Abstract
In contrast to animals, plant development involves continuous organ formation, which requires strict regulation of cell proliferation. The core cell cycle machinery is conserved across plants and animals, but plants have developed new mechanisms that precisely regulate cell proliferation in response to internal and external stimuli. Here, we report that the DOF transcription factor OBP4 negatively regulates cell proliferation and expansion. OBP4 is a nuclear protein. Constitutive and inducible overexpression of OBP4 reduced the cell size and number, resulting in dwarf plants. Inducible overexpression of OBP4 in Arabidopsis also promoted early endocycle onset and inhibited cell expansion, while inducible overexpression of OBP4 fused to the VP16 activation domain in Arabidopsis delayed endocycle onset and promoted plant growth. Furthermore, gene expression analysis showed that cell cycle regulators and cell wall expansion factors were largely down-regulated in the OBP4 overexpression lines. Short-term inducible analysis coupled with in vivo ChIP assays indicated that OBP4 targets the CyclinB1;1, CDKB1;1 and XTH genes. These results strongly suggest that OBP4 is a negative regulator of cell cycle progression and cell growth. These findings increase our understanding of the transcriptional regulation of the cell cycle in plants.
Similar content being viewed by others
Introduction
In contrast to animals, plants continue to generate new organs throughout their life cycles. Plant growth and development are closely coordinated to achieve the plant’s final size and shape49. Plant morphogenesis relies on spatially and temporally coordinated cell proliferation and differentiation44. Cell proliferation is increase the number of cells through the cell growth and division producing daughter cells. The core cell cycle regulatory mechanisms are conserved in eukaryotes. The cell cycle consists of the replication phase and the mitotic phase, which are separated by G1 and G2, two gap phases6. Cyclin-dependent kinases (CDKs) and their phosphorylated target genes, which trigger the onset of DNA replication and mitosis at the post-transcriptional level, play a central role in cell cycle regulation. Cell differentiation converts dividing cells into non-dividing cells and also determines cell fate. In many cases, cell differentiation occurs simultaneously with the endocycle. During the endocycle, the cell replicates its genome without cell division, resulting in cells with more than 4C of DNA content49. Previous study has shown that many genes are involved in endocycle initiation. CDKB1;1, a key regulatory gene in the G2-M phase transition, has been shown to negatively regulate the onset of endocycle4. High expression level of ICK/KRP1 may suppress the endocycle and inhibit G2-M phase transition45,65. RBR1 regulates the switch from proliferation to endocycles by targeting the CCS52A1 and CSS52A2 genes33.
Cell cycle progression is also controlled at the transcriptional level in response to developmental and environmental signals15. A number of MYB transcription factors9,27, TCP transcription factors1,29 and E2F transcription factors23,34 can affect cell proliferation in a spatial, temporal or quantitative manner. Previous research has shown that TCP20 and MYB59 bind to the promoter of CYCB1;1 and modulate its expression in G2-M phase29,38. TCP15 controls endocycle onset by directly targeting the CYCA2;3 and RBR (RETINOBLASTOMA-RELATED) genes31. The AP2 transcription factor ANT (AINTEGUMENTA) gene can maintain the meristematic competence of cells by regulating CYCD3;1 expression37. However, the mechanisms by which developmental signals interact with plant cell cycle progression remain unclear.
DNA binding with one finger (DOF) proteins are a group of plant-specific transcription factors. A conserved N-terminal DNA-binding domain is present in typical DOF proteins and the N-terminal domain can bind to DNA sequences harbouring an AAAG core motif and interact with other proteins. A divergent transcription regulation domain is present at the C terminus, this divergent C terminus is used for transcriptional regulation51,58. In Arabidopsis, the DOF transcription factor family has 37 members that play diverse roles in plant development and in response to environmental stimuli. In a screen for proteins that interact with the bZIP transcription factor OCS element binding factor 4 (OBF4), the OBF binding protein 1 (OBP1) was the first to be reported. Furthermore, other DOF transcription family proteins, OBP2 and OBP3, were identified to have the ability to bind OBF4 and to enhance their binding to the OCS element in the downstream target genes21. OBP1 is involved in the control of cell division by targeting the core cell cycle regulator CYCB3;3 and the S phase-specific transcription factor DOF2.347. OBP2 plays a role in the regulation of indole glucosinolate metabolism48 and OBP3 has been characterized as a novel component of light signalling54.
This study identifies the function of DOF transcription factor OBP4/DOF5.4 in cell cycle regulation. OBP4 controlled plant growth by regulating core cell cycle genes involved in the replication machinery and cell expansion regulators. Correspondingly, OBP4 overexpression resulted in the early onset of endocycle progression. Based on the expression profile and genetic results, we propose that OBP4 is a novel regulator of cell cycle progression and cell expansion.
Materials and Methods
General methods
Arabidopsis seeds were sown in soil and grown in a growth chamber with a 16-h day length provided by fluorescent light at 120 μmol m−2s−1 and a day:night temperature of 22 °C-18 °C and relative humidity of 60–75%. Agrobacterium tumefaciens strain GV3101 was used to transform Arabidopsis thaliana Col-0 (35S::OBP4, RNAi::OBP4, pOBP4::GUS). Chemicals were purchased from Roche (Basel, Switzerland) or Sigma (St. Louis, MO). We used NCBI (http://www.ncbi.nlm.nih.gov/) and TAIR (http://www.arabidopsis.org/) to analyze gene sequences. We used GraphPad primer 5 (GraphPad Software Inc., San Diego, CA) to make diagrams. For induction experiments, plants were sprayed with 20 μm estradiol solution or grown in MS medium containing 20 μm estradiol.
