBC receptor, we show here that it does indeed causes the induction of eryptosis, confirmed by morphological changes.
RBCs and platelets are particularly sensitive to systemic inflammatory changes. Ultrastructure of RBCs, platelets as well as viscoelastic properties of clots formed with whole blood, might give great insight on effects that cytokines may have on the development of conditions like atherosclerosis, thrombosis and hypercoagulability. These biophysical readouts are therefore excellent health indicators and may be exploited in therapeutic studies.
Additional Information
How to cite this article: Bester, J. and Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 6, 32188; doi: 10.1038/srep32188 (2016).
References
Choi, G., Schultz, M. J., Levi, M. & van der Poll, T. The relationship between inflammation and the coagulation system. Swiss Med Wkly 136, 139–144 (2006).
Anžej, S. et al. Evidence of hypercoagulability and inflammation in young patients long after acute cerebral ischaemia. Thromb Res 120, 39–46, doi:10.1016/j.thromres.2006.08.005 (2007).
Karabudak, O. et al. Inflammation and hypercoagulable state in adult psoriatic men. Acta Derm Venereol. 88, 337–340, doi:10.2340/00015555-0456 (2008).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Levi, M. & van der Poll, T. Inflammation and coagulation. Crit Care Med. 38, S26–S34, doi:10.1097/CCM.0b013e3181c98d21 (2010).
Tantry, U. S. et al. Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic RIsk Progression (TRIP) study. Platelets 21, 360–367, doi:10.3109/09537100903548903 (2010).
Borissoff, J. I., Spronk, H. M. H. & ten Cate, H. The Hemostatic System as a Modulator of Atherosclerosis. N Engl J Med. 364, 1746–1760 (2011).
Kitchens, C. S. et al. Thrombotic Storm Revisited: Preliminary Diagnostic Criteria Suggested by the Thrombotic Storm Study Group. Am J Med 124, 290–296, doi:10.1016/j.amjmed.2010.10.018 (2011).
Petäjä, J. Inflammation and coagulation. An overview. Thromb Res. 127 Suppl 2, S34–S37, doi:10.1016/S0049-3848(10)70153-5 (2011).
van der Poll, T., de Boer, J. D. & Levi, M. The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis. 24, 273–278, doi:10.1097/QCO.0b013e328344c078 (2011).
Esmon, C. T. Molecular circuits in thrombosis and inflammation. Thromb Haemost 109, 416–420, doi:10.1160/Th12-08-0634 (2013).
Levi, M. & van der Poll, T. Disseminated intravascular coagulation: a review for the internist. Int Emerg Med. 8, 23–32, doi:10.1007/s11739-012-0859-9 (2013).
Levi, M. & Poll, T. Coagulation in patients with severe sepsis. Semin Thromb Hemost 41, 9–15, doi:10.1055/s-0034-1398376 (2015).
Ozeren, A. et al. Levels of serum IL-1beta, IL-2, IL-8 and tumor necrosis factor-alpha in patients with unstable angina pectoris. Mediators Inflamm. 12, 361–365, doi:10.1080/09629350310001633360 (2003).
Dahlbäck, B. Blood coagulation. The Lancet 355, 1627–1632 (2000).
Levi, M., van der Poll, T. & Büller, H. R. Bidirectional relation between inflammation and coagulation. Circulation 109, 2698–2704 (2004).
Levi, M. & van der Poll, T. Two-way interactions between inflammation and coagulation. Trends in cardiovascular medicine 15, 254–259 (2005).
Carroll, M. B. & Bond, M. I. Use of tumor necrosis factor-alpha inhibitors in patients with chronic hepatitis b infection. Semin Arthritis Rheum 38 (3), 208–217 (2008).
Marinos, G. et al. Tumor necrosis factor receptors in patients with chronic hepatitis B virus infection. Gastroenterology 108, 1453–1463 (1995).
Koulentaki, M. et al. Nitric oxide and pro-inflammatory cytokines in acute hepatitis B. European journal of internal medicine 15, 35–38 (2004).
Wang, J.-Y., Wang, X.-L. & Liu, P. Detection of serum TNF-, IFN-, IL-6 and IL-8 in patients with hepatitis B. World Journal of Gastroenterology 5, 38–40 (1999).
Beaulieu, L. M. et al. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 34, 552–564, doi:10.1161/atvbaha.113.302700 (2014).
Dinarello, C. A. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 41, 1203–1217, doi:10.1002/eji.201141550 (2011).
Burger, D., Dayer, J. M., Palmer, G. & Gabay, C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. 20, 879–896, doi:10.1016/j.berh.2006.06.004 (2006).
