Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) has emerged as a therapeutic target in many diseases, because it can induce antioxidant enzymes and other cytoprotective enzymes. Moreover, some Nrf2 activators have strong anti-inflammatory activities. Oxidative stress and inflammation are major components involved in the pathology of diabetic nephropathy. In the present study, we evaluated the Nrf2-dependent anti-oxidative and anti-inflammatory effects of digitoflavone in streptozotocin-induced diabetic nephropathy. The molecular mechanisms of digitoflavone were investigated in vitro using SV40-transformed mouse mesangial cells (SV40-Mes13). For the in vivo experiment, diabetes was induced in Nrf2+/+ and Nrf2−/− mice by STZ injection and digitoflavone was administered 2 weeks after the STZ injection. Digitoflavone induced Nrf2 activation and decreased oxidative damage, inflammation, TGF-β1 expression, extracellular matrix protein expression and mesangial cell hyperplasia in SV40-Mes13 cells. Digitoflavone-treated Nrf2+/+ mice, but not Nrf2−/− mice, showed attenuated common metabolic disorder symptoms, improved renal performance, minimized pathological alterations and decreased oxidative damage, inflammatory gene expression, inflammatory cell infiltration, TGF-β1 expression and extracellular matrix protein expression. Our results show that the anti-oxidative and anti-inflammatory effects of digitoflavone are mediated by Nrf2 activation and that digitoflavone can be used therapeutically to improve metabolic disorders and relieve renal damage induced by diabetes.
Similar content being viewed by others
Introduction
Mortality associated with diabetes doubled to 1.3 million deaths worldwide from 1990–20101. Diabetic nephropathy is the major determinant of morbidity and mortality in patients with diabetes. Chronic hyperglycemia is a major initiator of diabetic nephropathy (DN)2; however, current therapies aimed at lowering blood glucose do not prevent the ultimate progression of DN and co-treatment with renoprotective drugs often results in toxicity, limiting their efficacy. Therefore, the use of effective medications that specifically target DN represents a useful and needed addition to treatment strategies involving strict glycemic control.
Oxidative stress and inflammation induced by hyperglycemia and dyslipidemia play significant roles in the development of vascular complications in DN patients and animals3,4,5,6,7. Oxidative stress contributes to the onset and pathogenesis of diabetic nephropathy and high glucose-induced renal damage is associated with excessive production of reactive oxygen species (ROS) under hyperglycemic conditions in vitro2,8. The development of DN is associated with significant inflammatory cell infiltration and increased plasma levels of C-reactive protein (CRP) and inflammatory cytokines such as vascular cell adhesion molecule-1 (VCAM-1) and IL-19,10. Oxidative stress and inflammation are inseparably linked, as each begets and amplifies the other. Hence, therapies targeting oxidative stress and inflammation may effectively preserve normal renal function and prevent or slow the progression of DN.
The Nrf2-Keap1 (nuclear factor erythroid 2-related factor 2-Kelch-like ECH-associated protein 1) system is one of the most critical cytoprotective mechanisms protecting the body against oxidative stress and it also plays an important role in reducing inflammation11,12,13,14. It regulates intracellular antioxidants, phase II detoxifying enzymes and many other proteins that detoxify xenobiotics and neutralize ROS and thus promotes cell survival and maintains cellular redox homeostasis15. Moreover, some Nrf2 activators also have strong anti-inflammatory activities16,17,18. A recent study found that Nrf2 is involved in the progression of renal disease19 and Nrf2 has been demonstrated to be an emerging therapeutic target for DN20,21. However, lessons gleaned from the phase II clinical trial of Nrf2 activator bardoxolone methyl suggest that new candidate Nrf2 activators and further mechanistic studies are needed before Nrf2 activators can be considered as a clinical treatment for DN.
Digitoflavone (3,0,4,5,7-tetrahydroxyflavone, Fig. 1A) is a member of the flavone subclass of flavonoids, which are enriched in vegetables and fruits22,23,24,25. Plants rich in digitoflavone have been used in Chinese traditional medicine to treat hypertension, inflammatory diseases and cancer26. In recent studies, we found that digitoflavone produces several biological effects, including induction of cell cycle arrest, angiogenesis inhibition, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) down-regulation in apoptosis and Nrf2 activation in chemoprevention27,28,29,30.
Digitoflavone activated Nrf2 diminishes mesangial ROS generation under hyperglycemic conditions.