Plasmid constructs
To construct the 35S::OBP4 vector, polymerase chain reaction (PCR) was used to amplify the OBP4 CDS using Col-0 leaf cDNA as the template. The OBP4 cDNA was cloned into a modified pHB plant transformation vector via the PstI and SacI sites. To construct the pER8::OBP4 and pER8::OBP4::HA vectors, OBP4 cDNA or OBP4 cDNA fused to an HA tag were cloned into the pER8 vector via the SpeI and XhoI sites. To construct the pER8::OBP4::VP16 vector, OBP4 cDNA was fused to the VP16 domain and cloned into the pER8 vector via the SpeI and XhoI sites. To construct the RNAi::OBP4 vector, the forward and reverse fragments of a 3′-UTR OBP4 sequence that does not have similarity with the UTRs of the other genes were linked to the pCAMBIA1301-RNAi vector. To construct the Promoter::GUS fusion vector, an approximately 2.0 kb 5′-UTR upstream of the ATG start codon was fused to the Escherichia coli GUS reporter gene in the pCAMBIA1300 vector. The primer sequences are shown in Supplementary Table S1.
RNA isolation and Quantitative real-time PCR
The roots, stems, leaves, flowers and siliques of five-week-old plant were harvested for RNA isolation using TRIzol reagent (Invitrogen, Grand Island, NY). 2 μg RNA was digested with DNase and reverse-transcribed by Superscript III reverse transcriptase (Invitrogen) to generate first-strand cDNA in a 20 μl reaction volume. Quantitative RT-PCR was performed in 96-well plates with 1 μl of a 1:5 dilution of the first-strand cDNA reaction and in a 10 μl volume on a LightCycler 96 Sequence Detection System (Roche). Transcript levels were measured based on SYBR Green technology using SYBR Green reagent (Applied Biosystems Applera, Darmstadt, Germany) according to the manufacturer’s instructions. Data were analyzed using the ΔΔCT method. The qPCR results were analyzed using LightCycler 96 analysis software 1.1 (Roche). The Arabidopsis ACTIN2 gene was used as a control. Primer sequences are shown in Supplementary Table S2.
Subcellular localization of OBP4
OBP4 sequences were cloned into PBI121 vector to construct fusion plasmid by using primers containing BamH1 and Sal1 sites. PBI121 plasmid was used as a control. The fusion construct and the control plasmid were transformed into Arabidopsis and tobacco leaf epidermal cells. Transformed cells were observed under a OLYMPUS FV1000 microscope.
Culture and synchronization of Arabidopsis suspension cell
Col Arabidopsis Seeds were surface-sterilized and vernalized. Callus formation was induced under continuous darkness at 23 °C by placing root explants excised from 2-week-old seedlings onto callus induction medium. The rapidly dividing callus was inoculated into MS medium containing 3% w/v sucrose, 0.5 mg/L 2,4-D, 0.05 mg/L kinetin to establish suspension cultures, and incubated on a rotary shaker at 110 rpm. The method to achieve synchronization was as previous described62.
Flow Cytometry of Cell Cycle Progression
WT, pER8::OBP4 and pER8::OBP4::VP16 transgenic Arabidopsis seeds were sterilized with 0.15% mercuric chloride and germinated on a plate. After induction with estradiol, nuclears were isolated from Arabidopsis leaves at different time points55. The DNA content of individual transgenic cells was determined by flow cytometry. Each sample was prepared three times and subjected to Beckman Coulter MoFlo XDP. A total of 10,000 nuclei were measured per analysis.
ChIP assays
An HA-coding sequence was fused in frame to the end of the OBP4 gene and the gene fusion was sub-cloned under the estradiol-inducible promoter in the pER8 vector. The expression construct was then transformed into Col-0 plants. Two-week-old pER8::OBP4::HA transgenic plants that were grown on MS agar plates that expressed OBP4::HA via induction by estradiol for a period were used to conduct ChIP experiments according to a previously described method using anti-HA polyclonal antibodies (Roche:118674231)64. The qRT-PCR primers used are listed in Supplemental Table 2.
For scanning electron microscopy
The leaves and hypocotyls were cut and sputter-coated with gold and further visualized by using a Hitachi JEOL JSM-6360LV SEM (scanning electron microscope).
Microscopy and GUS staining
After removal of chlorophyll using 100% ethanol, epidermal cells were observed under a Olympus light microscope (Tokyo, Japan) and the size of cells were analyzed by cell^P Software, about thirty cells were observed of each genotype. β-Glucuronidase activity was determined histochemically as described20 and ten lines of transgenic plants were observed.
Results
OBP4 is a DOF transcription factor family protein
The OBP1 protein was previously isolated47,61. Other members of the OBP family were identified using the sequence of the OBP1 DOF domain to screen an Arabidopsis cDNA library. OBP4 is a previously uncharacterized, new member of the DOF family of proteins in Arabidopsis. The amino acid sequences of the OBPs (OBP1, OBP2, OBP3 and OBP4) are shown in Fig. S1. The OBP4 protein has 308 amino acids and its DOF domain is located at the N terminus (boxed)32,38. The homology among the OBP proteins is restricted to the DOF domain. The specific characteristics of the OBPs are indicated in Fig. S1. In OBP1 and OBP2, a serine-rich domain is observed, while in OBP2, an asparagine-rich domain is present. We further compared the OBPs to DOF proteins from other species in the database. We did not identify any significant homology between the OBP proteins and other DOF members other than the DOF domain. As shown in Fig. 1A, OBP4 is closely related to the Brassica napus BnaC02g41820D protein.
Homologous alignment and analysis of OBP4 levels.
(A) Phylogenetic comparison of DOF protein sequences from Arabidopsis thaliana, Brassica napus, Oryza sativa, Capsella rubella, Camelina sativa and Eutrema salsugineum in the database. Alignments were made by ClustalW2 and MEGA5.1 software using the default parameters and excluding positions with gaps. To determine OBP4 expression in response to hormone treatment, eight-day-old seedlings were transferred to fresh medium (mock), or medium that contained (B) IAA (0.25 μM) (C) GA (0.25 μM) (D) JA (0.25 μM) (E) SA (0.5 μM) (F) ABA (0.1 μM) or (G) CK (0.1 μM) for the indicated period. RNA was then isolated from whole seedlings and analysed. Three biological and technical replicates were used. Asterisks indicate significant differences, p < 0.05.