Dinarello, C. A. Blocking IL-1 in systemic inflammation. J Exp Med. 201, 1355–1359, doi:10.1084/jem.20050640 (2005).
Gabay, C., Lamacchia, C. & Palmer, G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 6, 232–241, doi:10.1038/nrrheum.2010.4 (2010).
Dinarello, C. A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 83, 447s–455s (2006).
Permin, H. & Andersen, L. P. Inflammation, immunity and vaccines for Helicobacter infection. Helicobacter 10 Suppl 1, 21–25, doi:10.1111/j.1523-5378.2005.00337.x (2005).
Brown, G. T., Narayanan, P., Li, W., Silverstein, R. L. & McIntyre, T. M. Lipopolysaccharide stimulates platelets through an IL-1beta autocrine loop. J Immunol. 191, 5196–5203, doi:10.4049/jimmunol.1300354 (2013).
Hottz, E. D., Monteiro, A. P., Bozza, F. A. & Bozza, P. T. Inflammasome in platelets: allying coagulation and inflammation in infectious and sterile diseases? Mediators Inflamm. 2015, 435783, doi:10.1155/2015/435783 (2015).
Mihara, M., Hashizume, M., Yoshida, H., Suzuki, M. & Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 122, 143–159, doi:10.1042/cs20110340 (2012).
Rose-John, S. The soluble interleukin-6 receptor and related proteins. Best Pract Res Clin Endocrinol Metab. 29, 787–797, doi:10.1016/j.beem.2015.07.001 (2015).
Wolf, J., Rose-John, S. & Garbers, C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 70, 11–20, doi:10.1016/j.cyto.2014.05.024 (2014).
Hashizume, M. & Mihara, M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis 2011, 765624, doi:10.1155/2011/765624 (2011).
Rose-John, S., Scheller, J., Elson, G. & Jones, S. A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 80, 227–236, doi:10.1189/jlb.1105674 (2006).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813, 878–888, doi:10.1016/j.bbamcr.2011.01.034 (2011).
Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 8, 1237–1247, doi:10.7150/ijbs.4989 (2012).
Scheller, J. & Rose-John, S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 195, 173–183, doi:10.1007/s00430-006-0019-9 (2006).
Marino, M. et al. Novel path to IL-6 trans-signaling through thrombin-induced soluble IL-6 receptor release by platelets. J Biol Regul Homeost Agents 27, 841–852 (2013).
Tedgui, A. & Mallat, Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 86, 515–581, doi:10.1152/physrev.00024.2005 (2006).
Rabinovich, A., Cohen, J. M. & Kahn, S. R. Predictive value of markers of inflammation in the postthrombotic syndrome: a systematic review: inflammatory biomarkers and PTS. Thromb Res. 136, 289–297, doi:10.1016/j.thromres.2015.06.024 (2015).
Ueland, T. et al. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 443, 71–77, doi:10.1016/j.cca.2014.09.001 (2015).
Konrad, F. M. & Reutershan, J. CXCR2 in acute lung injury. Mediators Inflamm 2012, 740987, doi:10.1155/2012/740987 (2012).
Waugh, D. J. & Wilson, C. The interleukin-8 pathway in cancer. Clin Cancer Res. 14, 6735–6741, doi:10.1158/1078-0432.ccr-07-4843 (2008).
Regnault, V. et al. Platelet activation induced by human antibodies to interleukin-8. Blood 101, 1419–1421, doi:10.1182/blood-2002-02-0620 (2003).
Antonelli, A. et al. Serum levels of proinflammatory cytokines interleukin-1beta, interleukin-6 and tumor necrosis factor alpha in mixed cryoglobulinemia. Arthritis Rheum 60, 3841–3847, doi:10.1002/art.25003 (2009).
Arican, O., Aral, M., Sasmaz, S. & Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17 and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005, 273–279, doi:10.1155/mi.2005.273 (2005).
Bester, J., Buys, A. V., Lipinski, B., Kell, D. B. & Pretorius, E. High ferritin levels have major effects on the morphology of erythrocytes in Alzheimer’s disease. Front Aging Neurosci. 5, 88 (2013).
Pretorius, E. et al. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol. 14, 30, doi:10.1186/s12933-015-0192-5 (2015).
Gasparyan, A. Y., Ayvazyan, L., Pretorius, E. & Kitas, G. D. Platelets in rheumatic diseases: friend or foe? Curr Pharm Des. 20, 552–566 (2014).