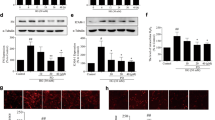
(A) Effects of digitoflavone on ARE-driven luciferase reporter activity in SV40-Mes13 cells. SV40-Mes13 cells were treated with various concentrations of digitoflavone for 24 h. Luciferase activity and cell viability were assayed in parallel as described in the materials and methods section. T-BHQ treatment represented an internal positive control. Top: chemical structure of digitoflavone. (B) and (C) Protein expression of Nrf2 and downstream targets was analyzed in SV40-Mes13 cells incubated in NG with various concentration of digitoflavone (B), HG, or HG + digitoflavone (C) in DMEM media for 24 h. (D) Nrf2 localization was assessed in SV40-Mes13 cells incubated in NG, HG, or HG + digitoflavone DMEM media for 24 h. (E) and (F) Total ROS levels (E) and O2·− levels (F) in SV40-Mes13 cells incubated in NG, HG, or HG + digitoflavone DMEM media are reported. All data represent the mean ± SD of triplicate independent experiments. *P < 0.05 vs. NG group. **P < 0.01 vs. control mice. #P < 0.05 vs. HG group.
In the current study, we screened digitoflavone as an effective Nrf2 activator in mouse mesangial cells and explored its therapeutic potential to slow the progression of diabetic nephropathy using a streptozotocin (STZ)-induced model of diabetes.
Materials and Methods
Material
Digitoflavone, 2′, 7′-Dichlorofluorescin diacetate (DCFH-DA), Periodic (PAS) kit, Trichrom estain (Masson) kit, butylhydroquinone (T-BHQ) were obtained from Sigma-aldrich, USA. Digitoflavone was dissolved in DMSO for in vitro study. Maxima® SYBR Green/ROX qPCR Master Mix (2×) and Maxima® First Strand cDNA Synthesis Kit were purchased from Fermentas life science (Fermentas, MBI). RIPA lysis buffer, dihydroethidium (DHE) was purchased from Beyotime, China. Antibodies for immunoblot and immunohistochemical (IHC) analysis included anti-7, 8-dihydro-8-oxo-2’-deoxyguanosine (8-oxo-dG) (Millipore, USA); anti-Nrf2, glutamate-cysteine ligase catalytic subunit (GCLC), glutamate—cysteine ligase regulatory subunit (GCLM), heme oxygenase-1 (HO-1), β-actin, Fibronectin(FN), nitrotyrosine (Santa Cruz Biotechnology, CA, USA); anti-Collagen IV, TGF-β1 (Abcam, USA); anti-phosphorylation-p65 (p-p65), phosphorylation-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (p-IκBα), phosphorylation-IκB kinase beta (IKKβ) (Cell Signaling Technology, MA, USA). Goat anti-rabbit IgG and goat anti-mouse IgG antibodies were purchased from LI-COR, Lincoln, NE, USA.
Cell culture and MTT assay
The SV40-transformed mouse mesangial cells (SV40-Mes13) cells were purchased from Cell Bank of Shanghai Institute of Biochemistry and Cell Biology. Cells were normally cultured in DMEM containing 5% fetal bovine serum and 5.6 mM glucose, unless indicated otherwise. Cell viability was determined using the MTT assay.
Transient transfection and analysis of luciferase reporter gene activity
The luciferase reporter assay was applied to investigate the transcriptional activity of Nrf2. The transient transfection and the ARE-luciferase reporter gene activity were carried out as previously described30. T-BHQ was set as a positive control.
SDS –PAGE, Western blot analysis, Immunofluorescence staining and RT-PCR
Cultured cells or kidney tissues were lysed in sample buffer (Beyotime, China) and protein concentration was determined using Nanodrop 1000 Spectrophotometer (Thermo, USA). Protein samples were analyzed by Western blot as previously described30. Cells in logarithmic phase were seeded at the density of 60 ~ 70% confluence per well into 24-well chamber slides. After treatment with test samples for the indicated times, cells were analyzed by immunofluorescence staining as previously described30. Cultured SV40-Mes13 cells or kidney tissues were lysed in TRIZOL reagent (Invitrogen, Carlsbad, CA) and total RNA was isolated. Then RNA was analyzed by RT-PCR as previously described30. Primers used for the reactions were purchased from Genscript and the primer sequences are listed as followed: MCP-1(Forward: 5'-AGGTGTCCCAAAGAAGCTGTA-3', Reverse: 5'-ATGTCTGGACCCATTCCTTCT-3'), CSF-1(Forward: 5'-CCCATATTGCGACACCGAA-3', Reverse: 5'-AAGCAGTAACTGAGCAACGGG-3'), ICAM-1(Forward: 5'-GCCTTGGTAGAGGTGACTGAG-3', Reverse: 5'-GACCGGAGCTGAAAAGTTGTA-3'), VCAM-1(Forward: 5'-TGCCGAGCTAAATTACACATTG-3', Reverse: 5'-CCTTGTGGAGGGATGTACAGA-3').
Cell proliferation assay
The rate of cell proliferation was measured two ways: 1) The iCELLigence system (Roche Applied Science, Germany and ACEA Biosciences, USA) allows real-time cell analysis (RTCA) via a system consisting of a microelectronic sensor array (MESA) 16× E-Plates coupled to a device station and an electronic sensor analyzer. The cell number, viability, morphology and degree of adherence of cells in contact with the electrodes affects the local ionic environment and leads to an increase in the electrode impedance, which is presented as the cell index (CI). The SV40 MES13 cells (8,000 per well) were cultured in NG or HG medium with or without DIG and cell growth was monitored for 30 h using the iCELLigence system. 2) Detection of Ki67 using indirect immunofluorescence.