OBP1 was first isolated through interactions with OBF proteins by an ocs element61. Ocs elements respond to salicylic acid (SA) and OBP1-3 expression has been reported to be induced by SA. Furthermore, we assessed whether OBP4 is affected by hormone treatment. After IAA and SA treatment, OBP4 expression in the leaves increased substantially at 3 h and remained high at 24 h (Fig. 1B,E), while after 8 h of GA treatment, OBP4 expression increased only slightly (Fig. 1D).
OBP4 gene expression and OBP4 protein localization
To investigate the expression pattern of OBP4 during development, qPCR was used to examine the OBP4 mRNA levels in different plant organs, including seedlings, roots, shoots, flowers, rosette leaves, cauline leaves, buds and siliques. Our results showed that OBP4 was ubiquitously expressed at different levels in all of the examined organs (Fig. 2A). Then, approximately 2.0 kb of the 5′-UTR promoter region of OBP4 was fused to the β-glucuronidase (GUS) reporter gene to investigate OBP4 expression at the tissue level. The GUS signal was observed in the silique, trichomes, flower organs and leaves (Fig. 2F–H). GUS was also detected in the seedlings, hypocotyls and roots (Fig. 2B–E). Furthermore, the subcellular localization of the OBP4 protein was analyzed. Figure 2I shows that the GFP control protein was observed in both the nuclei and the cytoplasm, whereas the 35 S::OBP4::GFP protein was localized in the nuclei when transiently expressed in tobacco epidermal cells. The same results were observed in transgenic roots stably expressing OBP4::GFP. These results indicate that OBP4 is a nuclear protein.
Tissue and cell cycle-dependent OBP4 expression patterns and subcellular localization.
(A) qPCR analysis of OBP4 gene expression level. (B–G) GUS promoter analysis of OBP4 tissue-specific expression. (B) Seedling at 6 days after germination. (C) Roots. (D) Hypocotyls. (E) Rosette leaves. (F) Trichome. (G) Sepal of flower and anther. (H) Siliques. (I) Subcellular localization of the vector control and OBP4 in tobacco epidermal cells and transgenic Arabidopsis root cells. DIC, differential interference contrast, referring to bright-field images of the cells. Asterisks indicate significant differences, p < 0.05.
Constitutive and inducible overexpression of OBP4 alters cell proliferation and results in a dwarf phenotype
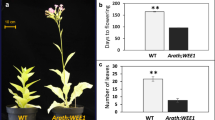
To elucidate the mechanisms underlying the effects of OBP4 on cell cycle progression and cell expansion, which influence plant development, we performed a phenotypic analysis of plants that were constitutively overexpressing OBP4. OBP4 transgenic lines were screened and eight transgenic lines overexpressing OBP4 were obtained, with the highest expression level approximately 32-fold to that of the WT at mRNA level (Fig. S2). We further attempted to increase the OBP4 expression in transgenic Arabidopsis without success. Constitutive OBP4 overexpression resulted in obvious defects in the plant development. Mature 35S::OBP4 plants had a greatly reduced plant size with small and curly rosette leaves (Fig. 3A,B). To investigate the reason for the arrested plant development, leaf cells and hypocotyl cells were counted and measured. In the one-week-old 35S::OBP4 seedling hypocotyls and the fourth leaves from three-week-old 35S::OBP4 plants, the cell numbers were decreased and epidermal cell size was also reduced compared to that of the wild-type plants (Fig. 3C–F,O,P). The petals were shorter, the siliques produced were shorter and fewer and seed setting did not occur (Fig. 3G–L). Furthermore, transgenic plants were characterized by shorter plant height and reduced flower number (Fig. 3M,N).
Phenotypes of transgenic plants overexpressing OBP4.
Six-week-old (A) wild-type and (B) 35 S::OBP4 plants grown under long-day conditions. The heights of the inflorescence stems of at least 7 plants were measured. (M) The plant height and (N) the flower number of the wild-type and 35 S::OBP4 plants. Two-week-old adaxial epidermis of and basal part of hypocotyls from (C,E) wild-type and (D,F) 35 S::OBP4 leaves were visualized by scanning electron microscopy. The red outlines indicate the cell boundary of one epidermal cell. Cell area analysis of (O) epidermal cells and (P) cotyledon cells. The bars represent the SD. Inflorescence morphology of (G) wild-type and (H) 35 S::OBP4 plants. Silique analysis of (I) wild-type and (J) 35 S::OBP4 plants. Flower morphology of (K) wild-type and (b) 35 S::OBP4 plants. (Q) Seedlings of control (left) and pER8::OBP4 (right) plants grown on MS medium supplied with 20 μM estradiol in the dark. Hypocotyls of (S) control and (R) pER8::OBP4 dark-grown seedlings (Scale bar = 100 μm). The red outlines indicate the cell boundary of one hypocotyl cell. (T) Analysis of epidermal cells of cotyledons and the fourth rosette leaf of wild-type and pER8::OBP4 plants (data are the mean ± SD, n = 7). Scale bars = 5 cm in (A,B); 0.4 cm in (K,L); 0.3 cm in (G–J); 100 μm in (C-F). Asterisks indicate significant differences, p < 0.05.