Pretorius, E., du Plooy, J., Soma, P. & Gasparyan, A. Y. An ultrastructural analysis of platelets, erythrocytes, white blood cells and fibrin network in systemic lupus erythematosus. Rheumatol Int. 34, 1005–1009, doi:10.1007/s00296-013-2817-x (2014).
Pretorius, E. et al. Eryptosis as a marker of Parkinson’s disease. Aging-US 6, 788–818 (2014).
Nielsen, V. G., Kirklin H. K., Hoogendoorn H., Ellis T. C. & Holman W. L. Thromboelastographic method to quantify the contribution of factor XIII to coagulation kinetics. Blood coagulation & Fibrinolysis: an international journal in haemostasis and thrombosis 18, 145–150, doi:10.1097/MBC.0b013e32802f7d91 (2007).
Nielsen, V. G. Beyond cell based models of coagulation: analyses of coagulation with clot “lifespan” resistance-time relationships. Thromb Res. 122, 145–152, doi:10.1016/j.thromres.2007.09.003 (2008).
Nielsen, V. G., Gurley, W. Q. & Burch, T. M. The Impact of Factor XIII on Coagulation Kinetics and Clot Strength Determined by Thrombelastography. Anesthesia & Analgesia 99, 120–123, doi:10.1213/01.ane.0000123012.24871.62 (2004).
Nielsen, V. G., Lyerly, R. T. & Gurley, W. Q. The effect of dilution on plasma coagulation kinetics determined by thrombelastography is dependent on antithrombin activity and mode of activation. Anesthesia and Analgesia 99, 1587–1592, doi:10.1213/01.ANE.0000136843.58799.AB (2004).
Nielsen, V. G. & Pretorius, E. Iron-enhanced coagulation is attenuated by chelation A thrombelastographic and ultrastructural analysis. Blood Coagul Fibrinolysis 25, 845–850, doi:10.1097/mbc.0000000000000160 (2014).
Nielsen, V. G. et al. Carbon monoxide and iron modulate plasmatic coagulation in Alzheimer’s disease. Curr Neurovasc Res. 12, 31–39 (2015).
Haemonetics. TEG 5000 Hemostasis analyzer system 3–5 (Haemonetics Corporation, 2011).
Nielsen, V. G. et al. Qualitative thrombelastographic detection of tissue factor in human plasma. Anesth Analg 104, 59–64, doi:10.1213/01.ane.0000248223.05152.a1 (2007).
Bester, J. & Pretorius, E. Viscoelasticity as a measurement of clot structure in poorly controlled type 2 diabetes patients: towards a precision and personalized medicine approach. Oncotarget: Precision Medicine In Press (2016).
Swanepoel, A. C., Nielsen, V. G. & Pretorius, E. Viscoelasticity and Ultrastructure in Coagulation and Inflammation: Two Diverse Techniques, One Conclusion. Inflammation eprint (ahead of print), doi:10.1007/s10753-015-0148-7 (2015).
Buys, A. V. et al. Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc Diabetol. 12, 25, doi:10.1186/1475-2840-12-25 (2013).
Lang, E. et al. Accelerated apoptotic death and in vivo turnover of erythrocytes in mice lacking functional mitogen- and stress-activated kinase MSK1/2. Sci Rep. 5, 17316, doi:10.1038/srep17316 (2015).
Ran, Q. et al. Eryptosis Indices as a Novel Predictive Parameter for Biocompatibility of Fe3O4 Magnetic Nanoparticles on Erythrocytes. Sci Rep. 5, 16209, doi:10.1038/srep16209 (2015).
Tesoriere, L. et al. Oxysterol mixture in hypercholesterolemia-relevant proportion causes oxidative stress-dependent eryptosis. Cell Physiol Biochem. 34, 1075–1089, doi:10.1159/000366322 (2014).
Lupescu, A. et al. Enhanced suicidal erythrocyte death contributing to anemia in the elderly. Cell Physiol Biochem 36, 773–783, doi:10.1159/000430137 (2015).
Briglia, M. et al. Fucoxanthin Induced Suicidal Death of Human Erythrocytes. Cell Physiol Biochem. 37, 2464–2475, doi:10.1159/000438599 (2015).
Zierle, J., Bissinger, R., Egler, J. & Lang, F. Lapatinib Induced Suicidal Death of Human Erythrocytes. Cell Physiol Biochem. 37, 2275–2287, doi:10.1159/000438583 (2015).
Lang, E. et al. Impact of cyclin-dependent kinase CDK4 inhibition on eryptosis. Cell Physiol Biochem. 37, 1178–1186, doi:10.1159/000430241 (2015).