Animal experiments
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Jiangsu Province Institute of Traditional Chinese Medicine and written up following the ARRIVE guidelines. Experiments were performed in accordance with published National Institutes of Health guidelines. Nrf2+/+ and Nrf2−/− C57BL/6 mice were purchased from the Jackson Lab and obtained through breeding of Nrf2+/−. All animals received water and food ad libitum. Eight-week-old mice received either sodium citrate (control) or STZ (50 mg/kg, pH 4.5, dissolved in sodium citrate) through intraperitoneal injection for 5 consecutive days. Two weeks following STZ injection, fasting glucose levels (4 h fast) were measured and mice with a fasting glucose level above 250 mg/dL were considered diabetic and used for this study. Mice were randomly allocated into four groups (n = 8 per group) to receive treatment: 1) control = carboxymethyl cellulose (CMC), 2) STZ = CMC, 3) Digitoflavone (DIG) 1 = 25 mg/kg digitoflavone, 4) DIG 2 = 50 mg/kg digitoflavone. Digitoflavone at 25/50 mg/kg dose suspended in 0.5% CMC was given as gavage to mice and mice of control group and STZ group were given 0.2 mL 0.5% CMC solution every day from week 2 to week 12. Doses of digitoflavone were guided by published literature30 and tested in pilot studies to ensure Nrf2 activation within 24 h after gavage. Blood glucose level (4 h fast) and body weight were monitored throughout the course of treatment. At the end of 12 weeks of treatment, mice were put into clean metabolic cages one day before sacrifice and urine was collected for 24 h. At week 12, mice were sacrificed and the kidney, blood and urine were isolated for analysis.
Measurements of creatinine, BUN, blood insulin and urinary albumin
Blood glucose level (4 h fast) was determined by glucometer (Ascensia Elite, Bayer, Leverkusen, Germany). Serum creatinine and usea nitrogen clearance were measured as an index of GFR. Urinary and blood creatinine, BUN, blood insulin and urinary albumin were detected by standard diagnostic kits (Jiancheng Biotech Co., Ltd., Nanjing, China) according to the manufacturer’s instructions.
Renal morphology assessment and IHC analysis
Tissue sections from paraffin-embedded kidney were stained with HE, PAS and Masson’s trichrome. For PAS-stained tissue sections, a five-grade method was used to evaluate the sclerosis in glomeruli as described31. For IHC analysis, the deparaffinized sections were boiled in sodium citrate buffer and primary antibody was used in a dilution of 1:100 for 4 °C overnight. The immunostaining was visualized using diaminobenzidine tetrahydrochloride (DAB) and the slides were counterstained with hematoxylin.
Oxidative stress test
Oxidative DNA damage in the glomerulus was measured by the amount of 8-oxo-dG, deparaffinized kidney sections were treated with proteinase K (10 mg/mL ), RNase A (100 mg/mL) and 2 N HCl then stained by IHC with an anti-8-oxo-dG antibody. Oxidative protein damage was measured by the IHC analysis of nitrotyrosine.
Cellular ROS contents were measured by incubating SV40-Mes13 cells with 10 μM DCFH-DA for 30 min and the fluorescence intensity was measured by flow cytometry. O2·− level was measured by flow cytometry using 10 μM DHE for 30 min to obtained sufficient fluorescence signal.
Statistical analysis
Results are expressed as mean ± SD. Statistical tests were performed using SPSS 15.0. Unpaired Student t tests were used to compare the means of two groups. For multiple comparisons between groups, a one-way ANOVA was performed to detect statistical differences. Differences within the ANOVA were determined using a Tukey’s post-hoc test. P value of less than 0.05 was considered to be statistically significant.
Results
Digitoflavone activates the Nrf2 pathway and diminishes mesangial ROS generation under hyperglycemic conditions
Mesangial cells play a crucial role in dictating the function of glomeruli. SV40-Mes13 cells were used to study the molecular mechanism by which digitoflavone preserves renal function during the progression of diabetic nephropathy. Treatment of SV40-Mes13 cells with digitoflavone for 24 h increased antioxidant-response element (ARE)-luciferase activity (Fig. 1A, bottom). Parallel cell viability assays revealed no obvious cytotoxic effects (62.5 to 250 nM) (Fig. 1A, bottom). Western blot analysis demonstrated that digitoflavone significantly and dose-dependently increased protein abundance of Nrf2 and its downstream anti-oxidant targets (Fig. 1B).