We also observed a similar seedling phenotype by inducing OBP4 expression in pER8::OBP4 transgenic plants. pER8::OBP4 plants treated with 20 μM estradiol were smaller than WT and showed growth arrest 4 days after induction in dark (Fig. 3Q,R,S). At the cellular level, the hypocotyl cells of pER8::OBP4 plants were smaller than those of WT (Fig. 3T). The situation was similar for the 35S::OBP4 plants which were smaller than WT. Unfortunately, OBP4 loss-of-function mutant was not available in the public seed collections and RNAi approaches were unsuccessful. To convert OBP4 into a transcriptional activator, we fused it with the strong activation domain VP16. The resulting OBP4::VP16 causes strong transcriptional activation. Therefore, we transformed pER8::OBP4::VP16 as a potential negative OBP4 allele into plants. Further we obtained pER8::OBP4::VP16 transgenic plants and after four days of estradiol induction, the leaves were obviously larger than those of the control (Fig. S4). These results demonstrated that OBP4 can regulate plant development.
OBP4 negatively regulates G2-to-M phase transition and promotes endocycle onset
We hypothesized that G2-to-M phase transition is defective in pER8::OBP4 plants. To investigate the mechanism underlying OBP4 regulation of G2-to-M phase transition, we determined whether the transgenic plant cells showed defects during cell cycle progression, particularly G2-to-M phase transition, such as G2 phase arrest2,39 and early endocycle entry49. Overexpression of OBP4 affected cell proliferation in suspension cells (Fig. S5). To assess whether G2-to-M phase transition is defective in pER8::OBP4 plants, we measured the DNA content in pER8::OBP4 plant cells by flow cytometry. Before induction, at 7 days after germination (DAG), the ploidy level distribution was similar to that of wild-type (Fig. 4D,H); Nuclei isolated from both WT and pER8::OBP4 seedlings displayed four primary peaks that corresponded to 2 C, 4 C, 8 C and 16 C DNA content. After 1-day induction with estradiol, pER8::OBP4 plants showed an increased proportion of 8 C cells and a decreased proportion of 2 C and 4 C cells (Fig. 4E,I). After 4-day induction, the proportion of 8 C cells greatly increased in the pER8::OBP4 plants and the proportion of 4 C and 2 C cells further decreased. Additionally, 32 C cells appeared in the β-estradiol-induced plants (Fig. 4F,J). These results suggest that more pER8::OBP4 plant cells escaped from the cell cycle compared to control plants and that early endocycle onset occurred.
Flow cytometric analysis of cell cycle progression.
(A–C) Plant phenotypes in two induced OBP4 overexpression lines (pER8 line 1, pER8 line 2). The images are from the mid-portion of each root of 13-day-old plants grown on MS solid medium (A) without or (B) with 20 μm estradiol induction for 5 days. Scale bar = 0.5 cm. (C) The root lengths and lateral root number were measured before and after induction. Flow cytometric analysis of wild-type (D–G) and pER8::OBP4 (H–K) in DAG 7, DAG 8 and DAG 11 seedlings after 0, 1 and 4 days of 20 μm estradiol induction, respectively. 2 C, 4 C, 8 C, 16 C and 32 C represent the DAPI signals that correspond to nuclei with different DNA contents. Flow cytometric analysis was repeated three times. The average ( ± SD) from more than 5 seedlings for each time point is presented. Asterisks indicate significant differences, p < 0.05.
We further measured the DNA content in pER8::OBP4::VP16 transgenic plant cells by flow cytometry. Before induction, at 9 days after germination (DAG), the ploidy level distribution was similar to that of the control. After the plants were induced for 4 days, pER8::OBP4::VP16 transgenic plants had a decreased proportion of 8 C and 32 C cells. In contrast, the vector control and the wild-type plants showed similar DNA content before and after estradiol treatment (Fig. S4 D–G). Thus, Delayed endocycle onset was observed in the OBP4::VP16 plants.
OBP4 negatively regulates cell cycle genes and cell wall expansion factors
Because the OBP4 protein is a nuclear-localized DOF transcription factor, we hypothesized that OBP4 controls plant development by regulating gene expression. To verify this hypothesis, we investigated the ability of OBP4 to regulate downstream target genes. We analyzed differentially expressed genes between wild-type plants and plants that constitutively overexpressed OBP4. We identified the genes encoding cell wall expansion factors, such as xyloglucan endo -transglucosylation genes (XTH3, XTH9, XTH17) and expansion genes (EXPA8, EXPA9, EXPB3) and cell cycle-related genes, such as cyclin genes (CYCA2;1, CYCA2;3, CYCB1;1, CYCB2;1, CYCB2;3, CYCB3;1,) cyclin-dependent kinase genes CDKB1;1, CDKB1;2, CDKB2;1) and cell cycle-related transcription factors (MYB3R4). These genes were markedly down-regulated in the 35 S::OBP4 transgenic lines as determined by qPCR analysis (Fig. 5).
OBP4 regulates the expression of core cell cycle genes and cell expansion factors.
(A) Using qRT-PCR analysis, we confirmed that the cyclin genes CYCA2;1, CYCA2;4, CYCB1;1, CYCB1;4, CYCB2;2, CYCB2;3, CYCB2;4, CYCB3;1, CDKB1;1, CDKB1;2, CDKB2;1 and a G2-specific regulator, MYB3R4, were repressed strongly in OBP4-OE plants. (B) The cell expansion factor genes XHT3, XTH9, XTH17, EXPA9, EXPA8 and EXPB3 were strongly repressed in OBP4-OE. Expression levels were normalized to ACTIN2 expression as an internal control. The values shown are the means ± SD. Experiments were performed three times. Asterisks indicate significant differences, p < 0.05.