Lang, E. et al. Vitamin D-Rich Diet in Mice Modulates Erythrocyte Survival. Kidney Blood Press Res. 40, 403–412, doi:10.1159/000368517 (2015).
Zidova, Z. et al. DMT1-mutant erythrocytes have shortened life span, accelerated glycolysis and increased oxidative stress. Cell Physiol Biochem. 34, 2221–2231, doi:10.1159/000369665 (2014).
Lang, F., Abed, M., Lang, E. & Föller, M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal 21, 138–153, doi:10.1089/ars.2013.5747 (2013).
Lang, F., Gulbins, E., Lang, P. A., Zappulla, D. & Föller, M. Ceramide in suicidal death of erythrocytes. Cell Physiol Biochem. 26, 21–28, doi:10.1159/000315102 (2010).
Lang, F., Jilani, K. & Lang, E. Therapeutic potential of manipulating suicidal erythrocyte death. Expert Opin Ther Targets 19, 1219–1227, doi:10.1517/14728222.2015.1051306 (2015).
Lang, F., Lang, E. & Föller, M. Physiology and pathophysiology of eryptosis. Transfus Med Hemother 39, 308–314, doi:10.1159/000342534 (2012).
Lang, F. & Qadri, S. M. Mechanisms and significance of eryptosis, the suicidal death of erythrocytes. Blood Purif 33, 125–130, doi:10.1159/000334163 (2012).
Qadri, S. M. et al. Sphingosine but not sphingosine-1-phosphate stimulates suicidal erythrocyte death. Cell Physiol Biochem. 28, 339–346, doi:10.1159/000331750 (2011).
Qadri, S. M. et al. Phosphatidylserine externalization and procoagulant activation of erythrocytes induced by Pseudomonas aeruginosa virulence factor pyocyanin, doi:10.1111/jcmm.12778 (2016).
Qadri, S. M. et al. Dicoumarol activates Ca2+-permeable cation channels triggering erythrocyte cell membrane scrambling. Cell Physiol Biochem. 28, 857–864, doi:10.1159/000335800 (2011).
Qadri, S. M. et al. Enhanced suicidal erythrocyte death in mice carrying a loss-of-function mutation of the adenomatous polyposis coli gene. J Cell Mol Med. 16, 1085–1093, doi:10.1111/j.1582-4934.2011.01387.x (2012).
Pretorius, E., Olumuyiwa-Akeredolu, O. O., Mbotwe, S. & Bester, J. Erythrocytes and their role as health indicator: Using structure in a patient-orientated precision medicine approach. Blood Rev. Epub ahead of print, doi:10.1016/j.blre.2016.01.001 (2016).
Lang, E., Qadri, S. M. & Lang, F. Killing me softly - suicidal erythrocyte death. Int J Biochem Cell Biol. 44, 1236–1243, doi:10.1016/j.biocel.2012.04.019 (2012).
Bester, J., Soma, P., Kell, D. B. & Pretorius, E. Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia and the possible role of bacterial lipopolysaccharides (LPS). Oncotarget 6, 35284–35303 (2015).
Narayanan, K. B. & Park, H. H. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 20, 196–209, doi:10.1007/s10495-014-1073-1 (2015).
Allen, T. C. & Kurdowska, A. Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 138, 266–269, doi:10.5858/arpa.2013-0182-RA (2014).
Acknowledgements
Thank you to Dr M Strydom who managed the patient recruitment and phlebotomy. National Research Foundation (NRF) of South Africa (91548: Competitive Program) and Medical Research Council (MRC) of South Africa (Self-Initiated Research Program: A0×331). Grant holder: E Pretorius.
Author information
Authors and Affiliations
Contributions
E.P. wrote the main manuscript text and prepared all figures; J.B. performed TEG analysis and prepared whole blood smears for SEM. Both authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bester, J., Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep 6, 32188 (2016). https://doi.org/10.1038/srep32188
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32188
This article is cited by
-
Impact of rise and fall phases of shear on platelet activation and aggregation using microfluidics
Journal of Thrombosis and Thrombolysis (2024)
-
Analysis of related factors of portal vein thrombosis in liver cirrhosis
BMC Gastroenterology (2023)
-
Association of placental histology and neonatal hematologic outcomes
Journal of Perinatology (2023)
-
Rat tight junction proteins are disrupted after subchronic exposure to okadaic acid
Environmental Science and Pollution Research (2023)
-
Axial spondyloarthritis may protect against poor outcomes in COVID-19: propensity score matched analysis of 9766 patients from a nationwide multi-centric research network
Clinical Rheumatology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.