To mimic hyperglycemic conditions in diabetic nephropathy, SV40-Mes13 grown in normal glucose (NG) (5.5 mmol/L) medium were shifted to either NG medium with 19.5 mmol/L mannitol or high glucose (HG) (25 mmol/L) medium in the presence or absence of digitoflavone. Increased Nrf2 expression and predominant nuclear localization of Nrf2 were observed in response to digitoflavone treatment (Fig. 1C,D). Similarly, protein levels of Nrf2 downstream targets—GCLM, GCLC and HO-1 were increased in response to HG and further enhanced by treatment with digitoflavone (Fig. 1C). Hyperglycemic conditions also activated the Nrf2 pathway (Fig. 1C,D) in cultured SV40-Mes13 cells, presumably through oxidative stress.
SV40-Mes13 cells cultured in HG media showed higher levels of ROS and the free radical superoxide anion (O2·−) in comparison with those cultured in NG media and activation of Nrf2 by digitoflavone reduced ROS (Fig. 1E) and O2− (Fig. 1F) levels.
High glucose-mediated mesangial cell hyperplasia and inflammation can be antagonized by digitoflavone
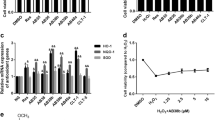
High glucose induced phosphorylation of NF-κB, its upstream kinase IκB kinase beta (IKKβ) and NF-κB inhibitor nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα); however, digitoflavone treatment reduced phosphorylation of NF-κB pathway members in a dose-dependent manner (Fig. 2A). High glucose also increased the expression of NF-κB downstream inflammatory cytokines MCP-1, as well as ICAM-1 and VCAM-1 and these effects were reversed by digitoflavone treatment (Fig. 2B).
High glucose-mediated mesangial cell hyperplasia and inflammation can be antagonized by digitoflavone induced Nrf2.
(A) Expression of NF-κB pathway proteins was assessed by immunoblot analysis in SV40-Mes13 cells incubated in NG, HG, or HG + digitoflavone DMEM media for 24 h. (B) Expression of NF-κB downstream genes was assessed by real-time PCR analysis in SV40-Mes13 cells. (C) Expression of TGF-β1 and downstream proteins was assessed by immunoblot analysis in SV40-Mes13 cells. (D) Cell growth of SV40-Mes13 cells incubated in NG, HG, or HG + digitoflavone DMEM media were monitored in real-time for 30 h (upper panel = average; lower panel = average with error). (E) Cell hyperplasia and death were assessed by Ki67 immunolabeling or Hoechst 33258 staining. All data represent the mean ± SD of triplicate independent experiments. *P < 0.05 vs. NG group. #P < 0.05 vs. HG group.
Expression levels of TGF-β1 and its downstream effectors were analyzed under hyperglycemic conditions. Hyperglycemia up-regulated TGF-β1, FN and collagen IV in SV40-Mes13 cells and these effects were suppressed by treatment with digitoflavone (Fig. 2C).
The SV40-Mes13 cells grown in HG media proliferated more over the course of the experiment than those grown in NG or in HG supplemented with digitoflavone (Fig. 2D). Immunofluorescence analysis of Ki67 showed that hyperglycemia caused mesangial cell hyperplasia, which was counteracted by digitoflavone-induced Nrf2 activation. No cell death was observed using Hoechst 33258 staining under any condition (Fig. 2E).
Activation of Nrf2 by digitoflavone improves metabolic disorder indices in an STZ-induced diabetic model
Nrf2 knockout mice (Nrf2−/−) were used to study the effectiveness of dietary Nrf2 activator digitoflavone in diabetic nephropathy. STZ was used to induce diabetes in Nrf2 wild-type (Nrf2+/+) and Nrf2−/− mice and diabetes-related common metabolic disorder indices were measured. Nrf2+/+ and Nrf2−/− mice treated with STZ showed significantly increased blood glucose levels (4 h fast) (Fig. 3A), urine production (Fig. 3C) and water uptake (Fig. 3D) and decreased weight gain (Fig. 3B). Importantly, only Nrf2+/+ animals showed significantly alleviated metabolic dysfunction indices following digitoflavone treatment, demonstrating that this effect of digitoflavone was Nrf2-dependent. In addition, STZ significantly decreased insulin levels and digitoflavone treatment did not alter insulin levels regardless of genotype (Fig. 3E), indicating that the protective effect of digitoflavone was insulin-independent.
Activation of Nrf2 by digitoflavone improves metabolic disorder in a STZ-induced diabetic model.
(A) The average level of blood glucose (4h fast) in each animal group is plotted. (B) Body weights of Nrf2+/+ (left panel) and Nrf2−/− (right panel) animals are shown. (C) Urine output from Nrf2+/+ (top panel) and Nrf2−/− (bottom panel) animals is shown. (D) Water consumption in the last 24 h before the mice were sacrificed is reported. (E) Blood insulin content is shown. Data are expressed as mean ± SD (n = 5). *P < 0.05 vs. control mice. **P < 0.01 vs. control mice. #P < 0.05 vs. STZ mice.