We further identified genes that were immediately expressed upon activation of OBP4. The estradiol induction system is successful at inducing plant transcription factors47. We generated a homozygous transgenic plant (pER8::ProOBP4::OBP4) with the OBP4 promoter driving a fusion of OBP4 cDNA under the β-estradiol-inducible promoter in the Col-0 background. The transgenic plant was similar to wild-type plants under normal conditions. In contrast, estradiol spray clearly inhibited leaf development; upward curling leaves were observed (Fig. 6A). Similar to stimulation of steroid receptors in animals, estradiol treatment led to transport of the OBP4 protein from the cytoplasm to the nucleus, resulting in downstream events. Furthermore, we performed qPCR analysis using RNA samples within 20 h of induction. Figure 6B indicates that the 16 isolated genes were clearly down-regulated after estradiol treatment for 20 h. The large number of expression changes at 20 h after estradiol induction suggests that secondary targets of OBP4 might exist. Genes with reduced expression were further examined for their induction kinetics. We profiled the selected genes at 3 h and 8 h after induction to identify direct targets. Among these genes, we identified 5 genes that were consistently down-regulated at 3 h and 8 h after induction: two core cell cycle proteins (CYCB1;1 and CDKB1;1) and three cell wall expansion factors (XTH3, XTH9 and XTH17). Moreover, their expression levels were higher in the OBP4::VP16 transgenic plants (Fig. S3). These results show that CYCB1;1, CDKB1;1, XTH3, XTH9 and XTH17 are putative genes immediately downstream of OBP4.
Search for immediate downstream genes of OBP4 using an estradiol-inducible transgenic plant.
(A) pER8::ProOBP4::OBP4 construct. The OBP4 gene was driven by the OBP4 promoter. The pER8 plant is shown before and after β-estradiol treatment. Leaf development was partly inhibited by estradiol induction in the seedlings. Scale bar = 0.5 cm. (B) Time-course induction analysis of the 10 cell cycle-related genes and 6 cell wall modification genes performed using real-time RT-PCR analysis. pER8::ProOBP4::OBP4 plant after a single addition of estradiol (20 μm). RNA samples were extracted from pER8::ProOBP4::OBP4 leaves at 0, 3, 8, or 20 h after mock (circles) or estradiol (dots) treatment. Vertical axis indicates the relative mRNA amount after β-estradiol treatment. Horizontal axis indicates the time after treatment. The bars indicate the SD. Asterisks indicate significant differences, p < 0.05.
The OBP4 transcription factor belongs to the DOF subfamily, whose predicted DNA binding sites are [A/T]AAAG and CTTT[A/T]57,58,59. Based on these DNA sequences, we found many DOF binding sites in the promoter regions of the CYCB1;1, CDKB1;1, XTH3, XTH9 and XTH17 genes (Fig. 7A). To verify whether OBP4 can bind to these consensus sequences in planta, we performed ChIP analysis to test the relative enrichment of OBP4 in the selected fragments. An antibody directed against HA was used to precipitate OBP4::HA and its crosslinked DNA, which was isolated from 2-week-old pER8::OBP4::HA seedlings after estradiol induction for 36 h and pER8::HA seedlings without induction served as a negative control. Immunoprecipitated DNA crosslinked with OBP4::HA was analyzed by real-time PCR using the gene-specific primers listed in Supplemental Table 2. OBP4 protein enrichment was indeed detected at the promoter regions of the CYCB1;1, CDKB1;1, XTH9 and XTH17 genes containing the predicted DOF binding sites (Fig. 7B). In sum, we conclude that OBP4 can transcriptionally regulate both cell cycle progression and cell expansion by regulating key cell cycle genes and cell expansion factors (Fig. 8).
ChIP analysis to verify OBP4 target genes.
(A) The sequence regions used for ChIP assays are marked in the gene promoters (A–K, top panel). DOF binding motifs are indicated as arrowheads. (B) pER8::OBP4::HA-1 and pER8::OBP4::HA-2 transgenic plants grown on MS-agar plates for 2 weeks were used for ChIP assays (bottom panel). The enrichment shown was calculated as the DNA level of each fragment in the β-estradiol-treated sample divided by that in the DMSO-treated sample. Anti-HA antibody was used to precipitate OBP4-HA. Three measurements were averaged for individual assays. Bars indicate the SD. The values in Col-0 plants were set to 1 after normalization to ACT2 for qPCR analysis. Asterisks indicate significant differences, p < 0.05.
Model of OBP4 function in cell cycle regulation and cell expansion.
Model of the OBP4 and OBP1 regulatory network. We inferred that the cell cycle regulators CYCB1;1 and CDKB1;1 and the cell expansion factors XTH9 and XTH17 are OBP4 target genes. We also showed that OBP4 negatively affects both cell cycle progression and cell expansion. OBP4 overexpression resulted in arrested development of Arabidopsis plants, with fewer and smaller cells. While OBP1 positively regulates CYCD3;3 and DOF2.3. OBP1 negatively affects cell expansion. The dotted line indicates entry to endoreplication.
Discussion
In most eukaryotic cells, cell cycle progression is an ordered series of events during which the chromosomes are duplicated and one copy of each duplicated chromosome segregates into each of the two daughter cells. Cell cycle regulation is critical for the normal development of multicellular organisms. The cell cycle is controlled by numerous mechanisms that ensure correct cell division, i.e., regulation of cyclin-dependent kinases (CDKs) by cyclins, CDK inhibitors and phosphorylation50. Fundamentally, the molecular mechanisms controlling the primary events in cell cycle progression are similar to those in eukaryotic cells. Thus, research on diverse organisms has contributed to the growing understanding of how these events are coordinated and controlled in plants10. However, the plant cell has a cell wall and the mechanisms of plant cell proliferation that are coordinated with cell wall expansion and contribute to plant growth remain unclear. A number of DOF proteins have been reported to participate in plant development and to respond to environmental stimuli. Previous results have shown that OBP1 regulates cell cycle re-entry by targeting CYCD3;3 and DOF2.347. Here, we report that another plant-specific OBP family protein, OBP4, is a negative regulator of cell division and plant growth. This study demonstrates the OBP4-mediated inhibition of plant growth via defective cell cycle progression and cell wall expansion.