Digitoflavone alleviates renal damage induced in the STZ diabetic model
Functional and pathological changes in the kidney were measured to investigate the therapeutic effects of digitoflavone on kidney function in the STZ diabetes model. The ratio of kidney weight to body weight in all STZ-injected groups was higher than that of the control group and this ratio was lowered significantly by treatment with digitoflavone in Nrf2+/+ mice (Fig. 4A). Diabetic mice exhibited higher serum creatinine and BUN, but chronic treatment with digitoflavone (50 and 100 mg/kg/day) significantly and dose-dependently reduced serum creatinine (Fig. 4B) and blood urea nitrogen (Fig. 4C). UAE and UACR were tested at week 12. STZ increased UAE and UACR in all treated groups; however, this increase was reversed by digitoflavone treatment in Nrf2+/+ mice only (Fig. 4D,E).
Digitoflavone alleviates renal damage induced in the STZ-diabetic model.
(A) Kidney-to-body weight ratios are provided. (B) and (C) Serum creatinine and BUN content is reported. (D) and (E) Albuminuria was assessed by measuring UAE (D) and UACR (E). Kidney tissue sections from each mouse were subjected to HE, PAS and trichrome staining. (F) A representative image from 1 mouse per group is shown. G: PAS-stained tissues were used for semiquantitative scoring to obtain the glomerulosclerotic index. Data are expressed as mean ± SD (n = 5). *P < 0.05 vs. control mice. **P < 0.01 vs. control mice. #P < 0.05 vs. STZ mice.
In agreement with the urine and blood analysis, histological examination showed that digitoflavone treatment suppressed STZ-induced pathological changes in the glomerulus. Glomerular lesions were observed in HE-stained tissue sections from STZ-injected mice (Fig. 4F, HE panel). Treatment with digitoflavone effectively restored the normal morphology of glomeruli in Nrf2+/+, but not Nrf2−/−, mice (Fig. 4F, HE panel). PAS staining showed glomerulosclerosis in the STZ-treated group, which was significantly ameliorated in the digitoflavone-treated Nrf2+/+, but not Nrf2−/−, mice (Fig. 4F, PAS panel and Fig. 2G). STZ treatment resulted in collagen deposition inside the glomeruli and in the periglomerular area, which was reduced in digitoflavone-treated Nrf2+/+, but not Nrf2−/−, mice (Fig. 4F, trichrome panel).
Digitoflavone-induced activation of the Nrf2 pathway confers protection against renal oxidative damage
To demonstrate that the beneficial effect of digitoflavone against renal damage was Nrf2-dependent, expression levels of Nrf2 and its downstream targets, as well as oxidative damage in the kidney, were assessed. Treatment with digitoflavone markedly increased protein levels of Nrf2, GCLC, GCLM and HO-1 (Fig. 5A,B). Importantly, digitoflavone treatment significantly reduced oxidative damage in the kidneys of Nrf2+/+ mice, as measured by the local formation of 8-oxo-dG and nitrotyrosine in glomerular tissue (Fig. 5C,D). Treatment with digitoflavone did not reduce oxidative damage or increase expression of GCLC, GCLM, or HO-1 in Nrf2−/− mice.
Digitoflavone-induced activation of Nrf2 pathway confers protection against renal oxidative damage.
(A) Whole kidney lysates from 3 mice per group were subjected to immunoblot analysis with antibodies against Nrf2, GCLC, GCLM, HO-1 and β-actin. The intensity of bands from replicate immunoblots was quantified and plotted (right panel, bar graphs). (B) Fixed kidney tissues were stained with antibodies against Nrf2, GCLC and GCLM. (C) 8-oxo-dG and nitrotyrosine staining assessed oxidative damage in the kidney and representative images are provided. Data are expressed as mean ± SD (n = 3). *P < 0.05 vs. control mice. #P < 0.05 vs. STZ mice.
Digitoflavone reduced interstitial inflammatory cell infiltration and inflammatory gene expression through an Nrf2-dependent mechanism in the diabetic kidney
The development of DN is associated with significantly increased inflammatory cytokine expression and inflammatory cell infiltration. Real-time PCR (RT-PCR) revealed that STZ-induced diabetes was associated with significantly up-regulated MCP-1 (Mcp1), CSF-1 (Csf1), ICAM-1 (Icam1) and VCAM-1 (Vcam1) expression in Nrf2+/+ and Nrf2−/− mice and these changes in expression were reversed by digitoflavone in Nrf2+/+ mice only (Fig. 6A). Immunohistochemical staining for CD45 and CD68 was performed in kidney tissue samples from digitoflavone-treated or untreated diabetic mice to observe focal interstitial inflammatory cell infiltration. Diabetic mice showed significantly increased infiltration of CD45-positive (CD45+) leukocytes and CD68-positive (CD68+) macrophages in interstitial areas, while the control kidneys showed fewer leukocytes and no macrophages. Digitoflavone dose-dependently reduced renal CD45+ leukocyte and CD68+ macrophage infiltration in Nrf2+/+ animals only (Fig. 6B).
Digitoflavone reduces renal inflammatory gene expression and inflammatory cell infiltration.
(A) Whole kidney RNA from 3 mice per group was subjected to RT-PCR analysis of MCP-1, CSF-1, ICAM-1 and VCAM-1 gene expression. (B) Renal inflammatory cell infiltration was analyzed by IHC. Data are expressed as mean ± SD (n = 3). *P < 0.05 vs. control mice. #P < 0.05 vs. STZ mice.
Nrf2 activation regulates TGF-β1 and extracellular matrix (ECM) deposition
A previous study found a negative association between Nrf2 expression and TGF-β1 expression21. Corroborating these previously published results, basal expression of TGF-β1 in Nrf2−/− mice was higher than that of Nrf2+/+ mice (Fig. 7A,B). Diabetes induced by STZ was associated with significantly up-regulated protein levels of TGF-β1 and its downstream effectors FN and collagen IV and these effects were significantly diminished by treatment with digitoflavone in the Nrf2+/+ animals only (Fig. 7A,B).
Digitoflavone activated Nrf2 reduces TGF-β1 expression and ECM deposition.
(A) Immunoblot analysis was performed on lysates from the whole kidney (left panel) and band intensities were quantified (right panel, bar graphs) and reported as relative expression to control animals. (B) Fixed kidney tissue was analyzed by IHC. Data are expressed as mean ± SD (n = 3). *P < 0.05 vs. control mice. #P < 0.05 vs. STZ mice.
Discussion
Oxidative stress and inflammation are critical mediators of the pathogenesis and progression of chronic kidney disease (CKD), acting in a self-perpetuating cycle in which oxidative stress causes inflammation by several mechanisms, including the activation of NF-κB. In turn, inflammation causes oxidative stress via production of reactive oxygen, nitrogen and halogen species by activated leukocytes and resident cells32. Our previous studies showed that digitoflavone served as an NF-κB inhibitor and Nrf2 activator in different disease models29,30. In the current study, the anti-oxidative and anti-inflammatory effect of digitoflavone in diabetic nephropathy was clearly demonstrated both in vitro and in vivo.
Constitutive Nrf2 activity is crucial in maintaining redox balance under normal conditions and its induction in response to oxidative stress, with consequent transcription of cytoprotective genes, represents an important defense system against oxidative stress32. Digitoflavone-induced Nrf2 activation in cultured mouse mesangial cells has been confirmed under NG and HG conditions and its downstream antioxidant proteins GCLC, GCLM, HO-1, which comprise the main antioxidant effectors of digitoflavone-induced Nrf2 activation, were also up-regulated30. Several reactive oxygen intermediates can be generated in the progression of diabetic nephropathy: O2·−, the non-radical hydrogen peroxide (H2O2), the highly reactive hydroxyl free radical (·OH), peroxynitrite (ONOO−) and singlet oxygen (1O2). Among the ROS, attention has mainly been focused on superoxide anion production33. Reduced superoxide anion and total ROS levels were found in the digitoflavone-treated mouse mesangial cells under HG conditions.
The digitoflavone-induced Nrf2-dependent antioxidant response is accompanied by a reduced inflammatory response, which was associated with decreased phosphorylation of members of the NF-κB signaling pathway (IKKβ, IκBα and NF-κB) and down-regulation of its downstream inflammatory cytokines MCP-1, ICAM-1 and VCAM-1. Several mechanisms have been demonstrated to explain the relationship between Nrf2 signaling and NF-κB signaling, including Keap1, which may bind to IKKβ and thus inhibit NF-κB signaling34 and HO-1, which may limit NF-kB activity by inhibiting IκBα degradation35. However, because IKKβ ETGE motifs do not exist in rodent cells34, Keap1 cannot negatively regulate IKKβ in mouse mesangial cells. Because of this limitation of the action of Keap1 in rodents, the anti-inflammatory effect of digitoflavone may be caused by its anti-oxidant activity in mouse mesangial cells. Further studies are merited to determine whether regulation of the Nrf2/Keap1 system by digitoflavone can inhibit IKKβ in human cells. We also showed that digitoflavone-induced Nrf2 activation can reduce cell hyperplasia and down-regulate expression of TGF-β1 and its downstream effectors in mouse mesangial cells under HG conditions.
In addition to demonstrating the therapeutic potential of digitoflavone in suppressing the pathogenesis of diabetic nephropathy, we also sought to determine the mechanism by which digitoflavone-induced Nrf2 activation produces beneficial effects. Nrf2 wild type and knockout mice were subjected to the STZ-induced diabetic model in an in vivo study. The observed mild reduction in blood glucose might have contributed to the effects of digitoflavone in Nrf2+/+ mice. We also found that digitoflavone had no effect on insulin levels and the reduction in blood glucose may have been insulin-independent. The altered gluconeogenesis and glycolysis observed in the Nrf2−/− mice36 suggests that the blood glucose reduction in the DIG-treated Nrf2+/+ mice may be partially attributable to the Nrf2-mediated glucose metabolism and glycogen formation in the liver. Alternatively, DIG might improve hepatic insulin sensitivity by suppressing the expression of the sterol regulatory element-binding protein (SREBP)-137. The published literature in this field supports the notion that Nrf2 activators influence glucose up-take in fibroblasts38 and play a central role in the acquisition of insulin resistance induced by oxidative stress in cardiomyocytes39.