OBP4 is a positive regulator of the endocycle during plant development
To characterize the function of OBP4, we examined transgenic plants with up-regulated or reduced OBP4 activity. The 35S constitutive and inducible overexpression transgenic plants presented obvious growth defects (Fig. 3A,B). While overexpression of OBP4 fused to an activation domain promoted plant growth (Fig. S3). OBP4 was expressed in all of the examined organs but at different levels (Fig. 2B–H). The OBP4 expression data from qPCR analysis were consistent with the GUS staining results (Fig. 2A). The timing and localization of OBP4 expression suggest that OBP4 has a role in cell cycle regulation. In inducible OBP4 transgenic plants, the cell size and other characteristics related to cell division were inhibited. OBP4 overexpression caused defective G2-to-M phase transition.
Furthermore, we hypothesized that OBP4 plays a role in the onset of the endocycle35. Indeed, OBP4 activation resulted in a longer G2 phase and early endoreduplication onset (Fig. 4). These findings support the conclusion that endoreplication entry involves the engagement of B-type cyclins and cyclin-dependent kinases19. Cell proliferation was negatively controlled by OBP4 activation and subsequently, the rate of cell proliferation was lower than that of the control, eventually resulting in fewer and smaller cells. The expression pattern of OBP4 together with its effect on cell cycle regulators, is a novel mechanism by which this protein controls cell proliferation.
OBP4 inhibits cell expansion by negatively regulating cell wall expansion factors
In contrast to animal cells, plant cells have cell walls. Cell morphogenesis involves the spatially controlled remodeling of the cell wall. Cell expansion is believed to be controlled by a balance between the drive for expansion and localized wall loosening43. There are two types of proteins that are involved in this process. Xyloglucan endotransglycosylases cleave the glycan backbone and transfer the cut end to an acceptor molecule. Expansins appear to loosen hydrogen bonds between cellulose microfibrils and cellulose fibers11,26. XTH9 may be associated with cell expansion in Arabidopsis18. A T-DNA insertion mutant of XTH17 showed low XET activity and had moderately shorter roots than wild-type plants66. Plants overexpressing AtEXP10 have large organs with enlarged cells, supporting the role of expansion in cell enlargement. Arabidopsis has 26 expansin genes and more than 5 expansin genes may act redundantly to influence plant growth8. To determine how OBP4 affects cell expansion, we examined the expression levels of several characterized Arabidopsis genes involved in cell expansion. The expression levels of the expansion genes AtEXPA3, AtEXPA5, AtEXPA8, AtEXPA9 and AtEXPB3 were significantly lower in 35S::OBP4 plants than in wild-type plants. AtEXP3 has been proposed to promote cell expansion24 and the atexp5 mutant has smaller leaves than wild-type40. These results suggest that reduced cell size in OBP4 overexpression lines might result from decreased expression of particular expansion genes. OBP4 may negatively regulate cell wall expansion factors to modify cell wall plasticity during the cell growth stage.
The well-accepted “compensated cell enlargement theory” indicates that a reduced cell number could be compensated by increased cell size and vice versa50. Constitutive and inducible activation of OBP4 in plants resulted in a significant reduction in both cell size and cell number. The leaves of 35S::OBP4 plants were characterized by fewer cells and a reduced cell size, which could not be explained by the compensation theory and which might suggest that OBP4 targets not only cell proliferation but also cell expansion. In contrast, the reduced cell size correlated well with the reduced level of the endocycle (fewer cells entering the endocycle), which has often been reported previously. To identify possible molecular mechanisms that could explain the changes in cell size, we examined the expression of genes encoding growth-related proteins among transcripts that were down-regulated in our profiling data and identified a large number of genes encoding cell wall-loosening enzymes, which are essential for cell growth and expansion30. Therefore, OBP4 can also affect growth by targeting the expression of genes encoding cell wall loosening enzymes. In conclusion, OBP4, as a DOF family transcription factor, can modify the onset of the endocycle and cell growth during plant development. This characterization of the function of OBP4 extends our understanding of the molecular mechanisms underlying cell cycle progression and cell expansion.
Additional Information
How to cite this article: Xu, P. et al. AtDOF5.4/OBP4, a Dof Transcription Factor Gene that Negatively Regulates Cell Cycle Progression and Cell Expansion in Arabidopsis thaliana. Sci. Rep. 6, 27705; doi: 10.1038/srep27705 (2016).
References
Aggarwal, P. et al. The TCP4 transcription factor of Arabidopsis blocks cell division in yeast at G1–>S transition. Biochem Biophys Res Commun 410, 276–281 (2011).
Bernstein, H. S. & Coughlin, S. R. A mammalian homolog of fission yeast Cdc5 regulates G2 progression and mitotic entry. Journal of Biological Chemistry 273, 4666–4671 (1998).
Boudolf, V. et al. B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. Plant Cell 16, 945–955 (2004a).
Boudolf, V. et al. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiol 150, 1482–1493 (2009).
Boudolf, V. et al. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 16, 2683–2692 (2004b).
Brodsky, W. Y. & Uryvaeva IV. Cell polyploidy: its relation to tissue growth and function. Int Rev Cytol 50, 275–332 (1977).
Chaubet, N., Flenet, M., Clement, B., Brignon, P. & Gigot, C. Identification of cis-elements regulating the expression of an Arabidopsis histone H4 gene. Plant Journal 10, 425–435 (1996).
Cho, H. T. & Cosgrove, D. J. Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 97, 9783–9788 (2000).
Cominelli, E. & Tonelli, C. A new role for plant R2R3-MYB transcription factors in cell cycle regulation. Cell Res 19, 1231–1232 (2009).
Cosgrove, D. J. Growth of the plant cell wall. Nat Rev Mol Cell Biol, 6, 850–861 (2005).
Cosgrove, D. J. et al. The growing world of expansins. Plant and Cell Physiology 43, 1436–1444 (2002).
De Veylder, L. et al. Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. Embo Journal 21, 1360–1368 (2002).