The Nrf2-dependent antioxidant response might be the main contributing factor to the protective effect of digitoflavone in diabetic nephropathy. The role of oxidative stress in the pathogenesis of diabetic nephropathy has gained increasing research attention in recent years40,41. In the current study, digitoflavone treatment significantly increased expression of Nrf2 and its downstream anti-oxidant proteins and this effect was accompanied by reduced oxidative damage in the glomerular tissues of STZ-treated Nrf2+/+ mice. These results indicate the importance of the Nrf2-dependent antioxidant response in ROS neutralization and alleviation of oxidative damage in the kidney.
We also showed that digitoflavone-induced Nrf2 activation decreased inflammation in mice with STZ-induced diabetic nephropathy. Inflammation is crucial in promoting the development and progression of DN42. Leukocytes produce pro-inflammatory cytokines that can induce resident renal cells to produce a spectrum of chemokines, including inflammatory cytokines MCP-1 and CSF-1 and adhesion molecules ICAM-1 and VCAM-142. In accordance with the in vitro study, digitoflavone treatment significantly alleviated STZ-induced changes in MCP-1, CSF-1, ICAM-1 and VCAM-1 gene expression in Nrf2+/+ mice only. In human DN, leukocytes and macrophages accumulate in the glomeruli and interstitium, even in the early stages of the disease43. In the STZ-induced diabetic nephropathy model, both Nrf2+/+ and Nrf2−/− mice showed accumulation of leukocytes and macrophages in the kidney and digitoflavone treatment significantly reduced inflammatory cell infiltration in Nrf2+/+ mice. Taken together, our in vitro and in vivo results show that digitoflavone induced Nrf2-dependent anti-oxidative effects that modulated inflammation in STZ-induced diabetic nephropathy, leading to down-regulation of inflammatory genes and reduced inflammatory cell infiltration.
Digitoflavone-mediated protection in diabetic nephropathy may also be mediated by the negative regulatory effect of Nrf2 activation on TGF-β1, a major profibrotic mediator of diabetic nephropathy. Subsequently, we found that digitoflavone treatment reduced expression of TGF-β1 and its downstream ECM proteins in mice with STZ-induced diabetic nephropathy and mouse mesangial cells.
Our findings indicate that the therapeutic benefit of digitoflavone in diabetic nephropathy is Nrf2-dependent. In addition to its antioxidant function, Nrf2 also negatively regulates inflammation and expression of TGF-β1 and ECM proteins. Our results provide convincing experimental evidence that dietary digitoflavone activates Nrf2 and can be used therapeutically to improve metabolic disorder and relieve kidney damage induced by diabetes. This study lays the foundation for clinical evaluation and the ultimate development of Nrf2 activator digitoflavone as a clinically used agent to prevent the onset and progression of diabetic nephropathy.
Additional Information
How to cite this article: Yang, Y. et al. Therapeutic potential of digitoflavone on diabetic nephropathy: nuclear factor erythroid 2-related factor 2-dependent anti-oxidant and anti-inflammatory effect. Sci. Rep. 5, 12377; doi: 10.1038/srep12377 (2015).
References
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Debnam, E. S. & Unwin, R. J. Hyperglycemia and intestinal and renal glucose transport: implications for diabetic renal injury. Kidney Int 50, 1101–1109 (1996).
Sassy-Prigent, C. et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49, 466–475 (2000).
Banba, N. et al. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int 58, 684–690 (2000).
Okada, S. et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52, 2586–2593 (2003).
Chow, F., Ozols, E., Nikolic-Paterson, D. J., Atkins, R. C. & Tesch, G. H. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 65, 116–128 (2004).
Gaede, P., Poulsen, H. E., Parving, H. H. & Pedersen, O. Double-blind, randomised study of the effect of combined treatment with vitamin C and E on albuminuria in Type 2 diabetic patients. Diabet Med 18, 756–760 (2001).
Fridlyand, L. E. & Philipson, L. H. Oxidative reactive species in cell injury: Mechanisms in diabetes mellitus and therapeutic approaches. Ann N Y Acad Sci 1066, 136–151 (2005).
Rivero, A. et al. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond) 116, 479–492 (2009).
Navarro-Gonzalez, J. F., Mora-Fernandez, C., Muros de Fuentes, M. & Garcia-Perez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7, 327–340 (2011).
Chen, X. L. et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290, H1862–1870 (2006).
Jin, W. et al. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm 2008, 725174 (2008).
Itoh, K., Ishii, T., Wakabayashi, N. & Yamamoto, M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res 31, 319–324 (1999).
Kensler, T. W., Wakabayashi, N. & Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47, 89–116 (2007).
Zhang, D. D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38, 769–789 (2006).
Jeong, W. S., Kim, I. W., Hu, R. & Kong, A. N. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 21, 661–670 (2004).
Shen, G. et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res 67, 9937–9944 (2007).
Xu, C., Shen, G., Chen, C., Gelinas, C. & Kong, A. N. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene 24, 4486–4495 (2005).
Zoja, C., Benigni, A. & Remuzzi, G. The Nrf2 pathway in the progression of renal disease. Nephrol Dial Transplant 29 Suppl 1, i19–i24 (2014).
Jiang, T. et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59, 850–860 (2010).
Zheng, H. et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60, 3055–3066 (2011).
Neuhouser, M. L. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer 50, 1–7 (2004).
Miean, K. H. & Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J Agric Food Chem 49, 3106–3112 (2001).
Gates, M. A. et al. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer 121, 2225–2232 (2007).
Sun, T., et al. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J Food Sci 72, S98–102 (2007).
Harborne, J. B. & Williams, C. A. Advances in flavonoid research since 1992. Phytochemistry 55, 481–504 (2000).
Cai, X. et al. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol In Vitro 25, 1385–1391 (2011).
Cai, X. et al. The molecular mechanism of luteolin-induced apoptosis is potentially related to inhibition of angiogenesis in human pancreatic carcinoma cells. Oncol Rep 28, 1353–1361 (2012).
Cai, X. et al. Digitoflavone inhibits IkappaBalpha kinase and enhances apoptosis induced by TNFalpha through downregulation of expression of nuclear factor kappaB-regulated gene products in human pancreatic cancer cells. PLoS One 8, e77126 (2013).
Yang, Y. et al. Chemoprevention of dietary digitoflavone on colitis-associated colon tumorigenesis through inducing Nrf2 signaling pathway and inhibition of inflammation. Mol Cancer 13, 48 (2014).
Taneda, S. et al. Amelioration of diabetic nephropathy in SPARC-null mice. J Am Soc Nephrol 14, 968–980 (2003).
Ruiz, S., Pergola, P. E., Zager, R. A. & Vaziri, N. D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83, 1029–1041 (2013).
Bondeva, T. & Wolf, G. Reactive oxygen species in diabetic nephropathy: friend or foe? Nephrol Dial Transplant 29, 1998–2003 (2014).
Lee, D. F. et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell 36, 131–140 (2009).
Wakabayashi, N., Slocum, S. L., Skoko, J. J., Shin, S. & Kensler, T. W. When NRF2 talks, who’s listening? Antioxid Redox Signal 13, 1649–1663 (2010).
Aleksunes, L. M., Reisman, S. A., Yeager, R. L., Goedken, M. J. & Klaassen, C. D. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J Pharmacol Exp Ther 333, 140–151 (2010).
Kwon, E. Y., Jung, U. J., Park, T., Yun, J. W. & Choi, M. S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 64, 1658–1669 (2015).
Plaisier, C. et al. Effects of cinnamaldehyde on the glucose transport activity of GLUT1. Biochimie 93, 339–344 (2011).
Tan, Y. et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 60, 625–633 (2011).
Kashihara, N., Haruna, Y., Kondeti, V. K. & Kanwar, Y. S. Oxidative stress in diabetic nephropathy. Curr Med Chem 17, 4256–4269 (2010).
Singh, D. K., Winocour, P. & Farrington, K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7, 176–184 (2011).
Lim, A. K. & Tesch, G. H. Inflammation in diabetic nephropathy. Mediators Inflamm 2012, 146154 (2012).
Galkina, E. & Ley, K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17, 368–377 (2006).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81274150, 81374018), Jiangsu Province Funds for Distinguished Young Scientists (No. BK20140049).
Author information
Authors and Affiliations
Contributions
Y.Y. Conception and design, acquisition of data, analysis and interpretation of data, writing of the manuscript. G.C. Acquisition of data, analysis and interpretation of data. X.C. Analysis and interpretation of data. Z.T. and X.C. Acquisition of data, analysis and interpretation of data, proof-reading of the manuscript. J.Y. Development of methodology, revision of the manuscript. X.S. Technical support, analysis and interpretation of data. X.W., Y.Y. and C.H. Conception and design, interpretation of data, revision of the manuscript. W.L. Statistical analysis. P.C. Obtained funding, conception and design, study supervision, revision of the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, Y., Chen, G., Cheng, X. et al. Therapeutic potential of digitoflavone on diabetic nephropathy: nuclear factor erythroid 2-related factor 2-dependent anti-oxidant and anti-inflammatory effect. Sci Rep 5, 12377 (2015). https://doi.org/10.1038/srep12377
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12377
This article is cited by
-
Therapeutic potential of ADAM17 modulation in gastric cancer through regulation of the EGFR and TNF-α signalling pathways
Molecular and Cellular Biochemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.