De Veylder, L., Beeckman, T. & Inze, D. The ins and outs of the plant cell cycle. Nature Reviews Molecular Cell Biology 8, 655–665 (2007).
Dewitte, W., Deveaux, Y., Huntley, R., Samland, A. & Murray, J. A. H. The role of D-type cyclins in plant growth and development. Plant Biotechnology 2002 and Beyond, Proceedings of the 10th IAPTC&B Congress 2002 Orlando, Florida, USA Dordrecht: Springer: Netherlands,, 247–253 (2003 June 23-28).
Gaamouche, T. et al. Cyclin-dependent kinase activity maintains the shoot apical meristem cells in an undifferentiated state. Plant Journal 64, 26–37 (2010).
Haga, N. et al. R1R2R3-Myb proteins positively regulate cytokinesis through activation of KNOLLE transcription in Arabidopsis thaliana. Development 134, 1101–1110 (2007).
Healy, J. M. S., Menges, M., Doonan, J. H. & Murray, J. A. H. The Arabidopsis D-type cyclins CycD2 and CycD3 both interact in vivo with the PSTAIRE cyclin-dependent kinase Cdc2a but are differentially controlled. Journal of Biological Chemistry 276(10), 7041–7047 (2001).
Hyodo, H. et al. Active gene expression of a xyloglucan endotransglucosylase/hydrolase gene, XTH9, in inflorescence apices is related to cell elongation in Arabidopsis thaliana. Plant Mol Biol, 52, 473–482 (2003a).
Inze, D. & De Veylder, L. Cell cycle regulation in plant development. Annual Review of Genetics 40, 77–105 (2006).
Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13), 3901–3907 (1987).
Kang, H. G. & Singh, K. B. Characterization of salicylic acid-responsive, Arabidopsis DOF domain proteins: overexpression of OBP3 leads to growth defects. Plant Journal 21(4), 329–339 (2000).
Kim, Y. S. et al. A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell 18, 3132–3144 (2006).
Kosugi, S. & Ohashi, Y. Functional analysis of Arabidopsis E2F and DP transcription factors. Plant and Cell Physiology 43, S212–S212 (2002).
Kwon, Y. R., Lee, H. J., Kim, K. H., Hong, S. W. & Lee, H. Ectopic expression of Expansin3 or Expansin beta 1 causes enhanced hormone and salt stress sensitivity in Arabidopsis. Biotechnology Letters 30, 1281–1288 (2008).
Lee, K. H., Kim, Y. S., Park, C. M. & Kim, H. J. Proteomic identification of differentially expressed proteins in Arabidopsis mutant ntm1-D with disturbed cell division. Molecules and Cells 25, 70–77 (2008).
Lee, Y., Choi, D. & Kende, H. Expansins: ever-expanding numbers and functions. Current Opinion in Plant Biology 4, 527–532 (2001).
Lai, L. B. et al. The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. Plant Cell 17, 2754–2767 (2005).
Lammens, T. et al. Atypical E2F activity restrains APC/C-CCS52A2 function obligatory for endocycle onset. P Natl Acad Sci USA 105, 14721–14726 (2008).
Li, C. X., Potuschak, T., Colon-Carmona, A., Gutierrez, R. A. & Doerner, P. Arabidopsis TCP20 links regulation of growth and cell division control pathways. P Natl Acad Sci USA 102, 12978–12983 (2005).
Li, Y., Jones, L. & McQueen-Mason, S. Expansins and cell growth. Current Opinion in Plant Biology 6, 603–610 (2003).
Li, Z. Y., Li, B. & Dong, A. W. The Arabidopsis transcription factor AtTCP15 regulates endoreduplication by modulating expression of key cell-cycle genes. Mol Plant 5, 270–280 (2012).
Lucas-Reina, E., Romero-Campero, F. J., Romero, J. M. & Valverde, F. An Evolutionarily Conserved DOF-CONSTANS Module Controls Plant Photoperiodic Signaling. Plant Physiol 168, 561 (2015).
Magyar, Z. et al. Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO J 31(6), 1480–1493 (2012).
Mariconti, L. et al. The E2F family of transcription factors from Arabidopsis thaliana - Novel and conserved components of the retinoblastoma/E2F pathway in plants. Journal of Biological Chemistry 277, 9911–9919 (2002).
Menges, M., Hennig, L., Gruissem, W. & Murray, J. A. H. Genome-wide gene expression in an Arabidopsis cell suspension. Plant Mol Biol 53, 423–442 (2003).
Menges M. & Murray, J. A. H. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant Journal 30, 203–212 (2002).
Mizukami, Y. & Fischer, R. L. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proceedings of the National Academy of Sciences of the United States of America 97, 942–947 (2000).
Moreno-Risueno, M. A., Martinez, M., Vicente-Carbajosa, J. & Carbonero, P. The family of DOF transcription factors: from green unicellular algae to vascular plants. Molecular Genetics and Genomics 277, 379–390 (2007).
Ohi, R. et al. The Schizosaccharomyces-Pombe Cdc5(+) Gene Encodes an Essential Protein with Homology to C-Myb. Embo Journal 13, 471–483 (1994).
Park, C. H. et al. Brassinosteroids control AtEXPA5 gene expression in Arabidopsis thaliana. Phytochemistry 71, 380–387 (2010).
Ramirez-Parra, E. & Gutierrez, C. E2F regulates FASCIATA1, a chromatin assembly gene whose loss switches on the endocycle and activates gene expression by changing the epigenetic status. Plant Physiol 144, 105–120 (2007).
Reichheld, J. P., Sonobe, S., Clement, B., Chaubet, N. & Gigot C. Cell-Cycle-Regulated Histone Gene-Expression in Synchronized Plant-Cells. Plant Journal 7, 245–252 (1995).
Roberts, K. The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol 6, 688–694 (1994).
Sabelli, P. A. et al. Control of cell proliferation, endoreduplication, cell size and cell death by the retinoblastoma-related pathway in maize endosperm. Proceedings of the National Academy of Sciences of the United States of America. 110, 1827–1836 (2013).
Schnittger, A., Weinl, C., Bouyer, D., Schobinger, U. & Hulskamp, M. Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 15, 303–315 (2003).
Sekine, M. & Iwakawa, H., Harashima H. Arabidopsis CDKA;1, a Cdc2 homolog, controls proliferation of generative cells in male gametogenesis. Plant and Cell Physiology 47, S12–S12 (2006).
Skirycz, A. et al. The DOF transcription factor OBP1 is involved in cell cycle regulation in Arabidopsis thaliana. Plant Journal 56, 779–792 (2008).
Skirycz, A. et al. DOF transcription factor AtDOF1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant Journal 47, 10–24 (2006).
Sugimoto-Shirasu, K. & Roberts, K. “Big it up” endoreduplication and cell-size control in plants. Current Opinion in Plant Biology 6, 544–553 (2003).
Tsukaya, H. Organ shape and size: a lesson from studies of leaf morphogenesis. Current Opinion in Plant Biology 6, 57–62 (2003).
Umemura, Y., Ishiduka, T., Yamamoto, R. & Esaka, M. The DOF domain, a zinc finger DNA-binding domain conserved only in higher plants, truly functions as a Cys2/Cys2 Zn finger domain. Plant Journal 37, 741–749 (2004).
Vlieghe, K. et al.The DP-E2F-like gene DEL1 controls the endocycle in Arabidopsis thaliana. Curr Biol 15, 59–63 (2005).
Walker, J. D., Oppenheimer, D. G., Concienne, J. & Larkin, J. C. SIAMESE, a gene controlling the endoreduplication cell cycle in Arabidopsis thaliana trichomes. Development 127, 3931–3940 (20000).
Ward, J. M., Cufr, C. A., Denzel, M. A. & Neff, M. M. The DOF transcription factor OBP3 modulates phytochrome and cryptochrome signaling in Arabidopsis. Plant Cell 17, 475–485 (2005).
Xu, P. & Cai, W. RAN1 is involved in plant cold resistance and development in rice (Oryza sativa). J Exp Bot, 65, 3277–3287 (2014).
Yamamoto, Y. & Ito, M. A group of R1R2R3-Myb proteins act as repressors on transcription of G2/M phase-specific genes in Arabidopsis thaliana. Plant and Cell Physiology 48, S87–S87 (2007).
Yanagisawa, S. The transcriptional activation domain of the plant-specific DOF1 factor functions in plant, animal and yeast cells. Plant and Cell Physiology 42, 813–822 (2001).
Yanagisawa, S. The DOF family of plant transcription factors. Trends Plant Sci 7(12), 555–560 (2002).
Yanagisawa, S. DOF domain proteins: plant-specific transcription factors associated with diverse phenomena unique to plants. Plant and Cell Physiology 45, 386–391 (2004).
Yang, X., Tuskan, G. A. & Cheng, M. Z. Divergence of the DOF gene families in poplar, Arabidopsis and rice suggests multiple modes of gene evolution after duplication. Plant Physiol 142, 820–830 (2006).
Zhang, B., Chen, W., Foley, R. C., Buttner, M. & Singh, K. B. Interactions between distinct types of DNA binding proteins enhance binding to ocs element promoter sequences. Plant Cell 7(12), 2241–2252 (1995).
Zhang, W. K. et al. Characterization of a novel cell cycle-related gene from Arabidopsis. Journal of Experimental Botany, 56, 807–816 (2005).
Zhang, Y. X. et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proceedings of the National Academy of Sciences of the United States of America 107(42), 18220–18225 (2010).
Zhang, Y. et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Journal of Agricultural Biotechnology, 107(42), 18220–18225 (2010).
Zhou, Y., Wang, H., Gilmer, S., Whitwill, S. & Fowke, L. C. Effects of co-expressing the plant CDK inhibitor ICK1 and D-type cyclin genes on plant growth, cell size and ploidy in Arabidopsis thaliana. Planta 216, 604–613 (2003).
Zhu, X. F. et al. Xyloglucan Endotransglucosylase-Hydrolase17 Interacts with Xyloglucan Endotransglucosylase-Hydrolase31 to Confer Xyloglucan Endotransglucosylase Action and Affect Aluminum Sensitivity in Arabidopsis. Plant Physiology, 165, 1566–1574 (2014).
Acknowledgements
This work was supported by National Natural Science Foundation of China (31500236), the National Basic Research Program of China (2011CB710902), the National Scientific Program (2012AA101103-04), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA04020202-15).
Author information
Authors and Affiliations
Contributions
W.C. and P.X. conceived the experiments. P.X. performed the experiments with the help of H.C., L.Y., W.C. and P.X. analyzed the data and wrote the manuscript. All authors have read and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xu, P., Chen, H., Ying, L. et al. AtDOF5.4/OBP4, a DOF Transcription Factor Gene that Negatively Regulates Cell Cycle Progression and Cell Expansion in Arabidopsis thaliana. Sci Rep 6, 27705 (2016). https://doi.org/10.1038/srep27705
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27705
This article is cited by
-
Comparative transcriptomics identifies candidate genes involved in the evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae)
BMC Plant Biology (2022)
-
Genome-wide identification of black pepper (Piper nigrum L.) Dof gene family and the differential gene screening in resistance to Phytophthora capsici
Biologia (2022)
-
Genome-wide binding analysis of the tomato transcription factor SlDof1 reveals its regulatory impacts on fruit ripening
Molecular Horticulture (2021)
-
Genome-wide identification and characterization of a plant-specific Dof transcription factor gene family in olive (Olea europaea) and its comparison with Arabidopsis
Horticulture, Environment, and Biotechnology (2021)
-
Utilizing MIKC-type MADS-box protein SOC1 for yield potential enhancement in maize
Plant Cell Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.